Abstract
OBJECTIVES:
To describe the spectrum of cognitive outcomes of children with and without cerebral palsy (CP) after neonatal encephalopathy, evaluate the prognostic value of early developmental testing and report on school services and additional therapies.
METHODS:
The participants of this study are the school-aged survivors of the National Institute of Child Health and Human Development Neonatal Research Network randomized controlled trial of whole-body hypothermia. Children underwent neurologic examinations and neurodevelopmental and cognitive testing with the Bayley Scales of Infant Development–II at 18 to 22 months and the Wechsler intelligence scales and the Neuropsychological Assessment–Developmental Neuropsychological Assessment at 6 to 7 years. Parents were interviewed about functional status and receipt of school and support services. We explored predictors of cognitive outcome by using multiple regression models.
RESULTS:
Subnormal IQ scores were identified in more than a quarter of the children: 96% of survivors with CP had an IQ <70, 9% of children without CP had an IQ <70, and 31% had an IQ of 70 to 84. Children with a mental developmental index <70 at 18 months had, on average, an adjusted IQ at 6 to 7 years that was 42 points lower than that of those with a mental developmental index >84 (95% confidence interval, −49.3 to −35.0; P < .001). Twenty percent of children with normal IQ and 28% of those with IQ scores of 70 to 84 received special educational support services or were held back ≥1 grade level.
CONCLUSIONS:
Cognitive impairment remains an important concern for all children with neonatal encephalopathy.
Keywords: neonatal encephalopathy, cognitive outcomes, hypoxic ischemic encephalopathy
What’s Known on This Subject:
Surviving infants with neonatal encephalopathy treated with hypothermia have lower rates of moderate to severe cerebral palsy and cognitive impairment at 18 to 24 months. Limited data exist on the association between cognitive functioning and neuromotor, behavioral, and school outcomes.
What This Study Adds:
Although the incidence of death or IQ <55 is reduced after therapeutic hypothermia, survivors of neonatal encephalopathy with and without cerebral palsy are at elevated risk for subnormal IQ and the need for specialized educational services at 6 to 7 years.
Cognitive impairment, with or without neuromotor impairment, is an important problem after neonatal encephalopathy. Significant cognitive delays were identified among survivors at 18 to 24 months in all major randomized controlled trials of hypothermia for hypoxic ischemic encephalopathy (HIE).1–7 Studies in other high-risk neonatal populations report variable correlations between early assessments and school-age developmental outcomes, however.8–12 The accurate assessment of cognitive functioning among the survivors of presumed HIE is important in appraising the impact of perinatal interventions, selecting children for referral to early intervention and support services, and in counseling parents and families on potential long-term outcomes.
The American College of Obstetricians and Gynecologists and the American Academy of Pediatrics task force on neonatal encephalopathy and cerebral palsy (CP) proposed that an acute intrapartum event could not result in isolated cognitive deficits.13 Yet early human studies reported neurocognitive and behavioral problems even among the nondisabled survivors of HIE.14–21 The use of more precise definitions of presumed HIE and the introduction of therapeutic hypothermia may have altered the association between neuromotor and cognitive outcomes, potentially altering the disease phenotype.
We previously reported on the childhood outcomes of participants in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) randomized controlled trial of whole-body hypothermia.22 The aims of the current study were to describe the spectrum of cognitive outcomes of children with moderate and severe HIE with and without CP and neuromotor impairment, evaluate the prognostic value of early developmental testing and other predictors of school-age cognition, and report on special educational services and additional therapies. We hypothesized that cognitive impairment, though strongly associated with neuromotor impairment, could occur without CP, and infants with severe mental developmental impairment at 18 to 22 months (Mental Developmental Index [MDI] <70) would continue to have severe developmental and cognitive impairment at school age.
Methods
This is a secondary analysis of prospectively collected data from the NICHD NRN multicenter trial of whole-body hypothermia that recruited participants between 2000 and 2003.2,22 Neonates with moderate to severe encephalopathy within the first 6 hours of life were randomly assigned to therapeutic hypothermia at 33.5°C for 72 hours or intensive care alone, and participants were followed longitudinally at 18 to 22 months and 6 to 7 years. All surviving children with follow-up outcomes at both time points are included. Demographic, medical history, and growth parameters were obtained during the newborn period and at follow-up. Parents were interviewed to obtain information on functional status (Functional Status II)23 and the need for special education, school, and other support services (Child Health Questionnaire).24 Detailed neurodevelopmental assessments were performed by certified examiners who were trained to reliability and recertified on an annual basis; examiners were blinded to treatment group assignment.
Neurodevelopmental assessments included neurologic examinations (at 18–22 months and 6–7 years), the Bayley Scales of Infant Development II (BSID-II) at 18 to 22 months,2 and the Wechsler intelligence scales25,26 and the Neuropsychological Assessment (NEPSY)–Developmental Neuropsychological Assessment27 at 6 to 7 years. CP was defined as a nonprogressive central nervous system disorder that affected tone, movement, posture, and age-appropriate activities.28 Severity of CP was classified according to the Gross Motor Function Classification System (GMFCS), with moderate to severe CP defined as levels II to V.29,30 Neurologic assessments at 6 to 7 years also included evaluations of complex motor function (eg, straight line walking, standing on 1 foot, hopping, Romberg test) and fine motor and coordination skills (eg, finger-to-nose, alternate finger thumb opposition). The Wechsler Preschool and Primary Scale of Intelligence, Third Edition (WPPSI-III) was administered to all English-speaking children within the test age. To minimize loss to follow-up, the Wechsler Intelligence Scale for Children, Fourth Edition also was permitted for all Spanish-speaking children or children seen beyond 7 years, 3 months, and 15 days who could not be tested with the WPPSI-III. Children who were so severely impaired that psychometric evaluation was precluded were assigned the lowest possible score (>3 SD below the mean) for MDI and Full Scale IQ.31 A mean score of 100 and an SD of 15 represents normal functioning for typically developing children. Two subscales of the NEPSY were administered to assess attention or executive function and visuospatial processing. The study protocol was approved by the investigational review board of each participating NRN site, and informed parental consent was obtained before enrollment and school-age follow-up.
Neonatal and follow-up data were collected at each participating center and transmitted to the Research Triangle Institute, the NICHD data coordinating center, for data management and analysis. We made preliminary unadjusted comparisons of baseline characteristics and neurodevelopmental outcomes by using χ2 or Fisher exact tests for categorical data and 2-sided t tests for continuous data. The primary outcome of this secondary study selected a priori was cognitive impairment at 6 to 7 years of age, defined as an IQ score <70 (2 SD below the mean). Developmental instrument cutoff scores were compared between the hypothermia and normothermia groups and among children with and without CP or other neuromotor impairments at 6 to 7 years. We assessed the relationship between MDI and composite IQ scores within 1 and 2 SD of the mean (<70, 70–84, >84) by using positive and negative predictive values and the Spearman’s rank-order correlation coefficient. Predictors of 6- to 7-year cognitive functioning (school-age IQ) were examined via regression models that included important covariates associated with neurodevelopmental outcomes (eg, maternal education and level of encephalopathy). The rates of special education, speech therapy, and behavior problems also were compared between 18 months and 6 to 7 years. Because there were no statistically significant differences in median IQ scores between the hypothermia and control groups, for the purposes of this analysis all children were analyzed together.
Results
Details of Study Participants and Baseline Cognitive Testing
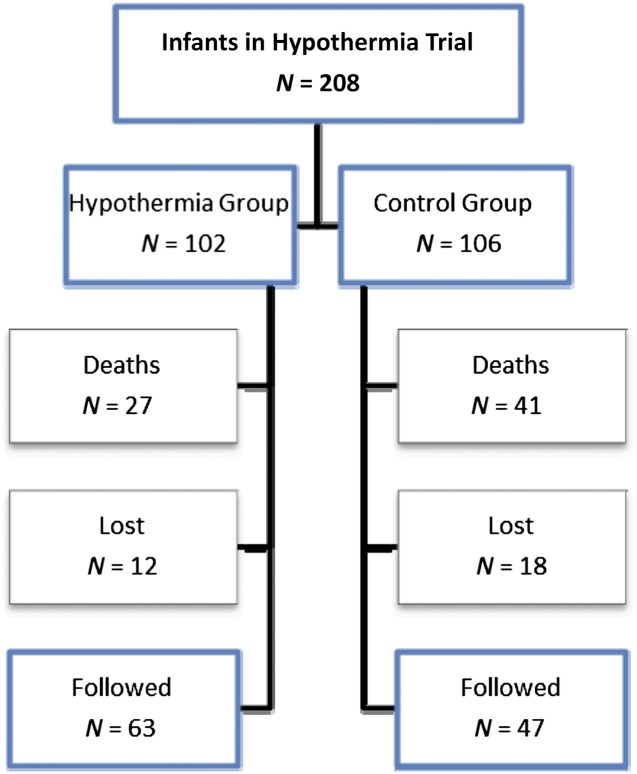
A total of 110 of 140 surviving children were evaluated at 18 months and 6 to 7 years and made up the study population (Figure 1). Twelve hypothermia participants and 18 control participants were lost to follow-up. Among these, 22 infants had BSID-II testing at 18 months but were missing IQ at 6 to 7 years. Four children had IQ testing at 6 to 7 years but were missing BSID-II, and 4 children had both assessments missing. Baseline characteristics were similar for children with and without assessments except for race; more participants who identified with white race underwent follow-up (Table 1). Seventy-eight percent of the study participants had moderate encephalopathy, and 22% had severe encephalopathy at randomization. Neither MDI nor IQ scores differed statistically between the hypothermia and normothermia groups; however, fewer children treated with hypothermia had scores <55 (Table 2). Verbal IQ scores were lower than performance IQ or processing speed quotients, with verbal IQ scores showing the greatest deficits among the individual measures of intelligence assessed. Among children able to undergo testing with the NEPSY, attention or executive functioning and visuospatial processing scores were similar between the 2 treatment groups (median core domain scores 95 and 97, respectively). However, 22% of children were deemed untestable as a result of neurologic or cognitive impairments (for attention or executive function, 17% hypothermia vs 28% normothermia; for visuospatial processing, 13% hypothermia vs 26% of children treated with normothermia; P not significant).
FIGURE 1.
Follow-up of a study population with moderate and severe neonatal encephalopathy. Among 140 survivors to 6–7 years, 110 infants (79%) had both 18- to 22-month and 6- to 7-year assessments and make up the participants of this study.
TABLE 1.
Maternal and Neonatal Characteristics of Study Participants
| Study Participants (survivors with 18-mo and 6- to 7-y assessments), N = 110 | Excluded (survivors without both assessments), N = 30 | |
|---|---|---|
| Maternal characteristics, (%) | ||
| Race, n* | ||
| Black | 38 (35) | 9 (30) |
| White | 45 (41) | 6 (20) |
| Other | 27 (25) | 15 (50) |
| Maternal age, y | 26.8 ± 5.8 | 27.3 ± 6.5 |
| Married, n | 65 (61) | 14 (48) |
| Maternal education less than high school (at birth) | 29 (35) | 9 (38) |
| Maternal education less than high school (at 18 mo) | 27 (26) | 8 (32) |
| Medicaid (at 18 mo) | 55 (50) | 16 (64) |
| Prenatal care | 103 (94) | 29 (97) |
| Gravida, median n | 2 | 2 |
| Parity, median n | 2 | 1.5 |
| Complications of pregnancy, n | ||
| Chronic hypertension | 18 (16) | 4 (13) |
| Antepartum hemorrhage | 20 (18) | 2 (7) |
| Thyroid disease | 0 (0) | 0 (0) |
| Diabetes | 10 (9) | 4 (13) |
| Intrapartum complications, n | ||
| Fetal heart decelerations | 82 (75) | 24 (80) |
| Cord prolapse | 20 (18) | 5 (17) |
| Uterine rupture | 17 (15) | 3 (10) |
| Maternal pyrexia | 11 (10) | 4 (13) |
| Shoulder dystocia | 14 (13) | 3 (14) |
| Maternal hemorrhage | 9 (8) | 0 (0) |
| Labor, h | 13.4 ± 10.1 | 11.7 ± 8.4 |
| Rupture of membrane, h | 6.5 ± 11.7 | 5.5 ± 6.8 |
| Emergency cesarean delivery, n | 79 (72) | 21 (70) |
| Neonatal characteristics | ||
| Age at randomization, h | 4.4 ± 1.3 | 4.4 ± 1.1 |
| Transferred from birth hospital, n | 48 (44) | 11 (37) |
| Male gender, n | 61 (55) | 17 (57) |
| Apgar score ≤5, n | ||
| At 5 min | 97 (88) | 25 (86) |
| At 10 min | 76 (76) | 18 (69) |
| Birth wt, g | 3359 ± 589 | 3458 ± 829 |
| Length, cm | 50.8 ± 3.0 | 51.5 ± 2.9 |
| Head circumference, cm | 34.1 ± 1.6 | 33.7 ± 2.3 |
| Intubation in delivery room, n | 101 (92) | 28 (93) |
| Continued respiratory support at 10 min, n | 100 (91) | 28 (93) |
| Time to spontaneous respiration ≥10 min, n | 66 (62) | 14 (54) |
| Cord blood | ||
| pH | 6.9 ± 0.2 | 6.9 ± 0.2 |
| Base deficit, mmol/L | 18.2 ± 7.3 | 17.5 ± 6.6 |
| Seizures, n | 51 (46) | 9 (30) |
| Moderate encephalopathy, n | 86 (78) | 22 (76) |
| Severe encephalopathy, n | 24 (22) | 7 (24) |
| Inotropic support, n | 34 (31) | 11 (37) |
| Anticonvulsants, n | 47 (47) | 10 (36) |
P < .05.
TABLE 2.
Cognitive Assessments at 18–22 mo and 6–7 y
| All Participants | Hypothermia | Normothermia | Hypothermia vs Normothermia | |
|---|---|---|---|---|
| P | ||||
| 18- to 22-mo neurodevelopmental assessment | ||||
| MDI | N = 110 | N = 63 | N = 47 | |
| Mean (SD) | 79.7 (20.1) | 81.9 (19.2) | 76.8 (21.0) | .19 |
| Median (Q1, Q3) | 83 (68, 94) | 85 (72, 93) | 79 (50, 94) | |
| (Minimum, maximum) | (49, 128) | (49, 128) | (49, 113) | |
| >84, n (%) | 53 (48) | 33 (52) | 20 (43) | .31 |
| 70–84, n (%) | 27 (25) | 17 (27) | 10 (21) | |
| 55–69, n (%) | 6 (5) | 2 (3) | 4 (9) | |
| <55, n (%) | 24 (22) | 11 (17) | 13 (28) | |
| 6- to 7-y neurodevelopmental assessment | ||||
| Full Scale IQa | N = 110 | N = 63 | N = 47 | |
| Mean (SD) | 78.5 (23.8) | 80.9 (23.3) | 75.3 (24.4) | .23 |
| Median (Q1, Q3) | 83 (65, 96) | 83 (69, 98) | 81 (40, 91) | |
| (Minimum, maximum) | (39, 121) | (39, 121) | (39, 116) | |
| >84, n (%) | 52 (47) | 30 (48) | 22 (47) | .75 |
| 70–84, n (%) | 27 (25) | 17 (27) | 10 (21) | |
| 55–69, n (%) | 8 (7) | 5 (8) | 3 (6) | |
| <55, n (%) | 23 (21) | 11 (17) | 12 (26) | |
| Verbal IQ | N = 91 | N = 55 | N = 36 | |
| Mean (SD) | 86.1 (17.1) | 85.9 (19.1) | 86.4 (13.7) | .88 |
| Median (Q1, Q3) | 85 (75, 101) | 85 (72, 102) | 85.5 (77, 96) | |
| (Minimum, maximum) | (46, 118) | (46, 118) | (46, 112) | |
| >84, n (%) | 51 (56) | 30 (55) | 21 (58) | .10 |
| 70–84, n (%) | 25 (27) | 12 (22) | 13 (36) | |
| 55–69, n (%) | 10 (11) | 9 (16) | 1 (3) | |
| <55, n (%) | 5 (5) | 4 (7) | 1 (3) | |
| Performance IQ | N = 91 | N = 55 | N = 36 | |
| Mean (SD) | 91.0 (16.8) | 91.3 (17.3) | 90.5 (16.3) | .82 |
| Median (Q1, Q3) | 90 (82, 101) | 93 (81, 104) | 89 (84, 100.5) | |
| (Minimum, maximum) | (45, 127) | (45, 127) | (45, 127) | |
| >84, n (%) | 59 (65) | 35 (64) | 24 (67) | .76 |
| 70–84, n (%) | 24 (26) | 16 (29) | 8 (22) | |
| 55–69, n (%) | 5 (5) | 2 (4) | 3 (8) | |
| <55, n (%) | 3 (3) | 2 (4) | 1 (3) | |
| Processing speed quotient | N = 87 | N = 53 | N = 34 | |
| Mean (SD) | 92.9 (17.0) | 93.2 (17.2) | 92.4 (17.0) | .84 |
| Median (Q1, Q3) | 94 (83, 103) | 94 (83, 104) | 91 (80, 102) | |
| (Minimum, maximum) | (45, 143) | (45, 123) | (49, 143) | |
| >84, n (%) | 62 (71) | 39 (74) | 23 (68) | .61 |
| 70–84, n (%) | 21 (24) | 11 (21) | 10 (29) | |
| 55–69, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| <55, n (%) | 4 (5) | 3 (6) | 1 (3) | |
| Attention or executive function core domain score | N = 77 | N = 46 | N = 31 | |
| Mean (SD) | 93.7 (16.6) | 94.5 (15.1) | 92.6 (18.7) | .63 |
| Median (Q1, Q3) | 95 (85, 104) | 95 (85, 104) | 95 (82, 110) | |
| (Minimum, maximum) | (50, 121) | (52, 121) | (50, 119) | |
| >84, n (%) | 60 (78) | 38 (83) | 22 (71) | .19 |
| 70–84, n (%) | 11 (14) | 6 (13) | 5 (16) | |
| 55–69, n (%) | 3 (4) | 0 (0) | 3 (10) | |
| <55, n (%) | 3 (4) | 2 (4) | 1 (3) | |
| No score, n (%) | 33/110 (30) | 17/63 (27) | 16/47 (34) | |
| Untestable | 29 (26) | 14 (22) | 15 (32) | |
| Due to neurologic or cognitive impairment | 24 (22) | 11 (17) | 13 (28) | |
| Other reasons | 5 (5) | 3 (5) | 2 (4) | |
| Missing, no reason | 4 (4) | 3 (5) | 1 (2) | |
| Visuospatial core domain score | N = 84 | N = 50 | N = 34 | |
| Mean (SD) | 97.7 (16.2) | 98.5 (15.6) | 96.5 (17.2) | .58 |
| Median (Q1, Q3) | 97 (85, 109) | 97 (88, 109) | 97 (85, 112) | |
| (Minimum, maximum) | (57, 132) | (57, 132) | (57, 127) | |
| >84, n (%) | 70 (83) | 42 (84) | 28 (82) | .89 |
| 70–84, n (%) | 11 (13) | 6 (12) | 5 (15) | |
| 55–69, n (%) | 3 (4) | 2 (4) | 1 (3) | |
| <55, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| No score, n (%) | 26/110 (24) | 13/63 (21) | 13/47 (28) | |
| Untestable | 23 (21) | 11 (17) | 12 (26) | |
| Due to neurologic impairment | 20 (18) | 8 (13) | 12 (26) | |
| Due to cognitive impairment | 3 (3) | 3 (5) | 0 (0) | |
| Due to other reasons | 3 (3) | 2 (3) | 1 (2) | |
| Missing, no reason |
At 18 mo, the BSID-II MDI was used to assess neurodevelopmental outcomes. At 6–7 y, the WPPSI-III (n = 93) or Wechsler Intelligence Scale for Children, Fourth Edition Full Scale IQ (n = 17) and the Neuropsychological Assessment (NEPSY) attention or executive function (n = 77) and visuospatial processing subscales (n = 84) were used to assess cognitive outcomes. Test score comparisons were conducted with Fisher exact tests for categorical data and 2-sided t tests for continuous data. Q1, quartile 1; Q3, quartile 3.
Children deemed so severely impaired as to preclude psychometric evaluation (n = 19) were assigned a Full Scale IQ of 39. No scores were imputed for verbal IQ, performance IQ, or processing speed quotient.
IQ Scores in Relation to CP and Neuromotor Function
Survivors free of CP tended to have higher developmental quotients than survivors afflicted by CP both at 18 months and 6 to 7 years; however, cognitive impairment occurred even in the absence of CP (Table 3). After neonatal encephalopathy, 96% of children (22/23) with CP had an IQ score <70 (all these children had moderate to severe CP at school age). Nine percent of children without CP had an IQ <70, and 31% had scores ranging from 70 to 84. Rates were similar among the subgroup of infants treated with hypothermia. The relationship between developmental quotients (MDI and IQ scores) and functional neuromotor outcomes is shown in Supplemental Tables 5 and 6. Among children with MDI scores <70, a wide range of functional gross motor outcomes was observed (ie, ability to walk, normal or level I GMFCS to total care, level V). For fine motor skills, more children with MDI scores <70 exhibited fine motor impairments (with only ∼17% exhibiting a fine pincer grasp). At 6 to 7 years, of children with IQ <70 (who underwent formal testing), 23% had normal gait, 6% to 16% had normal complex motor functions (ie, straight line walking, standing on 1 foot, hopping), and only ∼10% had intact fine motor and coordination skills (perhaps a gross indicator of developmental coordination problems, often linked with school difficulties).
TABLE 3.
Classification of Neurodevelopmental Outcomes at 18–22 mo and 6–7 y Among Survivors of HIE
| All Participants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Classification of MDI at 18–22 mo, n | Total Population, n Classification of IQ at 6–7 y | Survivors Free of CP at 6–7 y, n Classification of IQ at 6–7 y | Survivors With CP at 6–7 y, n Classification of IQ at 6–7 y | |||||||||
| >84 | [70, 84] | <70 | Total, n (%) | >84 | [70, 84] | <70 | Total, n (%) | >84 | [70, 84] | <70 | Total, n (%) | |
| All Participants | ||||||||||||
| >84 | 39 | 12 | 2 | 53 (48) | 38 | 12 | 2 | 52 (60) | 1a | 0 | 0 | 1 (4) |
| 70–84 | 12 | 13 | 2 | 27 (25) | 12 | 13 | 2 | 27 (31) | 0 | 0 | 0 | 0 (0) |
| <70 | 1 | 2 | 27 | 30 (27) | 1 | 2 | 4 | 7 (8) | 0 | 0 | 22b,c | 22 (96) |
| Total, n (%) | 52 (47) | 27 (25) | 31 (28) | 110b (100) | 51 (59) | 27 (31) | 8 (9) | 86 (100) | 1 (4) | 0 (0) | 22b (96) | 23 (100) |
| Change from MDI at 18–22 mo to IQ at 6–7 y | Categorical (>84, [70, 84], <70) | Numeric Values | Mean Numeric Change (SD) | |||||||||
| Unchanged | 79 | 3 | 0 | |||||||||
| Deteriorated | 16 | 64 | −10.2 (7.2) | |||||||||
| Improved | 15 | 43 | 12.0 (8.9) | |||||||||
| Hypothermia Participants | ||||||||||||
| >84 | 22 | 9 | 2 | 33 (52) | 22 | 9 | 2 | 33 (62) | 0 | 0 | 0 | 0 (0) |
| 70–84 | 8 | 7 | 2 | 17 (27) | 8 | 7 | 2 | 17 (32) | 0 | 0 | 0 | 0 (0) |
| <70 | 0 | 1 | 12 | 13 (21) | 0 | 1 | 2 | 3 (6) | 0 | 0 | 9b | 9 (100) |
| Total, n (%) | 30 (48) | 17 (27) | 16 (25) | 63b (100) | 30 (57) | 17 (32) | 6d (11) | 53 (100) | 0 (0) | 0 (0) | 9b (100) | 9 (100) |
| Normothermia Participants | ||||||||||||
| >84 | 17 | 3 | 0 | 20 (43) | 16 | 3 | 0 | 19 (58) | 1a | 0 | 0 | 1 (7) |
| 70–84 | 4 | 6 | 0 | 10 (21) | 4 | 6 | 0 | 10 (30) | 0 | 0 | 0 | 0 (0) |
| <70 | 1 | 1 | 15 | 17 (36) | 1 | 1 | 2 | 4 (12) | 0 | 0 | 13 | 13 (93) |
| Total, n (%) | 22 (47) | 10 (21) | 15 (32) | 47 (100) | 21 (64) | 10 (30) | 2 (6) | 33 (100) | 1 (7) | 0 (0) | 13 (93) | 14 (100) |
Percentages do not necessarily add up to 100% because of rounding.
Child with hemiplegia.
One child treated with hypothermia is missing CP status at 6–7 y. The child had CP at the 18-mo follow-up visit and was categorized as having the primary outcome based on severe developmental delay, blindness, and epilepsy.
Among children with IQ <70 with CP (N = 22), cranial MRI lesions included 16 with basal ganglia, thalamic, internal capsule, and cerebral lesions (NRN score 2B), 1 with basal ganglia, thalamic, and internal capsule lesions only (NRN score 2A), and 1 with hemispheric devastation (NRN score 3); 4 did not have an MRI.38
Of the 6 children treated with hypothermia who had IQ <70 but no CP, 4 had cranial MRI before NICU discharge. Two had normal scans, 1 had cerebral lesions only (NRN score 1B), and 1 had basal ganglia, thalamic, internal capsule, and cerebral lesions (NRN score 2B).
18-month Outcome in Relation to School-age IQ
Comparing 18- to 22-month and 6- to 7-year outcomes among all participants in the study, 79 of 110 (72%) children remained in the same developmental range (Table 3). Children with MDI <70 at 18 months had a high likelihood of cognitive impairment at 6 to 7 years of age (27/30 children had an IQ score <70). Children who tested within the normal range at 18 months (MDI >84), tended to have IQ scores >70 at school age (39 had scores >84, and 12 had scores in the 70–84 range). Children with intermediate MDI scores (70–84) had more variable outcomes. Those with declining scores often had comorbid neuromotor impairments (eg, complex or fine motor impairments) or lower socioeconomic status.
Even after we included other risk factors such as treatment, maternal education, and level of encephalopathy in the predictive models, a low MDI score (<70) at 18 to 22 months best predicted 6- to 7-year IQ <70 (Table 4). On linear regression, infants with a low MDI (<70) at 18 months had, on average, an adjusted IQ at 6 to 7 years that was 42 points lower than that of those with an MDI >84 (95% confidence interval [CI], −49.3 to −35.0, P < .001). In addition, children whose mothers had less than a high school (HS) education had, on average, an adjusted IQ that was 7.5 points (∼1/2 SD) lower than that of those whose mothers had more than an HS education (95% CI, −14.0 to −1.0, P = .02). Overall, an MDI score of <70 had a sensitivity of 0.87, specificity of 0.96, positive predictive value of 0.90, and negative predictive value of 0.95 for detecting cognitive impairment at school age (IQ <70) (Supplemental Table 7). The sensitivity was higher among infants treated with normothermia than those treated with hypothermia, which might have been mitigated by the severity of impairment. Spearman’s rank correlation for MDI and IQ scores was highly significant (ρ coefficient 0.74, P < .001).
TABLE 4.
Predictors of Cognitive Outcome as Measured by Full Scale IQ at 6–7 y
| Categorical | |||
|---|---|---|---|
| Odds Ratio | 95% CI | P | |
| Treatment group: hypothermia | 5.1 | 0.6 to 46.2 | .15 |
| MDI at 18 mo | <.001 | ||
| <70 vs >84 | 338 | 29.3 to >999 | <.001 |
| [70, 84] vs >84 | 1.8 | 0.2 to 14.0 | .60 |
| Maternal education less than high school at 18 mo | 2.5 | 0.4 to 14.7 | .32 |
| Level of encephalopathy: severe vs moderate | 2.2 | 0.3 to 15.4 | .43 |
| Continuous | |||
|---|---|---|---|
| Estimate | 95% CI | P | |
| Treatment group: hypothermia | −1.6 | (−7.2 to 4.1) | .58 |
| MDI at 18 mo | <.001 | ||
| <70 vs >84 | −42.1 | (−49.3 to −35.0) | <.001 |
| [70, 84] vs >84 | −5.6 | (−12.4 to 1.3) | .11 |
| Maternal education less than high school at 18 mo | −7.5 | (−14.0 to −1.0) | .02 |
| Level of encephalopathy: severe vs moderate | −2.1 | (−9.3 to 5.1) | .57 |
Rates of special educational services, speech therapy, and behavior problems are shown in Supplemental Table 8. After hypothermia, about one-third of the children followed were receiving early intervention or special educational assistance, as compared with 37% to 47% treated with normothermia. Rates were highest among children with an IQ <70 (100% of children with data available); however, even 20% of children with a normal IQ and 28% of those with IQ scores of 70 to 84 received some form of special educational services or were held back ≥1 grade level (P < .001). Behavior problems occurred in 21% of children at 18 months (as indicated by the BSID-II Behavior Rating Scale) and 7% of children at 6 to 7 years of age (by parental report) among those treated with hypothermia. Behavior problems were slightly higher in the normothermia group (32% and 9% at 18 months and 6 to 7 years, respectively). Maternal education less than HS was not predictive of resource utilization at 18 months or 6 to 7 years for either treatment group (P = NS); it was predictive of 6- to 7-year behavioral problems among hypothermia-treated children, however (3/15 infants with maternal education less than HS had behavior problems, compared with 1/45 infants with maternal education of HS or more, P = .046).
Discussion
Perinatal or postnatal hypoxic–ischemic insult models suggest learning impairment and memory and behavioral problems among the survivors of HIE.32–35 We observed some of these deficits in our school-age survivors who were treated for neonatal encephalopathy. More than 30% of the children followed to 6 to 7 years received special educational services. Furthermore, 7% to 9% had behavioral problems by parental report, a rate similar to the 5% to 7% reported among normally developing children.36 On formal IQ testing, cognitive impairment (IQ <70) was identified among nearly all children with CP (96%) and a small percentage of those without CP (9% of children without CP had an IQ score <70, and 31% had IQ scores of 70–84).
Miller and colleagues20 were among the first to identify an association between a watershed pattern of brain injury (greater abnormalities on T2 MRI in the intravascular boundary zones at a median age of 6 days) and mental developmental impairment at 30 months of age among children who had neonatal encephalopathy and normal motor outcomes. They also reported an association between deteriorating cognitive function and low socioeconomic status among children with mild to moderate impairments. We observed an association between maternal education less than HS and lower IQ scores. Whether genetically or environmentally influenced, these high-risk neonates might be targeted for early intervention services.
The same group of children reported by Miller et al was assessed at 4 years of age; increasing watershed injury pattern was associated with decreasing verbal IQ among children free of functional motor impairments.37 There was no association between brain injury pattern and performance IQ. Among the children with follow-up in our school-age study, verbal IQ was disproportionately affected as compared with performance IQ or processing speed quotient. However, the worst cognitive outcomes (across multiple domains) were observed among children with severe CP or neuromotor functional impairments. Unfortunately, MRI was performed only in a subset of the children we assessed.38
School-age follow-up after neonatal encephalopathy is limited mostly to studies conducted in the prehypothermia era. One exception is the recent follow-up study conducted by the TOBY study group.39 In that study, 6- to 7-year neurocognitive outcomes were reported for 184 of 325 trial participants randomly assigned to hypothermia or standard care alone. Survival with IQ ≥85 was higher for the hypothermia group (52%) than for control infants (39%) (relative risk, 1.31 [1.01–1.71]). Survival without CP or neurologic abnormalities also was higher for hypothermia-treated as compared with control infants. Mean IQ scores revealed no significant between-group differences, however. IQ scores were higher as compared with the NICHD follow-up, which might have been mitigated by loss to follow-up (nonparticipants had higher temperatures at randomization and were less likely to enter the study by 4 hours of age in the TOBY follow-up). Rates of utilization of special educational resources were comparable to ours (34.4% in the hypothermia group and 46.2% in the control group). Likewise, parentally reported behavioral problems were comparable to those of other school-age populations.
In contrast to the recent literature, follow-up studies conducted before the introduction of hypothermia provided comparisons with age-matched control children. This is important because rates of impairment might differ significantly when test reference norms are used as opposed to matched control groups.9 Robertson and colleagues14–17 studied 2 HIE birth cohorts (born 1974–1979 and 1982–1986) and age-matched controls assessed at 5.5 and 8 years of age. For both cohorts, all surviving children with severe HIE at birth were multiply handicapped, and 21% of surviving children with moderate HIE were disabled (8% had multiple disabilities). Nondisabled survivors of moderate neonatal encephalopathy were similar to healthy controls with respect to perceptual motor skills and receptive vocabulary but showed delays in school-related activities such as reading, spelling, and arithmetic. In fact, these children were likely to be 1 grade level behind the healthy age-matched controls. The outcomes of our neonates who received therapeutic hypothermia are better in terms of survival and neuromotor disabilities including CP; however, cognitive impairments remain. IQ scores were subnormal in 48% of participants treated with hypothermia (with 21% <70). Additionally, 32% of our hypothermia-treated children who were functioning at a level that permitted formal neurodevelopmental or IQ testing needed school assistance or special educational services.
Marlow et al40 evaluated the neurocognitive and behavioral outcomes of a cohort of school-age children from the United Kingdom born between 1992 and 1994 with HIE. Sixty-five of 130 eligible children were followed to 7 years along with a group of 49 matched comparison children who attended mainstream schools. In the 50 children without major disability, general cognitive ability was lowest for children with a history of severe encephalopathy (similar to our findings). Cognitive ability scores on the British Ability Scales–II were similar between children with moderate HIE at birth and those of their school-age peers. Despite no significant differences in developmental quotients among these children and their school-aged peers, children with neonatal encephalopathy had more special educational needs and lower achievement on assessments of national curriculum attainment. Behavioral problems were reported in 5 out of 22 (23%) of children with severe HIE, 2 out of 26 (8%) of children with moderate HIE, and 1 out of 42 (2%) of the comparison group. Seven percent of our school-age participants had behavioral problems, based on parental report. Twenty percent of our school-age participants with normal IQ scores needed special educational assistance, a finding that illustrates the importance of formal assessment before school entry. Careful neurodevelopmental follow-up coupled with advanced neuroimaging may advance our understanding of the neuropathology of neonatal encephalopathy and present new opportunities for intervention.
Important limitations of our work include the lack of power to detect significant differences in school-age IQ between the hypothermia and normothermia groups, the racial differences in survivors who underwent follow-up (which may have moderated our results in a favorable direction), the use of 2 instruments to assess IQ, and the failure to report functional outcomes or school achievement testing. Intellectual impairment requires impaired adaptive functioning. The NICHD NRN randomized trial of whole-body hypothermia was designed to detect a difference in death or disability at 18 to 22 months. Our school-age sample was not of sufficient size to detect a modest 5-point between-group difference in Full Scale IQ; a sample size of 142 per group would have been needed for this secondary study (based on a 2-tailed type 1 error of 0.05 and statistical power of 80%). Nevertheless, this is the largest study to date to address an unfortunate tenet of neuromythology regarding the lack of association between cognitive impairment and neonatal encephalopathy among survivors without CP. Our data show that cognitive impairment, though not specific to neonatal encephalopathy, is an important concern for all children with neonatal encephalopathy. Early intervention and school-age assessments are recommended regardless of motor impairment.
Supplementary Material
Acknowledgments
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC principal investigator) and Mr Scott A. McDonald (DCC statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (grant U10 HD27904): Abbot R. Laptook, MD; William Oh, MD; Theresa M. Leach, MEd, CAES; Angelita M. Hensman, RN, BSN; Lucy Noel; Victoria E. Watson, MS, CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (grants U10 HD21364, M01 RR80): Michele C. Walsh, MD, MS; Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Nancy Bass, MD; Harriet G. Friedman, MA; Nancy S. Newman, BA, RN; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center and University of Cincinnati Hospital (grants U10 HD27853, M01 RR8084): Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Jean J. Steichen, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Holly L. Mincey, RN, BSN; Jody Hessling, RN; Teresa L. Gratton, PA.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (grants U10 HD40492, M01 RR30): Ronald N. Goldberg, MD; C. Michael Cotten, MD, MHS; Kathryn E. Gustafson, PhD; Ricki F. Goldstein, MD; Kathy J. Auten, MSHS; Katherine A. Foy, RN; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Sandy Grimes, RN, BSN; Melody B. Lohmeyer, RN, MSN.
Emory University, Grady Memorial Hospital, and Emory University Hospital Midtown (grants U10 HD27851, M01 RR39): Barbara J. Stoll, MD; David P. Carlton, MD; Lucky Jain, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN, BS, CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development: Linda L. Wright, MD; Elizabeth M. McClure, MEd; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (grants U10 HD27856, M01 RR750): Brenda B. Poindexter, MD, MS; Anna M. Dusick, MD, FAAP; James A. Lemons, MD; Diana D. Appel, RN, BSN; Dianne E. Herron, RN; Lucy C. Miller, RN, BSN, CCRC; Leslie Richard, RN; Leslie Dawn Wilson, BSN, CCRC.
RTI International (grant U10 HD36790): W. Kenneth Poole, PhD; Jeanette O’Donnell Auman, BS; Margaret Crawford, BS; Betty K. Hastings; Jamie E. Newman, PhD, MPH; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN, BSN.
Stanford University and Lucile Packard Children’s Hospital (grants U10 HD27880, M01 RR70): Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS, CCRC; Maria Elena DeAnda, PhD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (grants U10 HD34216, M01 RR32): Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD, MPH; Kathleen G. Nelson, MD; Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN; Vivien A. Phillips, RN, BSN; Laurie Lou Smith, EdS, NCSP.
University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women (grant U10 HD40461): Neil N. Finer, MD; David Kaegi, MD; Maynard R. Rasmussen, MD; Yvonne E. Vaucher, MD, MPH; Martha G. Fuller, RN, MSN; Kathy Arnell, RNC; Chris Henderson, RCP, CRTT; Wade Rich, BSHS, RRT.
University of Miami Holtz Children’s Hospital (grants U10 HD21397, M01 RR16587): Shahnaz Duara, MD; Charles R. Bauer, MD; Sylvia Fajardo-Hiriart, MD; Mary Allison, RN; Maria Calejo, MS; Ruth Everett-Thomas, RN, MSN; Silvia M. Frade Eguaras, MA.
University of Rochester Medical Center, Golisano Children’s Hospital (grants U10 HD40521, M01 RR44): Dale L. Phelps, MD; Ronnie Guillet, MD, PhD; Gary J. Myers, MD; Diane Hust, MS, RN, CS; Linda J. Reubens, RN, CCRC.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (grants U10 HD40689, M01 RR633): Pablo J. Sánchez, MD; R. Sue Broyles, MD; Abbot R. Laptook, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Roy J. Heyne, MD; Catherine Twell Boatman, MS, CIMI; Cristin Dooley, PhD, LSSP; Gaynelle Hensley, RN; Jackie F. Hickman, RN; Melissa H. Leps, RN; Susie Madison, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Lizette E. Torres, RN; Alicia Guzman.
University of Texas Health Science Center at Houston Medical School, Children’s Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (grants U10 HD21373, M01 RR2588): Kathleen A. Kennedy, MD, MPH; Charles Green, PhD; Patricia W. Evans, MD; Esther G. Akpa, RN, BSN; Patricia Ann Orekoya, RN; Claudia I. Franco, RN, BSN; Anna E. Lis, RN, BSN; Georgia E. McDavid, RN; Patti L. Pierce Tate, RCP; Nora I. Alaniz, BS; Magda Cedillo; Susan Dieterich, PhD; Margarita Jiminez, MD; Terri Major-Kincade, MD, MPH; Brenda H. Morris, MD; M. Layne Poundstone, RN, BSN; Stacey Reddoch, BA; Saba Siddiki, MD; Maegan C. Simmons, RN; Laura L. Whitely, MD; Sharon L. Wright, MT.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (grant U10 HD21385): Yvette R. Johnson, MD, MPH; Laura A. Goldston, MA; Geraldine Muran, RN, BSN; Deborah Kennedy, RN, BSN; Patrick J. Pruitt, BS.
Yale University, Yale–New Haven Children’s Hospital (grants U10 HD27871, M01 RR125, UL1 RR24139): Patricia Gettner, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Elaine Romano, MSN; Joanne Williams, RN, BSN.
Footnotes
Dr Pappas conceptualized and designed the study, participated in data acquisition and interpretation, and drafted the initial manuscript; Dr Shankaran participated in the conceptualization and study design, data acquisition and interpretation, and review and revision of the manuscript; Mr McDonald and Dr Das participated in the study design and data interpretation, carried out the data analysis, and reviewed and revised the manuscript; Drs Vohr, Hintz, Ehrenkranz, Tyson, Yolton, Hammond, and Higgins and Ms Bara participated in the study design, data acquisition and interpretation, and review and revision of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Whole-Body Hypothermia Trial and its 6–7 Year School-age Follow-up through cooperative agreements. Although NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670 [DOI] [PubMed] [Google Scholar]
- 2.Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584 [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, et al. TOBY Study Group . Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361(14):1349–1358 [DOI] [PubMed] [Google Scholar]
- 4.Jacobs SE, Morley CJ, Inder TE, et al. Infant Cooling Evaluation Collaboration . Whole-body hypothermia for term and near-term newborns with hypoxic–ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165(8):692–700 [DOI] [PubMed] [Google Scholar]
- 5.Simbruner G, Mittal RA, Rohlmann F, Muche R, neo.nEURO.network Trial Participants . Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126(4). Available at: www.pediatrics.org/cgi/content/full/126/4/e771 [DOI] [PubMed] [Google Scholar]
- 6.Zhou WH, Cheng GQ, Shao XM, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic–ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157(3):367–372, e361–363 [DOI] [PubMed] [Google Scholar]
- 7.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341 [DOI] [PubMed] [Google Scholar]
- 9.Marlow N, Wolke D, Bracewell MA, Samara M, EPICure Study Group . Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19 [DOI] [PubMed] [Google Scholar]
- 10.Roberts G, Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group . The stability of the diagnosis of developmental disability between ages 2 and 8 in a geographic cohort of very preterm children born in 1997. Arch Dis Child. 2010;95(10):786–790 [DOI] [PubMed] [Google Scholar]
- 11.Barnett AL, Guzzetta A, Mercuri E, et al. Can the Griffiths scales predict neuromotor and perceptual–motor impairment in term infants with neonatal encephalopathy? Arch Dis Child. 2004;89(7):637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creighton DE, Robertson CM, Sauve RS, et al. Western Canadian Complex Pediatric Therapies Follow-up Group . Neurocognitive, functional, and health outcomes at 5 years of age for children after complex cardiac surgery at 6 weeks of age or younger. Pediatrics. 2007;120(3). Available at: www.pediatrics.org/cgi/content/full/120/3/e478 [DOI] [PubMed] [Google Scholar]
- 13.American College of Obstetricians and Gynecologists. Task Force on Neonatal Encephalopathy and Cerebral Palsy, American Academy of Pediatrics . Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology. Washington, DC: American College of Obstetricians and Gynecologists; 2003 [Google Scholar]
- 14.Robertson CM, Finer NN. Educational readiness of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Dev Behav Pediatr. 1988;9(5):298–306 [PubMed] [Google Scholar]
- 15.Robertson CM, Finer NN, Grace MG. School performance of survivors of neonatal encephalopathy associated with birth asphyxia at term. J Pediatr. 1989;114(5):753–760 [DOI] [PubMed] [Google Scholar]
- 16.Robertson CM, Grace MG. Validation of prediction of kindergarten-age school-readiness scores of nondisabled survivors of moderate neonatal encephalopathy in term infants. Can J Public Health. 1992;83(suppl 2):s51–s57 [PubMed] [Google Scholar]
- 17.Robertson CM. Long-term follow-up of term infants with perinatal asphyxia. In: Stevenson DK, Sunshine P, eds. Fetal and Neonatal Brain Injury: Mechanisms, Management and the Risks of Practice. 2nd ed. Oxford, England: Oxford University Press; 1997:615–630 [Google Scholar]
- 18.Moster D, Lie RT, Markestad T. Joint association of Apgar scores and early neonatal symptoms with minor disabilities at school age. Arch Dis Child Fetal Neonatal Ed. 2002;86(1):f16–f21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon G, Badawi N, Kurinczuk JJ, et al. Early developmental outcomes after newborn encephalopathy. Pediatrics. 2002;109(1):26–33 [DOI] [PubMed] [Google Scholar]
- 20.Miller SP, Newton N, Ferriero DM, et al. Predictors of 30-month outcome after perinatal depression: role of proton MRS and socioeconomic factors. Pediatr Res. 2002;52(1):71–77 [DOI] [PubMed] [Google Scholar]
- 21.Al-Macki N, Miller SP, Hall N, Shevell M. The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia. Pediatr Neurol. 2009;41(6):399–405 [DOI] [PubMed] [Google Scholar]
- 22.Shankaran S, Pappas A, McDonald SA, et al. Eunice Kennedy Shriver NICHD Neonatal Research Network . Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein RE, Jessop DJ. Functional Status II(R). A measure of child health status. Med Care. 1990;28(11):1041–1055 [DOI] [PubMed] [Google Scholar]
- 24.Landgraf JM, Abetz L, Ware JE., Jr The CHQ: A User’s Manual. Boston, MA: The Health Institute, New England Medical Center; 1996 [Google Scholar]
- 25.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3rd ed. San Antonio, TX: The Psychological Corporation; 2002 [Google Scholar]
- 26.Wechsler D. The Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: The Psychological Corporation; 2003 [Google Scholar]
- 27.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. San Antonio, TX: The Psychological Corporation; 1998 [Google Scholar]
- 28.Surveillance of Cerebral Palsy in Europe . Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol. 2000;42(12):816–824 [DOI] [PubMed] [Google Scholar]
- 29.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223 [DOI] [PubMed] [Google Scholar]
- 30.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48(6):424–428 [DOI] [PubMed] [Google Scholar]
- 31.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161(2):222–228, e223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jänicke B, Coper H. The effects of prenatal exposure to hypoxia on the behavior of rats during their life span. Pharmacol Biochem Behav. 1994;48(4):863–873 [DOI] [PubMed] [Google Scholar]
- 33.Arteni NS, Salgueiro J, Torres I, Achaval M, Netto CA. Neonatal cerebral hypoxia–ischemia causes lateralized memory impairments in the adult rat. Brain Res. 2003;973(2):171–178 [DOI] [PubMed] [Google Scholar]
- 34.Ramirez MR, Muraro F, Zylbersztejn DS, et al. Neonatal hypoxia–ischemia reduces ganglioside, phospholipid and cholesterol contents in the rat hippocampus. Neurosci Res. 2003;46(3):339–347 [DOI] [PubMed] [Google Scholar]
- 35.Ten VS, Bradley-Moore M, Gingrich JA, Stark RI, Pinsky DJ. Brain injury and neurofunctional deficit in neonatal mice with hypoxic–ischemic encephalopathy. Behav Brain Res. 2003;145(1–2):209–219 [DOI] [PubMed] [Google Scholar]
- 36.Pastor PN, Reuben CA, Duran CR. Identifying emotional and behavioral problems in children aged 4–17 years: United States, 2001–2007. Natl Health Stat Rep. 2012. Feb 24;(48):1–17 [PubMed] [Google Scholar]
- 37.Steinman KJ, Gorno-Tempini ML, Glidden DV, et al. Neonatal watershed brain injury on magnetic resonance imaging correlates with verbal IQ at 4 years. Pediatrics. 2009;123(3):1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankaran S, Barnes PD, Hintz SR, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Brain injury following trial of hypothermia for neonatal hypoxic–ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):f398–f404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzopardi D, Strohm B, Marlow N, et al. TOBY Study Group . Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140–149 [DOI] [PubMed] [Google Scholar]
- 40.Marlow N, Rose AS, Rands CE, Draper ES. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):f380–f387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.