Informed consent is essential for the conduct of ethical biomedical research.1 Despite its importance, obtaining informed consent is often a complex process, which raises concerns about the extent to which participants are truly informed. Effective implementation is especially difficult among research participants who have limited health literacy. Often, these potential participants are from traditionally high-risk groups, including underrepresented minorities and children. With this in mind, we suggest an innovative approach that uses low health-literacy communication strategies and visual aids to augment and potentially replace the traditional approach to informed consent.
The tension is clear. To provide a comprehensive review of the proposed research, the informed consent document and process are often lengthy, complex, and burdensome.2 Consequently, research participants who sign or verbalize consent often do so without truly understanding the form that they are being asked to sign. In a recent systematic review, participants in one-third of trials assessed did not have adequate understanding in the areas of risks, benefits, randomization, study aims, withdrawal, and voluntarism.3 There are no clear standards for “how much” understanding is adequate. Furthermore, we know that lower education levels, lower literacy, and a participant’s primary language are all associated with poor comprehension of the informed consent process.4 These issues are particularly important when studies are being done in children, adding an additional dimension to vulnerable populations.
Our approach was developed as a part of an ongoing randomized controlled trial (RCT) designed to prevent childhood obesity. As a part of the Growing Right Onto Wellness Trial (GROW; clinicaltrials.gov, NCT01316653),5 839 mother-child pairs consented to participate in this 3-year family-centered, community-based behavioral RCT. All 839 participants were from low-income, underserved populations at highest risk for obesity, and all children were between 3 and 5 years old. The institutional review board at Vanderbilt University Medical Center approved the trial.
The informed consent procedures for this population of mothers and children began with the “traditional” approach, by using a formal informed consent document that was reviewed in the participant’s language of choice, and was signed before participation. However, we supplemented the traditional informed consent approach by drawing on enhanced communication techniques from the low health-literacy and health-communication literature. GROW used a multifaceted approach to enhance informed consent that included (1) the use of effective health communication and low-literacy techniques, (2) the use of visual aids and graphics to promote understanding and guide the reader toward key study concepts, and (3) careful attention to child dissenting behaviors.
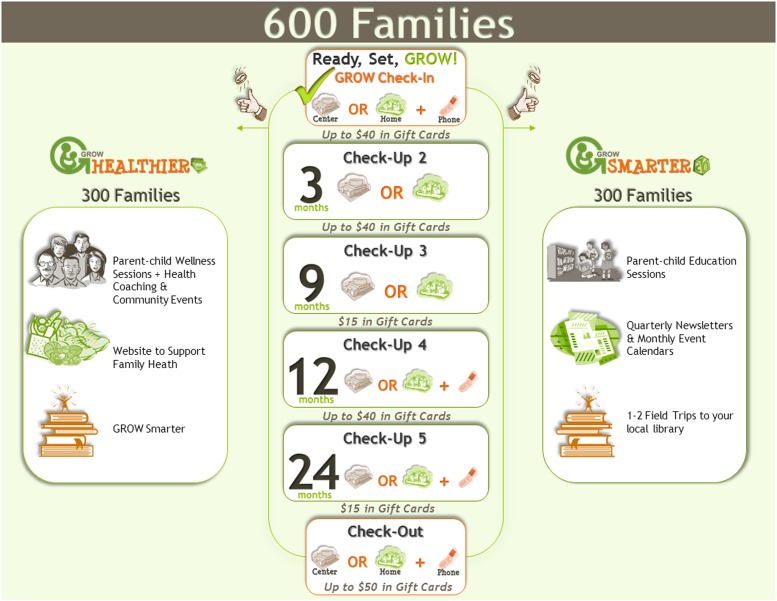
The GROW team developed 4 visual aids (see Fig 1 for representative example). These forms adhered to principles for development of low health-literacy print materials, including the use of white space, clear visual images, avoidance of jargon, and the use of easy-to-read figure captions.6 The study team used these visual aids while reading through the consent form with the participants, pointing to visual representations of important aspects of the informed consent process and the proposed research while talking through the written consent document. The first visual aid was an overview of study procedures and highlighted randomization, the purpose of each arm of the RCT, the study timeline, and potential compensation for participation. The second aid detailed what participants could expect from each of 6 data collection sessions throughout the study period by using pictures to explain specific types of data collected, including anthropometric collections, saliva samples for genetics, survey collection, and accelerometry. A third and fourth form were developed to explain in detail the specific activities of the 2 conditions (intervention and control groups).
FIGURE 1.
Representative example of visual informed consent detailing key principles, including randomization, incentives, and schedule of data collections.
Limited health literacy is an important determinant of patient understanding in clinical settings,7 and implementation of low health-literacy communication techniques has been shown to improve a broad range of patient understanding and clinical outcomes. Consequently, the GROW study team was trained to use the basic communication techniques of being seated while communicating, by using an open body position and eye contact, and avoiding medical jargon. Additionally, the study team was trained in the use of the teach-back technique. This technique asks participants to “teach-back” key concepts and allows the study team to ensure participant understanding before asking participants to sign the consent document. The study team also incorporated the use of the visual aids containing well-recognized images with ample white space and clear labeling, while pointing to and circling key components of the consent form alongside the visual aids.
Finally, because the children involved in this study were between 3 and 5 years old, the study team was trained to identify dissenting behaviors. An example of a dissenting behavior would include crying during data measurements. In these circumstances, children were encouraged to take a break from the data collection measurement, and were provided a healthy snack. Parents were encouraged not to force their children to participate in the data collection management, or to try to offer a reward for participation. If a child subsequently displayed dissenting behavior after redirection and encouragement, the child’s behavior was considered dissenting, and he or she was removed from the trial.
The emphasis of biomedical research targeting health disparities for traditionally underrepresented minorities and for broad socioeconomic strata has led to the inclusion of participants in research from a wide range of cultural and educational backgrounds. This creates a clear need to develop and test approaches, such as the use of low health-literacy principles in informed consent documents and communication delivery to ensure that we advance our ability to meet the needs of all potential research participants. We challenge the research community to build on the strong health-communication literature to not merely enhance but to replace the currently cumbersome and confusing informed consent process.
As national organizations continue to call for pediatric research to include participants from diverse populations, the informed consent process needs to incorporate a systematic approach to assess consent/assent for young children and their legal guardians interested in research participation. We need to know what works and what doesn’t in helping children and parents understand informed consent, so that we can continue to adhere to and advance the ethical bedrock of biomedical research that is informed consent.
Footnotes
Dr Heerman participated in the design and implementation of the study and drafted the initial manuscript; Dr White participated in the design of the study and reviewed and revised the manuscript; Dr Barkin conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01316653).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported, in part, by award U01 HL03620-03 from the National Heart, Lung, and Blood Institute, the Eunice Kennedy Shriver National Institute of Child Health and Development, and the Office of Behavioral and Social Sciences Research, as well as by Clinical and Translational Science Award UL1TR000445 from the National Center for Advancing Translational Sciences. Dr Heerman's time was supported in part by the Agency for Healthcare Research and Quality, grant number 1K12HS022990. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.US National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research . The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Bethesda, MD: The Commission; 1978 [PubMed] [Google Scholar]
- 2.Infectious Diseases Society of America. Grinding to a halt: the effects of the increasing regulatory burden on research and quality improvement efforts. Clin Infect Dis. 2009;49(3):328–335 [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Korbila IP, Giannopoulou KP, Kondilis BK, Peppas G. Informed consent: how much and what do patients understand? Am J Surg. 2009;198(3):420–435 [DOI] [PubMed] [Google Scholar]
- 4.Sherlock A, Brownie S. Patients’ recollection and understanding of informed consent: a literature review. ANZ J Surg. 2014;84(4):207–210 [DOI] [PubMed] [Google Scholar]
- 5.Po’e EK, Heerman WJ, Mistry RS, Barkin SL. Growing Right Onto Wellness (GROW): a family-centered, community-based obesity prevention randomized controlled trial for preschool child-parent pairs. Contemp Clin Trials. 2013;36(2):436–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doak CC, Doak LG, Root JH. Teaching Patients With Low Literacy Skills. 2nd ed. Philadelphia, PA: J.B. Lippincott; 1996 [Google Scholar]
- 7.DeWalt DA, Callahan LF, Hawk VH, et al. Health Literacy Universal Precautions Toolkit. AHRQ Publication No. 10-0046-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2010 [Google Scholar]