Abstract
Several reports suggest that Agrobacterium tumefaciens nucleic acids can induce transformation of the cells of susceptible host plants and that bacteria-free tissue cultures of transformed cells contain A. tumefaciens DNA, RNA, antigens, or bacteriophages. We assayed Vinca rosea tumor DNA for base sequence homologies with A. tumefaciens DNA by DNA·DNA solution enrichment and DNA·DNA filter saturation hybridization techniques. No homologies were found by either method. The filter saturation hybridization technique included model filters containing known percentages of bacterial DNA mixed with V. rosea leaf DNA. Using this sensitive technique, we found that no more than 0.02% of the crown gall tumor genome could be homologous to A. tumefaciens DNA. This upper estimate of homology corresponds to 0.2 bacterial genome equivalent per diploid tumor cell.
Keywords: DNA·DNA hybridization, Vinca rosea, callus culture
Full text
PDF

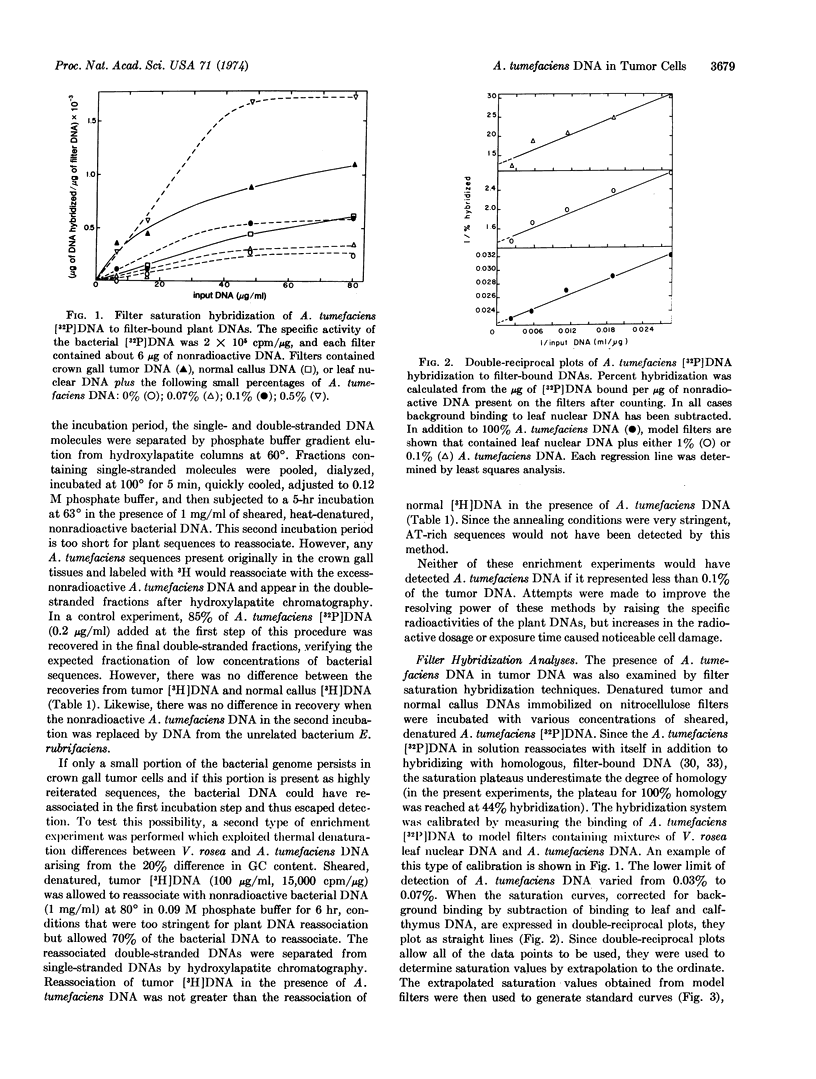
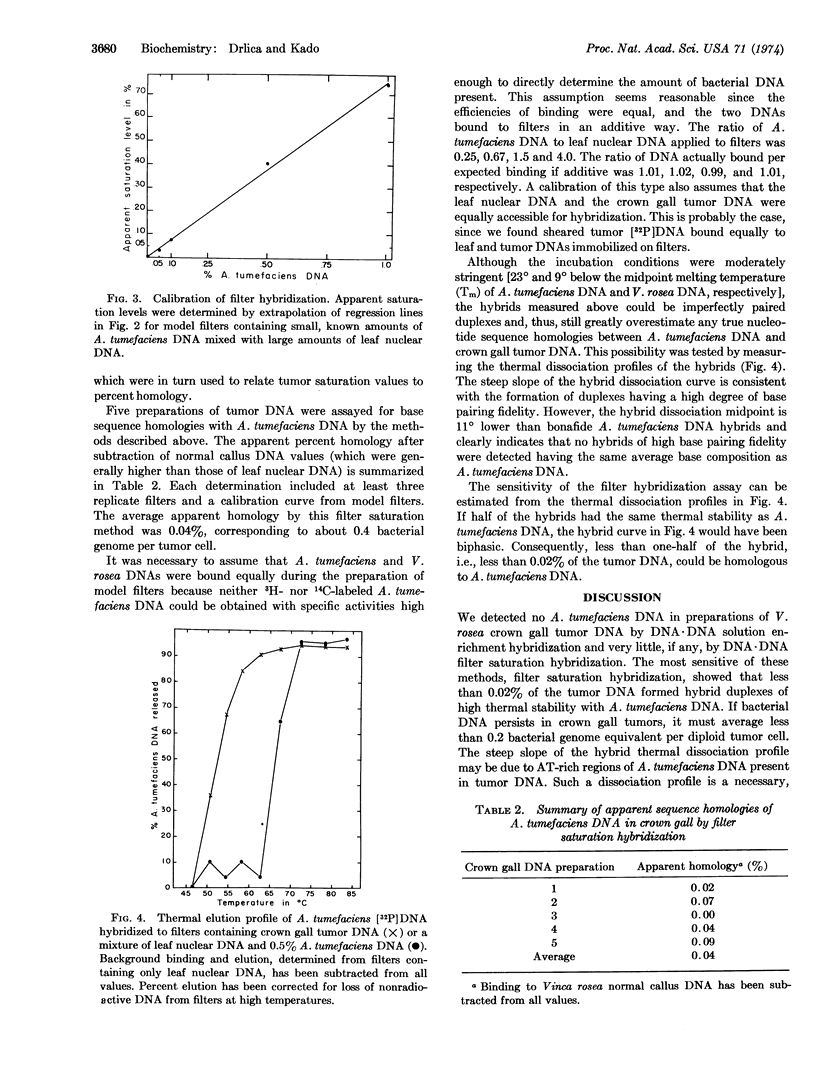

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUN A. C., WOOD H. N. On the activation of certain essential biosynthetic systems in cells of Vinca rosea L. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1776–1782. doi: 10.1073/pnas.48.10.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley R. E. The inception phase in the crown gall disease. Prog Exp Tumor Res. 1972;15:1–75. doi: 10.1159/000392507. [DOI] [PubMed] [Google Scholar]
- Beiderbeck R., Heberlein G. T., Lippincott J. A. On the question of crown-gall tumor initiation by DNA of bacteriophage PS8. J Virol. 1973 Feb;11(2):345–350. doi: 10.1128/jvi.11.2.345-350.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha K. C., Srivastava B. I. Evidence for the Presence of Bacteria-specific Proteins in Sterile Crown Gall Tumor Tissue. Plant Physiol. 1971 Aug;48(2):125–129. doi: 10.1104/pp.48.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ley J., Gillis M., Pootjes C. F., Kersters K., Tytgat R., Van Braekel M. Relationship among temperate Agrobacterium phage genomes and coat proteins. J Gen Virol. 1972 Aug;16(2):199–214. doi: 10.1099/0022-1317-16-2-199. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Drlica K. A., Knight C. A. Inhibition of chloroplast DNA synthesis by cycloheximide. J Mol Biol. 1971 Nov 14;61(3):629–641. doi: 10.1016/0022-2836(71)90068-4. [DOI] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Kovoor A. Sur la transformation de tissus normaux de Scorsonere provoquée in vitro par l'acide désoxyribonucléique d'Agrobacterium tumefaciens. C R Acad Sci Hebd Seances Acad Sci D. 1967 Nov 20;265(21):1623–1626. [PubMed] [Google Scholar]
- McCarthy B. J., McConaughy B. L. Related base sequences in the DNA of simple and complex organisms. I. DNA-DNA duplex formation and the incidence of partially related base sequences in DNA. Biochem Genet. 1968 Jun;2(1):37–53. doi: 10.1007/BF01458450. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Srivastava B. I. RNA-DNA hybridization studies with the crown gall bacteria and the tobacco tumor tissue. Biochem Biophys Res Commun. 1969 Jan 27;34(2):196–199. doi: 10.1016/0006-291x(69)90631-7. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sharp P. A., Sambrook J. Transcription of simian virus 40. II. Hybridization of RNA extracted from different lines of transformed cells to the separated strands of simian virus 40 DNA. J Virol. 1973 Jul;12(1):90–98. doi: 10.1128/jvi.12.1.90-98.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. L., Beardsley R. E. Bacteriphage activity in homogenates of crown gall tissue. J Virol. 1968 Jun;2(6):651–651. doi: 10.1128/jvi.2.6.651-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Quétier F., Huguet T., Guillé E. Induction of Crown-gall: partial homology between tumor-cell DNA, bacterial DNA and the G+C--rich DNA of stressed normal cells. Biochem Biophys Res Commun. 1969 Jan 6;34(1):128–133. doi: 10.1016/0006-291x(69)90538-5. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schilperoort R. A., Meijs W. H., Pippel G. M.W., Veldstra H. Agrobacterium tumefaciens cross-reacting antigens in sterile crown-gall tumors. FEBS Lett. 1969 May;3(3):173–176. doi: 10.1016/0014-5793(69)80127-4. [DOI] [PubMed] [Google Scholar]
- Schilperoort R. A., Van Sittert N. J., Schell J. The presence of both phage PS8 and Agrobacterium tumefaciens A 6 DNA base sequences in A 6 -induced sterile crown-gall tissue cultured in vitro. Eur J Biochem. 1973 Feb 15;33(1):1–7. doi: 10.1111/j.1432-1033.1973.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Schilperoort R. A., Veldstra H., Warnaar S. O., Mulder G., Cohen J. A. Formation of complexes between DNA isolated from tobacco crown gall tumours and RNA complementary to Agrobacterium tumefaciens DNA. Biochim Biophys Acta. 1967 Sep 26;145(2):523–525. doi: 10.1016/0005-2787(67)90075-5. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. DNA-DNA hybridization studies between bacterial DNA, crown gall tumor cell DNA and the normal cell DNA. Life Sci II. 1970 Aug 8;9(15):889–892. doi: 10.1016/0024-3205(70)90058-5. [DOI] [PubMed] [Google Scholar]
- Westphal H., Dulbecco R. Viral DNA in polyoma- and SV40-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1158–1165. doi: 10.1073/pnas.59.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]



