Highlights
-
•
Scarface is up-regulated in peripodial epithelium and peripodial stalk cells in wing disc.
-
•
Overexpression of JNK pathway causes up-regulation of scarface.
-
•
Down regulation of JNK pathway results in down regulation of scarface.
-
•
Scarface knockdown in wing disc phenocopies JNK pathway defect.
Abbreviations: SPH, serine protease homolog; JNK, Jun N-terminal Kinase; UAS, Upstream Activation Sequence; DPP, Decapentaplegic
Keywords: Drosophila, JNK pathway, Thorax closure, Serine protease homolog, Imaginal disc, Scarface
Abstract
The importance of the Jun N-terminal Kinase (JNK) pathway during normal development and tumor invasion has been well documented in Drosophila. Here, this pathway plays important roles in epithelial morphogenesis, wound healing, apoptosis, immunity and regulation of lifespan. However, which downstream molecules facilitate these effects is not very well elucidated. In this study, data are presented on a serine protease homolog (SPH), scarface. These data show that scarface is under regulatory control of the JNK pathway and that this pathway is both necessary and sufficient for its expression within the context of thoracic development. Consequently, down-regulation of scarface results in a thoracic-cleft phenotype that phenocopies the JNK pathway defect. A possible role of scarface during thoracic development in Drosophila is discussed.
1. Introduction
Development of Drosophila thorax has been an excellent model that has aided in our understanding of the morphogenetic processes involved in formation of an organism [1,2]. Several of the processes involved in thoracic development, like epithelial mesenchymal transition, migration, tissue invasion and extracellular matrix remodeling are also processes co-opted by cells that become cancerous and metastatic [3,4]. For this reason an understanding of gene functions, their regulation and processes they control during Drosophila thorax development has an added advantage of illuminating the underpinnings of tumor invasion and metastasis.
The adult thorax in drosophila develops from two contra-laterally positioned larval wing imaginal discs that undergo disc eversion and fuse at the midline during pupariation in a process that is referred to as thorax closure [2]. Defects in this closure result in a thoracic cleft phenotype and is observed in mutations of several key genes of the stress activated kinase pathway, called Jun N-Terminal Kinase (JNK) pathway [5]. JNK pathway along with DPP signaling regulates adhesiveness, cytoskeletal dynamics, extracellular matrix remodeling and tissue invasiveness during thorax closure [3,4,6]. Furthermore, it has been demonstrated that the JNK pathway also plays a critical role in tumor invasion and metastasis in Drosophila [4,7,8].
In response to upstream signals the JNK pathway phosphorylates AP-1 transcription factors D-Jun and D-Fos. These phosphorylated transcription factors translocate to the nucleus resulting in gene expression of downstream effectors. The core of this pathway is made up of the Drosophila JNK (DJNK) encoded by basket (bsk), the Drosophila JNKK encoded by hemipterous (hep) and the dual specificity phosphatase encoded by the puckered (puc) gene [5,9]. Puckered, through its phosphatase activity dephosphorylates the JNK, BSK, to negatively regulate JNK pathway in a feedback loop [5,9]. Thoracic cleft phenotypes have been reported for mutations in hep, dfos, underscoring the importance of JNK pathway in thoracic development of Drosophila [1,2,4,6].
An important downstream effector of the JNK pathway activity in thoracic development is the Matrix Metalloprotease [4,8]. Consequently, mutations in MMPs display the thoracic cleft phenotypes as well as disc eversion defects [4]. It has been shown that MMPs under the regulatory control of JNK pathway remodel a specialized extracellular matrix called Basement Membrane (BM). This BM remodeling is critical for disc eversion and thoracic closure in Drosophila [4].
Serine proteases (SP) are catalytically active enzymes that perform a host of functions in animals ranging from food digestion to immunity to development to blood coagulation [10,11]. Serine protease homologs (SPHs) on the other hand, while being similar to serine proteases, have a catalytic triad substitution rendering them catalytically compromised [12,13]. The exact function of these SPH molecules is not very well understood. However, it is suggested that these catalytically dead molecules have acquired new regulatory roles that they may perform in a context dependent manner [14]. Herein, it is demonstrated that a SPH in Drosophila encoded by the gene scarface [15] plays a role in thoracic development of Drosophila. It is shown that scarface is expressed in the Drosophila wing imaginal disc in regions important for thoracic development and is under regulatory control of the JNK pathway. A possible role for scarface during thoracic closure is discussed.
2. Materials and methods
2.1. Drosophila stocks and culture
Standard Drosophila culturing techniques were employed. All Drosophila cultures were raised on standard corn meal agar medium at 25 °C in vials and bottles unless stated otherwise. Few pellets of Red Star® active dry yeast were added to bottles and vials before culturing. The scarface protein trap (sfpbss) used in this study was a kind gift from Dr. Richard Mann and is described in Bonin and Mann (2004) [15]. UAS-Dcr-2 (FBti0100276), Tubulin-Gal80ts (FBti0027798), hepR75 (FBst0006761), Ptc-Gal4 (FBti0002124), Ap-Gal4 (FBti0002785), Pnr-Gal4 (FBti0004011), lines are described in indicated Flybase references and were obtained from the Bloomington Drosophila Stock Center. Deficiency lines [Df (2R) BSC630, Df (2R)BSC696 and Df (2R)BSC697] used in this study delete the scarface locus and were obtained from Bloomington Stock Center. sfpbss protein trap was placed in trans with these deficiencies to test for “scarface phenotype” enhancement. The UAS-scarface RNAi line used in this study was obtained from the Vienna Drosophila RNAi Center. UAS-dTAK1 is described in Mihaly et al. [16] and was used as described in Srivastava et al. [4].
2.2. Genotype used in various figures
| Fig. 1 | B, D: +/+ |
| C, E: w; sfpbss/+ | |
| F, I: sfpbss/Df(2R) BSC630 | |
| G, J: sfpbss/Df(2R) BSC696 | |
| H, K: sfpbss/Df(2R) BSC697 | |
| Fig. 2 | w; sfpbss/+ |
| Fig. 3 | y, hepR75/Y; sfpbss/+ |
| Fig. 4 | w; Ptc-Gal4, UAS-srcRFP, sfpbss/+; UAS-dTAK1/Tubulin-Gal80ts |
| Fig. 5 | A: Control w1118, phenotype indistinguishable from Ap-Gal4/+; UAS-Dcr-2/+ |
| B: Ap-Gal4/+; UAS-Dcr-2/UAS-scarface RNAi | |
| C: w; +/+; Pnr-Gal4, UAS-Dcr-2/+ | |
| D: w; +/+; Pnr-Gal4, UAS-Dcr-2, UAS-scarface RNAi/+ | |
| Supplementary Fig. 1 | A: w; +/+; Ubx-Gal4/UAS-GFP-nls |
| B–E: w; sfpbss/+ |
2.3. Induction of the JNK pathway, fixation of the imaginal discs and subsequent imaging
Drosophila larvae of the genotype w; Ptc-Gal4, UAS-srcRFP, sfpbss/+; UAS-dTAK1/Tubulin-Gal80ts were reared at 18 °C and then late second and early third instar larvae were shifted to 29 °C for 30–36 h. The third instar larvae were dissected, fixed and mounted in Vectashield mounting media with DAPI [4]. The mounted imaginal discs were then imaged on a LSM 510 Confocal microscope using sequential acquisition settings.
3. Results and discussion
3.1. Reagents used in this study and the scarface phenotype
The scarface gene encodes a protein that has been classified as a serine protease homolog (SPH) [12,13,15]. SPHs are similar to serine proteases but due to substitution of amino acids in the catalytic triad are rendered inactive [12,13]. Scarface protein contains a signal sequence and has been demonstrated to be a secreted protein [17,18]. To better understand the role of scarface in development and to understand its regulation we utilized available reagents for scarface (Fig. 1A). More specifically, in this study a previously published scarface protein trap line [15] and a RNA interference (RNAi) line available from the Vienna Drosophila RNAi Center (VDRC) have been used to understand scarface function. The sfpbss protein trap [15] is a semi lethal line where the animals die at pupal stage (Fig. 1B,C), however some escapers eclose as adults and exhibit a scar made up of necrotic tissue on the posterior side of head (Fig. 1D, E). This phenotype will be referred as the “scarface phenotype” for the rest of this study. In an attempt to generate a more severe scarface phenotype, we crossed the sfpbss protein trap to three deficiencies (Df (2R) BSC630, Df(2R) BSC696 and Df (2R) BSC697) that delete the scarface gene. Transheterozygous combinations of the protein trap with these deficiencies resulted in phenotypes that were indistinguishable from the sfpbss homozygous “scarface phenotype” (Fig. 1F–K). This suggests that the GFP tagged Scarface protein produced from the protein trap retains most of its function and is sufficient to allow animals to reach the pupal stage and in some cases adulthood. We next tested the scarface RNAi line obtained from the VDRC for lethality and for generation of the “scarface phenotype” when overexpressed ubiquitously using the UAS-Gal4 system [19]. Overexpression of scarface RNAi line under the influence of an Actin-Gal4 driver and in the presence of UAS-DCR2 (for enhancement of the RNAi effect) resulted in pupal lethality and scarring similar to what is shown in Fig. 1E. This suggests that the RNAi line targets the scarface locus and generates the “scarface phenotype”.
Fig. 1.
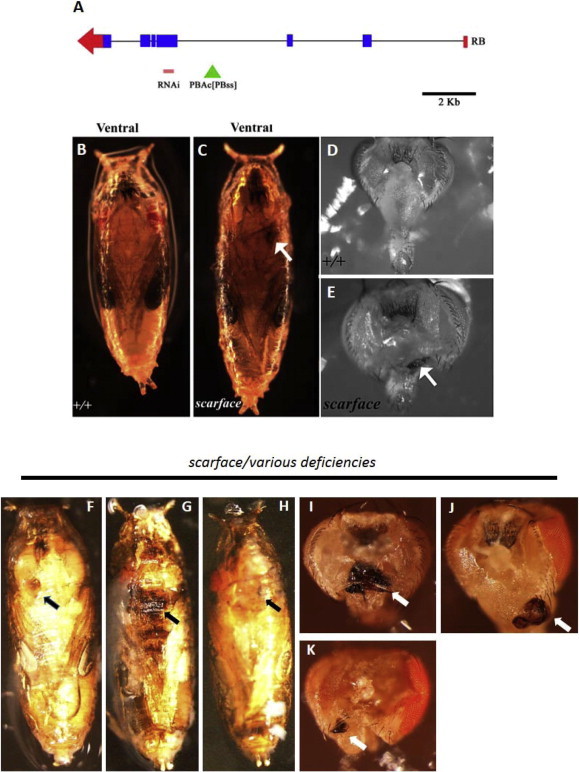
Scarface stocks used in this study and the “scarface phenotype”. (A) Location of scarface stocks used in this study superimposed on the genomic organization of the RB transcript. RNAi refers to the region targeted by the VDRC RNAi stock. PBAc[PBss] refers to the GFP protein trap line created by Bonin and Mann (2004) [15] and is referred to as sfpbss. (B–C) Ventral views of pupae from wild type (+/+) or scarface genotypes. The scarface mutant in C has necrotic scar near the proboscis (arrow). (D and E) Posterior views of head from wild type (+/+) adult and scarface adult escaper respectively. The necrotic black scar near the proboscis is marked with an arrow. (F–H) Ventral views of Pupae, (I–J) posterior view of head from sfpbss in transheterozygous combinations with various deficiencies that uncover the scarface locus. The scar associated with the “scarface phenotype” is marked with arrows. The transheterozygous combinations are as follows: (F and I) sfpbss/Df (2R) BSC630. (G and J) sfpbss/Df (2R) BSC696 (H and K) sfpbss/Df (2R) BSC697.
3.2. Scarface is expressed in Drosophila wing imaginal disc cells
It has been previously demonstrated that the sfpbss protein trap expression mimics the endogenous mRNA pattern during embryonic development (Sorrosal et al., EMBO Reports 2010 in Fig. 1B–D) [18]. Scarface has also been previously reported to be expressed in the wing imaginal disc cells in the future hinge region [15]. To better understand the distribution of Scarface protein in the developing wing disc and to confirm the previous observations in the hinge, we utilized the sfpbss protein trap as a readout of endogenous Scarface distribution in the wing imaginal disc. Confocal scans of wing discs from the protein trap demonstrated that the Scarface protein is expressed in the hinge region (Fig. 2B, arrowheads) as previously described. Additionally, we found expression of Scarface in the peripodial stalk (Fig. 2B, arrow and Supplementary Fig. 1) and the peripodial membrane (Fig. 2E, arrows and Supplementary Fig. 1) cells. In these peripodial cells, Scarface is excluded from the nucleus and is found predominantly in the cytoplasm (Fig. 2E, F, dashed circle and Supplementary Fig. 1). The peripodial stalk is the region of wing disc through which the imaginal disc is attached to the larval epidermis. On the other hand the peripodial epithelium is the region of wing disc that is made up of squamous epithelial cells and is folded over the columnar epithelial wing disc proper [20]. Both the peripodial stalk and the peripodial membrane cells play important roles during thorax development and are regions where the JNK pathway is also active [1].
Fig. 2.

Scarface is expressed in various regions of the wing disc. (A–F) Confocal scans of third instar larval wing disc. Scarface expression is marked with GFP from scarface GFP protein trap (sfpbss) in B, B’, E and nuclei are counterstained with DAPI in A, A’, D. The merged channels are presented in C, C’ and F. (A–C) Confocal scan through the columnar epithelium of the wing disc. (A’–C’) Close-up of the region boxed in A. Expression in the peripodial stalk is marked with an arrow in B and B’. The expression in the hinge region is indicated by arrowheads in B. (D–F) Region boxed in A is presented but of a focal plane from the side of peripodial epithelium. The expression of Scarface in E is marked with arrows and the circle in E and F highlight the nuclear exclusion of Scarface. The peripodial epithelium can be recognized by the presence of large nuclei in D and F.
3.3. JNK pathway is necessary and sufficient for scarface expression in the wing disc
The finding that Scarface protein is expressed in the peripodial membrane, and the peripodial stalk taken in combination with the known roles of JNK pathway in these tissues, suggested that scarface could be regulated by the JNK pathway. To test the necessity of JNK pathway for Scarface expression, we utilized a strong mutation in the D-JNKK encoded by the gene hemipterous (hepR75) [21]. Wing discs derived from larvae hemizygous for hepR75 and also carrying the scarface protein trap exhibited diminished Scarface expression as judged by reduced GFP levels in the peripodial stalk and peripodial membrane (Fig. 3, arrow compared to Fig. 2 B, B’, E). This demonstrated that JNK pathway is necessary for Scarface expression.
Fig. 3.
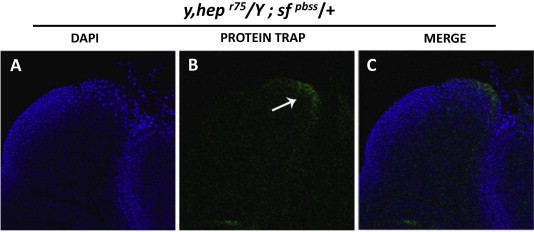
JNK pathway is necessary for Scarface expression. (A–C) Whole mount third larval instar wing disc from a male sfpbss bearing larva carrying a mutation in the hemipterous gene (hepr75). The region bounded by the box in Fig. 2A is presented. The disc is oriented with dorsal facing up and ventral facing down. Individual channels are labelled where nuclei are stained with DAPI (blue), Scarface protein trap with GFP. The GFP expression is lost in these wing discs in the region of the peripodial stalk (arrow in B) when compared to Fig. 2B’ suggesting that the JNK pathway is necessary for Scarface expression. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We next tested to see if JNK pathway was sufficient for the expression of Scarface. To test this we up-regulated this pathway using an upstream component of the JNK pathway called dTAK1 [16] and the UAS-Gal4 system [19]. Because JNK pathway activation results in larval lethality, we controlled the temporal expression of dTAK1 by placing it under the control of Gal80ts as previously described [4]. Induction of dTAK1 expression was achieved by shifting the larvae of the genotype Ptc-gal4, UAS-RFP, sfpbss/+; UAS-dTAK1/Tubulin-Gal80ts from 18 °C to 29 °C for 30–36 h (please see Section 2 for details). Wing discs derived from the temperature shifted larvae showed robust expression of Scarface in the Ptc-Gal4 domain along the anterior posterior compartment border (Fig. 4B, arrows). This up-regulation of Scarface in response to JNK pathway activation was also seen in the haltere and leg imaginal discs (Fig. 4D–F, arrow in E) suggesting that the JNK pathway is sufficient for Scarface expression within the context of these imaginal discs. Similar observations have been made for Scarface expression during embryonic development [17,18].
Fig. 4.

JNK Pathway is sufficient for Scarface expression. (A–F) Whole mount third instar imaginal discs from third instar larvae capable of activating the JNK pathway in a Ptc-Gal4 driver pattern along the antero-posterior compartment border. The domain of Ptc-Gal4 expression is marked by RFP (red channel in A, D) from a UAS-RFP transgene. The Scarface expression is marked with GFP (green channel, arrows in B and E) from sfpbss. The discs are counterstained with DAPI (blue) to label the nuclei. The merge of individual channels is also shown. Complete genotype of the discs can be found in materials and methods section. A–C are wing discs and D–F are leg and haltere discs labelled as L and H respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. scarface knockdown mimics JNK pathway mutant phenotype
Having established that Scarface is expressed in the peripodial stalk and peripodial membrane (JNK pathway is active here) cells under the regulatory control of JNK signaling, we reasoned that Scarface could be one of the effectors of JNK signaling within the context of thoracic development. If this were the case then Scarface knockdown in the developing thorax should result in phenotypes reminiscent of JNK pathway defect (thoracic cleft) [1,2,5]. To knockdown scarface in the developing thorax a UAS-scarface RNAi line was overexpressed in the domain of Pannier-Gal4 (Pnr-Gal4) expression and Apterous-Gal4 (Ap-Gal4) expression in combination with UAS-Dcr2 (for RNAi effect enhancement). The Pnr-Gal4 drives expression of a UAS transgene in the notum area of the wing disc destined to form the dorsal medio-lateral region of the adult thorax as well as thoracic bristles [22]. Apterous-Gal4, on the other hand expresses in the dorsal compartment of the wing disc which encompasses the region destined to form the future thorax [22].scarface knockdown using the Ap-Gal4 driver resulted in loss of bristles from the medio-lateral region of the thorax and the thorax displayed a mild thoracic cleft (arrow in Fig. 5B). scarface knockdown using the Pnr-Gal4 driver resulted in a much stronger thoracic cleft and loss of bristles from the medio-lateral region of the thorax (arrow in Fig. 5D). The thoracic cleft phenotype is reminiscent of JNK pathway defect and has been recovered for mutations in various components of the JNK pathway [5]. The regulation of scarface by the JNK pathway during thoracic development combined with the thoracic cleft phenotype generated when scarface is knocked down, suggests that part of the function of JNK during thoracic development is effected through SPH scarface.
Fig. 5.

Scarface knockdown in wing disc generates a thoracic cleft phenotype. (A–D) Adult thoraxes from control flies (A, C) and from flies where the scarface gene has been knocked down (B, D) using the Ap-Gal4 driver as in B or the Pnr-Gal4 driver as in D. The knockdown is achieved due to the presence of UAS-scarface RNAi and enhanced due to the presence of UAS-DCR2 transgenes. The complete genotype of these flies is provided in Section 2. Knockdown of scarface using the Ap-Gal4 driver results in mild cleft thorax phenotype with loss of bristles indicated with an arrow in B as compared to control thorax in A. Knockdown of scarface using the Pnr-Gal4 driver results in a more severe cleft thorax (D compared to B) phenotype with loss of bristles and the cleft indicated with arrows. The clefted thorax phenotype is reminiscent of phenotypes caused due to JNK pathway defect.
3.5. Possible function of scarface during Drosophila thorax development
JNK pathway plays a central role in thorax development and mediates this in at least two ways. First, it regulates the MMPs which in turn regulate the BM degradation, a step critical for the process of disc eversion [4]. Second, JNK signaling along with DPP also regulates the cytoskeletal dynamics and adhesion, properties important for the proper movement of epithelial sheets [6]. A third role of JNK pathway in thoracic development could be the regulation of BM integrity mediated by the SPH scarface during thoracic closure. While this needs to be unequivocally demonstrated during thoracic closure, evidence pointing to a similar function of JNK pathway in embryonic dorsal closure mediated by scarface already exists. It has been demonstrated by Sorrosal et al. (2010), that scarface is under regulatory control of the JNK pathway and is required for proper Laminin localization within the BM during embryonic dorsal closure [18].
While JNK regulation of scarface and its involvement in thoracic closure has been established in this study, what cellular role does scarface play during thoracic development in general and thoracic closure in particular (considering that it is not a catalytically active protein) is open to speculation. It has been suggested that SPHs have evolved into “catalytically dead regulatory molecules” and may exert their regulatory effects by sequestering substrates for serine proteases in a context dependent manner or by stabilizing cellular structures. For example, mutations in a SPH in Drosophila encoded by the gene masquerade (mas) [23] results in muscle attachment defects suggesting that this protein has a stabilizing effect on muscle-cell and matrix interaction. It is possible that, just as scarface stabilizes the integrity of BM by ensuring proper localization of Laminin during embryonic development [18], it may be performing a similar role during thoracic closure also. Future experiments may provide evidence confirming this idea.
During metamorphosis, the larval wing imaginal discs undergo a process of disc eversion where the imaginal discs are everted out of the larval body cavity. A hallmark of this disc eversion process is the degradation of BM mediated by the actions of MMPs under regulatory control of the JNK pathway [4]. However, once the disc eversion has occurred, the wing disc epithelial cells spread and move towards each other by crawling over the larval epidermis [3,6]. For spreading and movement to occur, modulation of the cell-matrix interaction with the underlying larval cells would be required. That this cell-matrix interaction modulation in thoracic closure could be brought about by SPH Scarface is evidenced by the thoracic cleft phenotype generated as a result of scarface knockdown. In the absence of this protein, (through RNAi knockdown) the cell-matrix interaction maybe destabilized, resulting in impeded and delayed movement of the two discs towards each other and generation of the thoracic cleft phenotype.
As mentioned earlier, experiments performed to study the function of Scarface in embryonic development have demonstrated that Scarface is indeed required for BM integrity through proper localization of Laminin [18] in the BM [24]. It has also been demonstrated in this study and others that scarface is under regulatory control of the JNK pathway [17,18]. Given that BM integrity is compromised during tumor metastasis and because JNK pathway is up-regulated in migrating tumors [4,8,25], it is attractive to suggest that SPH Scarface may play a role in tumor metastasis by regulating BM integrity. Future experiments will help us better understand the role of this SPH in tumor metastasis using a Drosophila model [26,27].
Author contributions
AS conceptualized, designed, performed the experiments, analyzed data and wrote the paper. QD helped with dissection, fixation and mounting of discs presented in Supplementary Fig. 1.
Acknowledgements
Many thanks are due to Dr. Tian Xu for his generous help with the continuation of this project in his laboratory and for his support. Thanks are also due to Dr. Richard Mann for the Scarface protein trap line. The BDSC and VDRC are acknowledged for various reagents used in this study. Part of this work was supported by an Anna Fuller Fund Postdoctoral Fellowship to AS at Yale University. Research in my laboratory at WKU is supported by the WKU Department of Biology startup funds, WKU Research Foundation RCAP-I grant # 11-8032 and by a KBRIN-AREA grant funded through a parent grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number 5P20GM103436-13.
Appendix A. Supplementary data
(A–E) Confocal scans of third instar larval wing discs. (A) Wing disc where Ubx-Gal4 drives UAS-GFP-nls in the peripodial epithelium. (B, C) Scarface is expressed in the peripodial membrane (arrows) as judged by comparison with the disc in A marked for peripodial expression bof Ubx. The nuclei are counterstained blue. (D, E) Scarface is also expressed in the peripodial stalk (arrow). The GFP from Scarface (B, D) and the merge (C, E) is shown.
References
- 1.Agnes F., Suzanne M., Noselli S. The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis. Development. 1999 Dec;126(23):5453–5462. doi: 10.1242/dev.126.23.5453. [DOI] [PubMed] [Google Scholar]
- 2.Zeitlinger J., Bohmann D. Thorax closure in Drosophila: involvement of Fos and the JNK pathway. Development. 1999 Sep;126(17):3947–3956. doi: 10.1242/dev.126.17.3947. [DOI] [PubMed] [Google Scholar]
- 3.Pastor-Pareja J.C., Grawe F., Martin-Blanco E., Garcia-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev. Cell. 2004 Sep;7(3):387–399. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava A., Pastor-Pareja J.C., Igaki T., Pagliarini R., Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl. Acad. Sci. U.S.A. 2007 Feb 20;104(8):2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kockel L., Homsy J.G., Bohmann D. Drosophila AP-1: lessons from an invertebrate. Oncogene. 2001 Apr 30;20(19):2347–2364. doi: 10.1038/sj.onc.1204300. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Blanco E., Pastor-Pareja J.C., Garcia-Bellido A. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 2000 Jul 5;97(14):7888–7893. doi: 10.1073/pnas.97.14.7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igaki T., Pagliarini R.A., Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 2006 Jun 6;16(11):1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Uhlirova M., Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006 Nov 15;25(22):5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y., Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004 Feb;14(2):94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Di Cera E. Serine proteases. IUBMB Life. 2009 May;61(5):510–515. doi: 10.1002/iub.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rawlings N.D., Barrett A.J. Evolutionary families of peptidases. Biochem. J. 1993 Feb 15;290(Pt 1):205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross J., Jiang H., Kanost M.R., Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003 Jan;30(304):117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- 13.Shah P.K., Tripathi L.P., Jensen L.J., Gahnim M., Mason C., Furlong E.E. Enhanced function annotations for Drosophila serine proteases: a case study for systematic annotation of multi-member gene families. Gene. 2008 Jan 15;407(1–2):199–215. doi: 10.1016/j.gene.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Pils B., Schultz J. Inactive enzyme-homologues find new function in regulatory processes. J. Mol. Biol. 2004 Jul 9;340(3):399–404. doi: 10.1016/j.jmb.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 15.Bonin C.P., Mann R.S. A piggyBac transposon gene trap for the analysis of gene expression and function in Drosophila. Genetics. 2004 Aug;167(4):1801–1811. doi: 10.1534/genetics.104.027557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihaly J., Kockel L., Gaengel K., Weber U., Bohmann D., Mlodzik M. The role of the Drosophila TAK homologue dTAK during development. Mech. Dev. 2001 Apr;102(1–2):67–79. doi: 10.1016/s0925-4773(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 17.Rousset R., Bono-Lauriol S., Gettings M., Suzanne M., Speder P., Noselli S. The Drosophila serine protease homologue scarface regulates JNK signalling in a negative-feedback loop during epithelial morphogenesis. Development. 2010 Jul;137(13):2177–2186. doi: 10.1242/dev.050781. [DOI] [PubMed] [Google Scholar]
- 18.Sorrosal G., Perez L., Herranz H., Milan M. Scarface, a secreted serine protease-like protein, regulates polarized localization of laminin A at the basement membrane of the Drosophila embryo. EMBO Rep. 2010 May;11(5):373–379. doi: 10.1038/embor.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993 Jun;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 20.Fristrom D., Fristrom J.W., editors. The Metamorphic Development of the Adult Epidermis. Cold Spring Harbor Laboratory Press; New York: 1993. [Google Scholar]
- 21.Glise B., Bourbon H., Noselli S. Hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell. 1995 Nov 3;83(3):451–461. doi: 10.1016/0092-8674(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 22.Calleja M., Herranz H., Estella C., Casal J., Lawrence P., Simpson P. Generation of medial and lateral dorsal body domains by the pannier gene of Drosophila. Development. 2000 Sep;127(18):3971–3980. doi: 10.1242/dev.127.18.3971. [DOI] [PubMed] [Google Scholar]
- 23.Murugasu-Oei B., Rodrigues V., Yang X., Chia W. Masquerade: a novel secreted serine protease-like molecule is required for somatic muscle attachment in the Drosophila embryo. Genes Dev. 1995 Jan 15;9(2):139–154. doi: 10.1101/gad.9.2.139. [DOI] [PubMed] [Google Scholar]
- 24.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer. 2003 Jun;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 25.Ebelt N.D., Cantrell M.A., Van Den Berg C.L. C-Jun N-Terminal Kinases mediate a wide range of targets in the metastatic cascade. Genes Cancer. 2013 Sep;4(9–10):378–387. doi: 10.1177/1947601913485413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava A. A protocol for genetic induction and visualization of benign and invasive tumors in cephalic complexes of Drosophila melanogaster. J. Vis. Exp. 2013;79 doi: 10.3791/50624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagliarini R.A., Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003 Nov 14;302(5648):1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–E) Confocal scans of third instar larval wing discs. (A) Wing disc where Ubx-Gal4 drives UAS-GFP-nls in the peripodial epithelium. (B, C) Scarface is expressed in the peripodial membrane (arrows) as judged by comparison with the disc in A marked for peripodial expression bof Ubx. The nuclei are counterstained blue. (D, E) Scarface is also expressed in the peripodial stalk (arrow). The GFP from Scarface (B, D) and the merge (C, E) is shown.


