Abstract
Introduction. The impact of preoperative BMI on surgical outcomes and long-term survival of gastric cancer patients was investigated in various reports with contrasting results. Materials & Methods. A total of 378 patients who underwent a surgical resection for primary gastric cancer between 1994 and 2011 were retrospectively studied. Patients were stratified according to BMI into a normal group (<25, group A), an overweight group (25–30, group B), and an obesity group (≥30, group C). These 3 groups were compared according to clinical-pathological characteristics, surgical treatment, and long-term survival. Results. No significant correlations between BMI and TNM (2010), UICC stage (2010), Lauren's histological type, surgical results, lymph node dissection, and postoperative morbidity and mortality were observed. Factors related to higher BMI were male gender (P < 0.05), diabetes (P < 0.001), and serum blood proteins (P < 0.01). A trend to fewer lymph nodes retrieved during gastrectomy with lymphadenectomy in overweight patients (B and C groups) was observed, although not statistically significant. There was no difference in overall survival or disease-specific survival between the three groups. Conclusion. According to our data, BMI should not be considered a significant predictor of postoperative complications or long-term result in gastric cancer patients.
1. Introduction
Gastric cancer represents one of the most frequent neoplasia worldwide, and specifically the fourth and fifth most common cancer in men and women and the third and fifth cause of cancer-related death [1]. To date the evidence shows a rising incidence of obesity, in particular in Western Countries, and an increased relative risk of cancer-related mortality at multiple sites with increased BMI [2]. Some authors suggest a relationship between increased BMI and esophageal and gastric cardia adenocarcinoma [3–5]. Some theories try to explicate the etiology of this relationship, as it seems to be related to the metabolic syndrome and the relative chronic inflammation [6] or to the higher abdominal pressure and the resulting gastroesophageal reflux [7, 8], but actually no clear etiology has been established between obesity and gastric carcinogenesis. Usually obese cancer patients have often been perceived as being at high risk of surgical complications. In fact, there are several technical disadvantages during a surgical procedure for obese patients, including poorer surgical visibility, blood oozing from soft tissues, a dissection plane hindered by adipose tissue, and difficulty with anastomosis. Another important aspect in the intricate relation between obesity and gastric cancer is the effect of BMI on the patient's outcomes following surgical gastric resection for adenocarcinoma. Many authors investigated this relation, with very different results in terms of survival, pathological findings, and results of surgical procedures [9–11]. The aim of this study was to examine the relationship between overweight and long-term survival of patients undergoing gastrectomy for gastric cancer in our center and to evaluate the postoperative complications and the adequacy of surgical therapy.
2. Materials and Methods
In this study 378 patients with primary gastric cancer surgically treated in the Unit of General Surgery and Surgical Oncology, University of Siena, between 1994 and 2011 were included. The histological confirmation of the neoplasia was preoperatively achieved by endoscopic biopsies. In order to perform the most appropriate surgical treatment an accurate preoperative staging was performed in all patients by computed tomography (CT) scan and, when necessary, by endoscopic ultrasound. Subtotal or total gastrectomy was performed according to tumor location and the possibility to obtain negative resection margins and a potentially curative (R0) resection. The extent of lymphadenectomy was classified according to the Japanese Gastric Cancer Association (JGCA) guidelines, as previously described [12, 13]. All the specimens were analyzed for pTNM determination. Tumor stage was defined according to the pathological tumor node metastasis (pTMN system) classification proposed by the International Union against Cancer (UICC/AJCC, 7th edition, 2010). All cases before 2010 were revised and the pTNM classification has been updated at the 7th edition [14]. Gastroesophageal junction tumors were defined according by the classification described by Siewert et al. [15], and type I tumors (distal esophageal) were excluded. Patients were stratified on the basis of BMI into three groups: normal group (BMI < 25 kg/m2, group A), overweight group (BMI 25–30 kg/m2, group B), and obesity group (BMI ≥ 30 kg/m2, group C). Clinical characteristics, surgical procedures, and histological findings were recorded in a specific database. Data were compared between the three groups, with special reference to postoperative outcome, morbidity, mortality, and long-term survival. Statistical analysis was performed with the χ 2 test or Fisher exact test to compare categorical variables. The Mann-Whitney U test and Kruskal-Wallis one-way analysis of variance (ANOVA) were used to compare continuous variables not normally distributed. Cumulative survival was calculated by the life table method of Kaplan and Meier, and the log-rank test was used to distinguish significant differences. Statistical significance was determined at P value of <0.05.
3. Results
218 patients were selected in group A, 121 in group B, and 39 in group C. The median BMI was 24.03 kg/m2 and the median age was 66 years. We performed a subtotal gastrectomy in 257 cases and a total gastrectomy in 121. In total, a median number of 35 lymph nodes (range: 3–140) was removed. Information regarding BMI, gender, age, and surgical data was available for all patients, whereas data regarding pT, pN, and tumor site were missing for some cases.
Clinicopathological features of the patients are summarized in Table 1.
Table 1.
Clinicopathological features of normal (BMI < 25 kg/m2), overweight (BMI 25–30 kg/m2), and obese (BMI ≥ 30 kg/m2) patients.
| Total | Group A | Group B | Group C | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Patient (n) | 378 | 218 | 121 | 39 | ||||
| BMI (median) | 24.03 | 22.05 | 27.00 | 31.62 | <0.001 | |||
| Age, y (median) | 66 | 67.2 | 67 | 63 | 0.111 (Kruskal) | |||
| Sex (male, female) | 223 : 155 | 116 : 102 | 84 : 37 | 23 : 16 | 0.015 (χ 2) | |||
| Diabetes (yes, no) | 51 : 319 | 23 : 192 | 15 : 101 | 13 : 26 | <0.001 (χ 2) | |||
| Missing | 8 | |||||||
| pT | 0.945 (χ 2) | |||||||
| 1a | 42 | 25 | 11.5% | 13 | 10.7% | 4 | 10.3% | |
| 1b | 30 | 17 | 7.8% | 10 | 8.3% | 3 | 7.7% | |
| 2 | 53 | 25 | 11.5% | 23 | 19.0% | 5 | 12.8% | |
| 3 | 72 | 44 | 20.2% | 21 | 17.4% | 7 | 17.9% | |
| 4a | 162 | 94 | 43.1% | 50 | 41.3% | 18 | 46.2% | |
| 4b | 18 | 12 | 5.5% | 4 | 3.3% | 2 | 5.1% | |
| Missing | 1 | 1 | 0.5% | |||||
| pN | 0.132 (χ 2) | |||||||
| 0 | 136 | 81 | 37.2% | 45 | 37.2% | 10 | 25.6% | |
| 1 | 39 | 16 | 7.3% | 15 | 12.4% | 8 | 20.5% | |
| 2 | 51 | 35 | 16.1% | 12 | 9.9% | 4 | 10.3% | |
| 3a | 72 | 35 | 16.1% | 28 | 23.1% | 9 | 23.1% | |
| 3b | 78 | 49 | 22.5% | 21 | 17.4% | 8 | 20.5% | |
| Missing | 2 | 2 | 0.9% | |||||
| Tumor site | 0.514 (χ 2) | |||||||
| Upper third | 53 | 28 | 12.8% | 17 | 14.0% | 8 | 20.5% | |
| Middle third | 104 | 57 | 26.1% | 39 | 32.2% | 8 | 20.5% | |
| Lower third | 193 | 113 | 51.8% | 59 | 48.8% | 21 | 53.8% | |
| Diffuse | 16 | 10 | 4.6% | 5 | 4.1% | 1 | 2.6% | |
| Gastric stump | 10 | 9 | 4.1% | 0 | 1 | 2.6% | ||
| Missing | 2 | 1 | 0.5% | 1 | 0.8% | 0 | ||
| Lauren | 0.318 (χ 2) | |||||||
| Diffuse | 104 | 65 | 29.8% | 24 | 19.8% | 15 | 38.5% | |
| Intestinal | 227 | 126 | 57.8% | 82 | 67.8% | 19 | 48.7% | |
| Mixed | 35 | 20 | 9.2% | 11 | 9.1% | 4 | 10.3% | |
| Missing | 12 | 7 | 3.2% | 4 | 3.3% | 1 | 2.6% | |
| Morbidity | 0.803 (χ 2) | |||||||
| Yes | 114 | 63 | 28.9% | 38 | 31.4% | 13 | 33.3% | |
| No | 264 | 155 | 71.1% | 83 | 68.6% | 26 | 66.7% | |
| Major morbidity | 0.696 (χ 2) | |||||||
| Yes | 87 | 47 | 21.6% | 31 | 25.6% | 9 | 23.1% | |
| No | 291 | 171 | 78.4% | 90 | 74.4% | 30 | 76.9% | |
| Postoperative death | 0.263 (χ 2) | |||||||
| Yes | 14 | 11 | 5.0% | 2 | 1.7% | 1 | 2.6% | |
| No | 364 | 207 | 95.0% | 119 | 98.3% | 38 | 97.4% | |
| Hospital stay, days: median | 11 | 12 | 12 | 11 | 0.356 (Kruskal) | |||
| Examined lymph nodes (median) | 35 | 37 | 33 | 35 | 0.059 (Kruskal) | |||
| Metastatic lymph nodes (median) | 4 | 4 | 3 | 5 | 0.586 (Kruskal) | |||
Preoperatively, higher BMI was associated with male gender (P < 0.05), type 2 diabetes (P < 0.001) (Table 1), and serum blood proteins (P < 0.01) (Figure 1). No significant relationships were found between BMI and TNM (2010), UICC stage (2010), Lauren's histological type, tumor site, major morbidity, and hospital stay. A trend to fewer retrieved lymph nodes was observed in overweight patients (B and C groups), as the median number of lymph nodes examined was 37 for group A (range 3–140), 33 for group B (range 5–108), and 35 for group C (range 4–68) (P = 0.059 Kruskal-Wallis). This difference was also not statistically significant when correlation analysis was used (P = 0.055) (Figure 2). The median number of metastatic lymph nodes was 4 for group A (range 0–86), 3 for group B (range 0–55), and 5 for group C (range 0–63) (P = 0.586).
Figure 1.
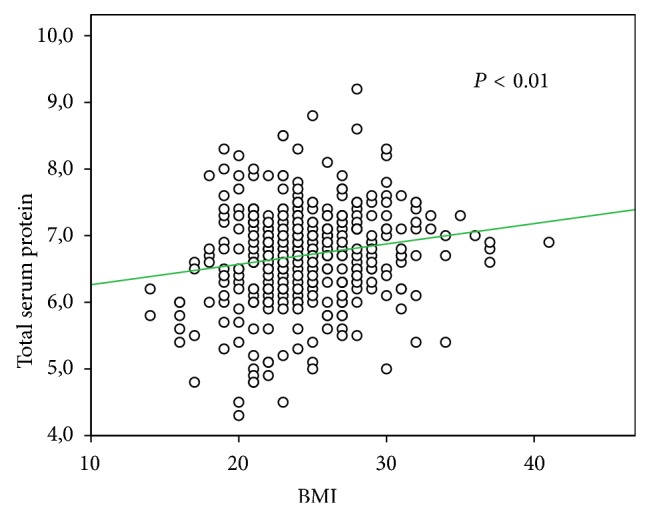
Correlation between BMI and serum blood protein (P < 0.01).
Figure 2.
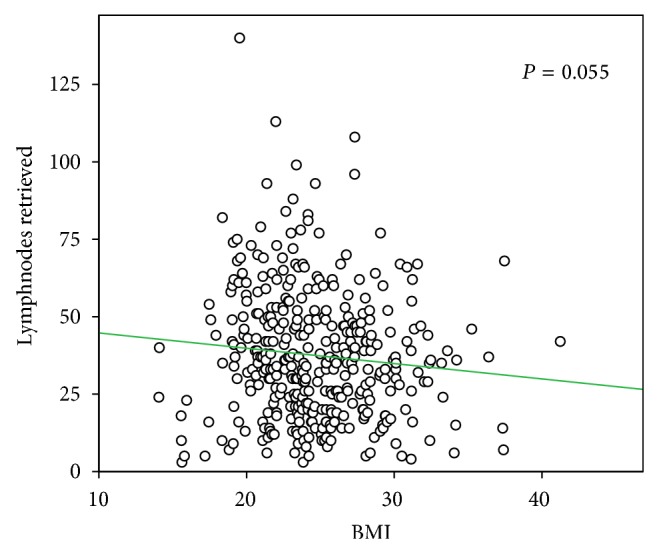
Correlation between BMI and number of lymph nodes harvested (P = 0.055).
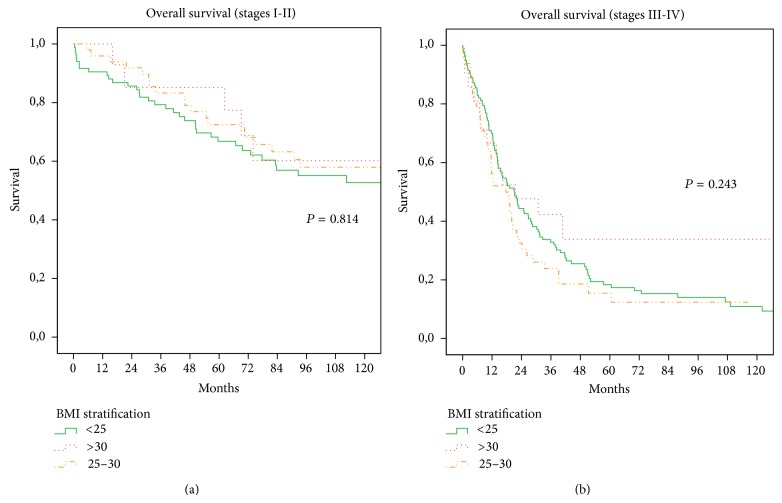
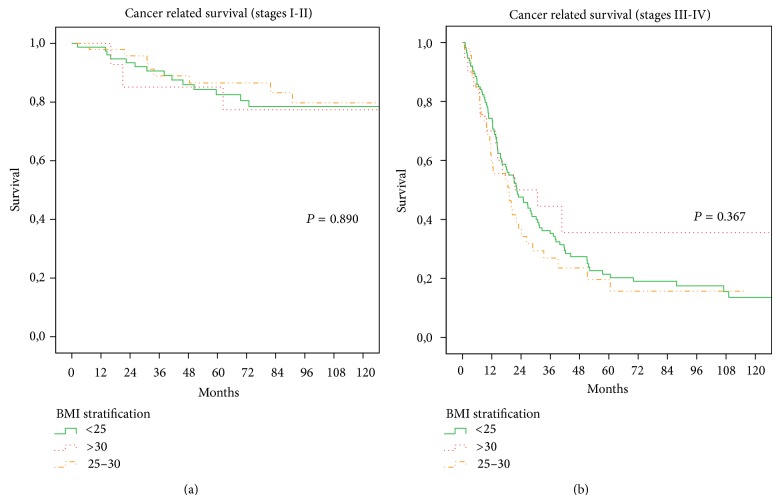
The rates of postoperative complications in the A, B, and C groups were 28.9%, 31.4%, and 33.3%, respectively. These differences did not reach statistical significance (P = 0.803). The rate of patients who died during postoperative hospital stay was 5.0% in group A, 1.7% in group B, and 2.6% in group C, and the difference was not significant (P = 0.230). In this analysis, median follow-up was 35 months (range 1–199). No statistical differences were found between the obesity, overweight, and normal groups. The 10-year overall survival rates were 28% in group A versus 36% in group B versus 44% in group C (P = 0.457). The 10-year disease specific survival rates were 40%, 50%, and 55% in group A, B, and C patients, respectively (P = 0.42). The overall survival and disease specific survival, after stratification based on tumor staging (I-II, III-IV), are shown in Figures 3 and 4; no significant differences were also observed in this stratified analysis according to BMI groups.
Figure 3.
(a)-(b) Overall survival for overweight group, obesity group, and normal group according to tumour stage. (a) Stages I and II (P = 0.814 log-rank test). (b) Stages III and IV (P = 0.243 log-rank test).
Figure 4.
(a)-(b) Cancer related survival for overweight group, obesity group, and normal group according to tumour stage. (a) Stages I and II (P = 0.890 log-rank test). (b) Stages III and IV (P = 0.367 log-rank test).
4. Discussion
The BMI is a measure for human body shape based on an individual's weight and height. It is well known that obesity is associated with cancer incidence and mortality; however the role of high BMI in cancer survival is less well understood.
The prevalence of overweight and obesity in the United States rose from the 1960s to 2010 [16]. The obesity and overweight rate was 45.8 per cent in Italy, 63.1 per cent in United States, and only 23.3 per cent in Korea. In fact, the mean or median BMI in Asian population is lower than that observed for white or European populations, but they have a higher percentage of body fat than white people of the same age, sex, and BMI. Furthermore, white people in Europe generally have a higher percentage of body fat for the same BMI than do those in USA [17]. Obesity is associated with a poor overall and disease free survival or advanced stage in multiple malignancies, as gastric [3], pancreatic [18], breast [19], and colorectal cancer [20]. Several recent studies have investigated the relationship between BMI and surgical outcomes of gastric cancer patients, with contrasting results in terms of operating time, number of dissected lymph nodes, postoperative morbidity and mortality, hospital stay, and long-term survival [21–24] (Table 2). This rather low originality may be considered a limitation to the present paper. However, many of these studies come from Eastern Countries, and few experiences from Western centers specialized in extended lymphadenectomy have been reported in literature.
Table 2.
Summary of studies from Europe, Asia, and USA.
| Year | Author | Country |
BMI stratification |
Cohort size | Outcome | ||
|---|---|---|---|---|---|---|---|
| Number of lymph nodes retrieved | Morbidity | Survival | |||||
| 2000 | Inagawa et al. [23] | Japan | <20; 20–25; >25 | 293 | No difference | No difference | |
| 2003 | Gretschel et al. [28] | Germany | <25; 25–30; >30 | 199 | No difference | No difference | |
| 2004 | Murphy et al. [37] | UK | <20; 20–25; 25–30; >30 | 50 | No difference | ||
| 2008 | Yamada et al. [31] | Japan | <25; >25 | 248 | No difference | No difference | No difference |
| 2009 | Oh et al. [26] | Korea | <25; >25 | 410 | Significant difference | Significant difference | No difference |
| 2009 | Tokunaga et al. [34] | Japan | <25; >25 | 7925 | Significant difference | ||
| 2009 | Ojima et al. [36] | Japan | <25; >25 | 689 | Significant difference | No difference | |
| 2011 | Nobuoka et al. [35] | Japan | <25; >25 | 644 | Significant difference | No difference (except for stage IV) | |
| 2010 | Kulig et al. [9] | Poland | <25; >25 | 1992 | Significant difference | No difference | Significant difference |
| 2012 | Lee et al. [38] | Korea | <25; >25 | 243 | No difference | No difference | |
| 2012 | Oh et al. [39] | Korea | <25; >25 | 61 | No difference | No difference | |
| 2013 | Bickenbach et al. [10] | USA | <25; >25 | 1853 | Significant difference | Significant difference | No difference |
| 2013 | Pata et al. [27] | Italy | <25; 25–30; >30 | 161 | Significant difference | Significant difference | |
| 2013 | Lin et al. [25] | Taiwan | <25, 25–30, >30 | 947 | Significant difference | Significant difference | No difference |
| 2014 | Wong et al. [29] | USA | <18.5; 18.5–25; 25–30; >30 |
222 | Significant difference | No difference | Significant difference |
| 2014 | Kim et al. [30] | Korea | <18.5; 18.5–23; 23–25; 25–30; >30 |
304 | Significant difference | No difference | No difference |
| 2014 | Present experience | Italy | <25; 25–30; >30 | 378 | No difference | No difference | No difference |
The technical difficulties due to obesity in many abdominal surgical procedures have been described, and obesity has been associated with a higher risk of perioperative morbidity after gastric cancer surgery in some series [25–27]. Obesity is related to a greater incidence of comorbidities, such as hypertension and diabetes mellitus, and this could justify, in part, these results. In our experience, we found no significant association between BMI and perioperative complications, morbidity, and mortality. Other authors, in Western and Eastern series, obtained the same results [9, 28–30]. In our opinion, this may be due to the high experience of our center in extended lymphadenectomy, which has been introduced as a standard treatment for gastric cancer several years ago [12, 13]. As other authors [29, 31] we did not find any relationship between increased BMI and any of the cancer-related parameters such as tumor site, Lauren classification, TNM stage, and metastatic lymph nodes. Additionally, we found no significant difference in the number of retrieved lymph nodes between normal, overweight, and obesity groups. According to the National Comprehensive Care Network (NCCN) guidelines [32], which recommend examination of 15 lymph nodes or more for adequate staging, we retrieved a median number of 35 lymph nodes. Even if BMI was not statistically related with the number of harvested lymph nodes, a trend to fewer retrieved lymph nodes in obese and in overweight patients was observed. This could indicate the isolation of lymph nodes from the specimen with abundant fat might be more difficult than their retrieval in nonobese patients. In the literature very discordant data are reported, in particular in terms of race and geographic origin; European [9, 27] and American authors [29, 33] described an inverse relationship between harvested lymph nodes and BMI, whereas Asiatic authors showed contradictory results [10, 26, 30, 31]. It is relevant, in our opinion, that despite the high number of removed lymph nodes, no significant differences in postoperative morbidity and mortality according to preoperative BMI were observed in the present study.
As a confirmation of the oncological effectiveness of lymphadenectomy in overweight patients, in our study we did not find any statistical difference between groups in terms of overall and disease free survival, in early as well as advanced stage of disease. In the literature different results are reported [9, 10, 23, 25, 26, 29–31, 34–36]. However, the general opinion is that increased BMI does not affect long-term outcomes of gastric cancer patients. In our experience long-term outcome of patients with high BMI was similar to or even better than other patients. This may underline the importance of centers experienced in gastric cancer surgery to perform an adequate lymphadenectomy with good long-term oncological outcome, without increasing postoperative complications or mortality, even in overweight or obese patients.
5. Conclusion
In conclusion, according to our data higher preoperative BMI is not related to postoperative outcome and long-term results in gastric cancer patients and therefore should not be considered a factor affecting surgical radicality, at least in specialized centers.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.American Cancer Society. Global Cancer Facts & Figures. 2nd. Atlanta, Ga, USA: American Cancer Society; 2011. [Google Scholar]
- 2.Calle E. E., Rodriguez C., Walker-Thurmond K., Thun M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England Journal of Medicine. 2003;348(17):1625–1638. doi: 10.1056/nejmoa021423. [DOI] [PubMed] [Google Scholar]
- 3.Abnet C. C., Freedman N. D., Hollenbeck A. R., Fraumeni J. F., Jr., Leitzmann M., Schatzkin A. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. European Journal of Cancer. 2008;44(3):465–471. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho Y., Lee D. H., Oh H. S., et al. Higher prevalence of obesity in gastric cardia adenocarcinoma compared to gastric non-cardia adenocarcinoma. Digestive Diseases and Sciences. 2012;57(10):2687–2692. doi: 10.1007/s10620-012-2095-6. [DOI] [PubMed] [Google Scholar]
- 5.O'Doherty M. G., Freedman N. D., Hollenbeck A. R., Schatzkin A., Abnet C. C. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP diet and health study. Gut. 2012;61(9):1261–1268. doi: 10.1136/gutjnl-2011-300551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kant P., Hull M. A. Excess body weight and obesity—the link with gastrointestinal and hepatobiliary cancer. Nature Reviews Gastroenterology and Hepatology. 2011;8(4):224–238. doi: 10.1038/nrgastro.2011.23. [DOI] [PubMed] [Google Scholar]
- 7.Lindblad M., Rodríguez L. A. G., Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes & Control. 2005;16(3):285–294. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 8.Lagergren J., Bergström R., Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Annals of Internal Medicine. 1999;130(11):883–890. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kulig J., Sierzega M., Kolodziejczyk P., et al. Implications of overweight in gastric cancer: a multicenter study in a Western patient population. European Journal of Surgical Oncology. 2010;36(10):969–976. doi: 10.1016/j.ejso.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Bickenbach K. A., Denton B., Gonen M., Brennan M. F., Coit D. G., Strong V. E. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Annals of Surgical Oncology. 2013;20(3):780–787. doi: 10.1245/s10434-012-2653-3. [DOI] [PubMed] [Google Scholar]
- 11.Park S. M., Lim M. K., Shin S. A., et al. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. Journal of Clinical Oncology. 2006;24(31):5017–5024. doi: 10.1200/jco.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 12.Roviello F., Pedrazzani C., Marrelli D., et al. Super-extended (D3) lymphadenectomy in advanced gastric cancer. European Journal of Surgical Oncology. 2010;36(5):439–446. doi: 10.1016/j.ejso.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Roviello F., Marrelli D., Morgagni P., et al. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Annals of Surgical Oncology. 2002;9(9):894–900. doi: 10.1007/bf02557527. [DOI] [PubMed] [Google Scholar]
- 14.Marrelli D., Morgagni P., de Manzoni G., et al. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized western centers. Annals of Surgery. 2012;255(3):486–491. doi: 10.1097/sla.0b013e3182389b1a. [DOI] [PubMed] [Google Scholar]
- 15.Siewert J. R., Feith M., Werner M., Stein H. J. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Annals of Surgery. 2000;232(3):353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegal K. M., Carroll D., Kit B. K., Ogden C. L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA—Journal of the American Medical Association. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 17.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. The Lancet. 2004;363(9403):157–163. doi: 10.1016/s0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 18.Aune D., Greenwood D. C., Chan D. S. M., et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Annals of Oncology. 2012;23(4):843–852. doi: 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 19.Lu Y., Ma H., Malone K. E., et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. Journal of Clinical Oncology. 2011;29(25):3358–3365. doi: 10.1200/JCO.2010.34.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bardou M., Barkun A. N., Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–947. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 21.Kodera Y., Sasako M., Yamamoto S., Sano T., Nashimoto A., Kurita A. Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. British Journal of Surgery. 2005;92(9):1103–1109. doi: 10.1002/bjs.4979. [DOI] [PubMed] [Google Scholar]
- 22.Dhar D. K., Kubota H., Tachibana M., et al. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000;59(1):18–23. doi: 10.1159/000012131. [DOI] [PubMed] [Google Scholar]
- 23.Inagawa S., Adachi S., Oda T., Kawamoto T., Koike N., Fukao K. Effect of fat volume on postoperative complications and survival rate after D2 dissection for gastric cancer. Gastric Cancer. 2000;3(3):141–144. doi: 10.1007/PL00011708. [DOI] [PubMed] [Google Scholar]
- 24.Lee J. H., Paik Y. H., Lee J. S., et al. Abdominal shape of gastric cancer patients influences short-term surgical outcomes. Annals of Surgical Oncology. 2007;14(4):1288–1294. doi: 10.1245/s10434-006-9235-1. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y.-S., Huang K.-H., Lan Y.-T., et al. Impact of body mass index on postoperative outcome of advanced gastric cancer after curative surgery. Journal of Gastrointestinal Surgery. 2013;17(8):1382–1391. doi: 10.1007/s11605-013-2238-x. [DOI] [PubMed] [Google Scholar]
- 26.Oh S. J., Hyung W. J., Li C., et al. Effect of being overweight on postoperative morbidity and long-term surgical outcomes in proximal gastric carcinoma. Journal of Gastroenterology and Hepatology. 2009;24(3):475–479. doi: 10.1111/j.1440-1746.2008.05704.x. [DOI] [PubMed] [Google Scholar]
- 27.Pata G., Solaini L., Roncali S., Pasini M., Ragni F. Impact of obesity on early surgical and oncologic outcomes after total gastrectomy with ‘over-d1’ lymphadenectomy for gastric cancer. World Journal of Surgery. 2013;37(5):1072–1081. doi: 10.1007/s00268-013-1942-8. [DOI] [PubMed] [Google Scholar]
- 28.Gretschel S., Christoph F., Bembenek A., Estevez-Schwarz L., Schneider U., Schlag P. M. Body mass index does not affect systematic D2 lymph node dissection and postoperative morbidity in gastric cancer patients. Annals of Surgical Oncology. 2003;10(4):363–368. doi: 10.1245/aso.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Wong J., Rahman S., Saeed N., et al. Effect of body mass index in patients undergoing resection for gastric cancer: a single center US experience. Journal of Gastrointestinal Surgery. 2014;18(3):505–511. doi: 10.1007/s11605-014-2455-y. [DOI] [PubMed] [Google Scholar]
- 30.Kim J. H., Chin H. M., Hwang S. S., Jun K. H. Impact of intra-abdominal fat on surgical outcome and overall survival of patients with gastric cancer. International Journal of Surgery. 2014;12(4):346–352. doi: 10.1016/j.ijsu.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Yamada H., Kojima K., Inokuchi M., Kawano T., Sugihara K. Effect of obesity on technical feasibility and postoperative outcomes of laparoscopy-assisted distal gastrectomy-comparison with open distal gastrectomy. Journal of Gastrointestinal Surgery. 2008;12(6):997–1004. doi: 10.1007/s11605-007-0374-x. [DOI] [PubMed] [Google Scholar]
- 32.Sinh P., Sharma P. Gastric cardia cancer: how much is it from fat? Digestive Diseases and Sciences. 2012;57(10):2493–2496. doi: 10.1007/s10620-012-2324-z. [DOI] [PubMed] [Google Scholar]
- 33. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Gastric Cancer, Version 2.2012, http://www.nccn.org/
- 34.Tokunaga M., Hiki N., Fukunaga T., Ohyama S., Yamaguchi T., Nakajima T. Better 5-year survival rate following curative gastrectomy in overweight patients. Annals of Surgical Oncology. 2009;16(12):3245–3251. doi: 10.1245/s10434-009-0645-8. [DOI] [PubMed] [Google Scholar]
- 35.Nobuoka D., Gotohda N., Kato Y., Takahashi S., Konishi M., Kinoshita T. Influence of excess body weight on the surgical outcomes of total gastrectomy. Surgery Today. 2011;41(7):928–934. doi: 10.1007/s00595-010-4397-7. [DOI] [PubMed] [Google Scholar]
- 36.Ojima T., Iwahashi M., Nakamori M., et al. Influence of overweight on patients with gastric cancer after undergoing curative gastrectomy an analysis of 689 consecutive cases managed by a single center. Archives of Surgery. 2009;144(4):351–358. doi: 10.1001/archsurg.2009.20. [DOI] [PubMed] [Google Scholar]
- 37.Murphy P. M., Blackshaw G. R. J. C., Paris H. J., Edwards P., Barry J. D., Lewis W. G. Prospective evaluation of nutritional status related to body mass indices and outcomes after modified D2 gastrectomy for carcinoma. Clinical Nutrition. 2004;23(4):477–483. doi: 10.1016/j.clnu.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Lee S. S., Ryu S. W., Kim I. H., Sohn S. S. Impact of gender and body mass index on surgical outcomes following gastrectomy: an Asia-Pacific perspective. Chinese Medical Journal. 2012;125(1):67–71. doi: 10.3760/cma.j.issn.0366-6999.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Oh C. A., Kim D. H., Oh S. J., et al. Impact of body mass index on surgical outcomes in radical total gastrectomy. Hepato-Gastroenterology. 2012;59(115):934–937. doi: 10.5754/hge11169. [DOI] [PubMed] [Google Scholar]