Abstract
Background
Arachidonic acid (ARA) is a precursor of various lipid mediators. ARA metabolites such as thromboxane A2 cause platelet aggregation and vasoconstriction, thus may lead to atherosclerotic disease. It is unclear whether dietary ARA influences the ARA-derived lipid mediator balance and the risk for atherosclerotic diseases, such as cerebral ischemia. Considering the function of ARA in atherosclerosis, it is reasonable to focus on the atherothrombotic type of cerebral ischemia risk. However, no systematic reviews or meta-analyses have been conducted to evaluate the effect of habitual ARA exposure on cerebral ischemia risk. We aimed to systematically evaluate observational studies available on the relationship between ARA exposure and the atherothrombotic type of cerebral ischemia risk in free-living populations.
Summary
The PubMed database was searched for articles registered up to June 24, 2014. We designed a PubMed search formula as follows: key words for humans AND brain ischemia AND study designs AND ARA exposure. Thirty-three articles were reviewed against predefined criteria. There were 695 bibliographies assessed from the articles that included both ARA and cerebral ischemia descriptions. Finally, we identified 11 eligible articles and categorized them according to their reporting and methodological quality. We used the Strengthening the Reporting of Observational Studies in Epidemiology Statement (STROBE) checklist to score the reporting quality. The methodological quality was qualitatively assessed based on the following aspects: subject selection, ARA exposure assessment, outcome diagnosis, methods for controlling confounders, and statistical analysis. We did not conduct a meta-analysis due to the heterogeneity among the studies. All eligible studies measured blood ARA levels as an indicator of exposure. Our literature search did not identify any articles that evaluated dietary ARA intake and tissue ARA as assessments of exposure. Seven of the 11 eligible articles were considered to be of low quality. No articles reported a dose-dependent positive association between an increased cerebral ischemia risk and ARA exposure. However, most studies did not assess the risk in each subtype of cerebral ischemia, thus various etiological types of cerebral ischemia risk were involved in their results.
Key Messages
We did not find a positive association between ARA exposure and cerebral ischemia risk. Eligible studies reported inconsistent findings: cerebral ischemia risk did not change or significantly decreased. We could not draw any conclusions due to the limited number of eligible high-quality studies. Further evidence from well-designed observational studies is required. Simultaneously, in order to develop effective preventive measures against cerebral ischemia, it is imperative to establish standardized definitions, nomenclatures, classifications, and diagnostic procedures.
Key Words: Epidemiology, Cerebral ischemia, Dietary fatty acids, Arachidonic acid, Free-living populations, Systematic review, Cohort studies, Nested case-control studies, Case-control studies, Cross-sectional studies
Introduction
Cerebrovascular disease is a leading cause of mortality worldwide. It has been estimated that cerebrovascular diseases (stroke) accounted for 5.5 million deaths, equivalent to 9.6% of all deaths in the world. In the absence of any meaningful preventive measures, it is expected that stroke-derived deaths will increase to 7.8 million by 2030 [1]. Given these considerations, stroke prevention is a major public health issue around the world.
The pathological background for stroke may be either ischemic or hemorrhagic disturbances of the cerebral blood circulation. With respect to cerebral ischemia, several modifiable risk factors have been established, including hypertension, diabetes mellitus, dyslipidemia, and smoking [2,3,4,5,6,7,8,9,10,11].
Previous research revealed that dietary fat intake could influence cerebral ischemia risk. For example, epidemiological studies indicated that fish consumption, especially n-3 fatty acids, might reduce cerebral ischemia risk [12,13]. In contrast, arachidonic acid (ARA) might be associated with atherosclerosis based on its biological function. Atherosclerosis is a chronic inflammatory process involving the recruitment and accumulation of monocytes, macrophages, and dendritic cells in artery walls [14,15]. In this process, various ARA-derived mediators (mainly thromboxane A2, prostaglandin E2, and leukotrienes) serve important functions in the development of atherosclerosis and plaque instability via increased leukocyte chemotaxis, vascular inflammation, and subsequent matrix degeneration [16,17]. Cerebral ischemia results from the atherosclerotic obstruction of large cervical and cerebral arteries or from embolism originating in other parts of the arterial system. In this context, it may seem plausible that ARA exposure may increase cerebral ischemia risk. However, previous reports proposed that beneficial ARA metabolites such as prostaglandin I2 and epoxyeicosatrienoic acid have a vasodilator function and inhibit platelet aggregation, leukocyte adhesion, and vascular smooth muscle cell proliferation [18,19,20,21]. Therefore, the balance between pro- and anti-inflammatory ARA metabolites may be important in vascular homeostasis.
It is still unclear whether dietary ARA influences the ARA-derived lipid mediator balance and the risk for atherosclerotic diseases such as cerebral ischemia. However, no systematic reviews or meta-analyses have been conducted to evaluate the effect of habitual ARA exposure on cerebral ischemia risk in free-living populations. Therefore, we systematically evaluated available observational studies on the relationship between ARA exposure and cerebral ischemia risk.
Methods
Search Strategy
The PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) was searched for observational studies investigating the relationship between dietary or blood ARA levels and cerebral ischemia risk that were published up to June 24, 2014. We designed a PubMed search formula as follows: key words for humans AND brain ischemia AND study designs AND ARA exposure (see Appendix).
Study Selection
Inclusion criteria were English-language articles published after 1966 reporting original data on the relationship between ARA exposure and cerebral ischemia risk in free-living populations. Eligible ARA exposure assessments were the amount of dietary intake, blood levels, or tissue levels. We included cohort, case-cohort, nested case-control, case-control, and cross-sectional studies in this review.
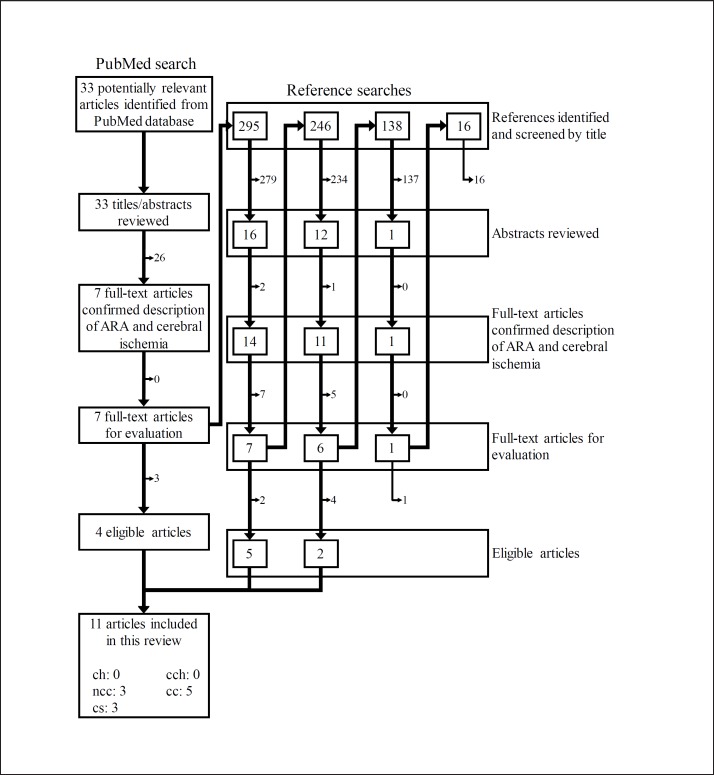
Figure 1 represents the study selection process. The PubMed search yielded 33 publications. Their titles and abstracts were assessed according to the following exclusion criteria: (1) nonhuman studies, (2) limited to special populations such as persons with unusual eating habits, (3) examined after intervention, (4) not about cerebral ischemia and fatty acids, or (5) published prior to 1965. Next, each full-text article was reviewed against the following inclusion criteria: (1) eligible study design and (2) reporting original data of ARA exposure and cerebral ischemia risk. Four eligible articles were obtained from the initial PubMed search.
Fig. 1.
Flow diagram for the literature search and study selection. ch = Cohort study; cch = case-cohort study; ncc = nested case-control study; cc = case-control study; cs = cross-sectional study.
For the full-text articles that included both ARA and cerebral ischemia descriptions, the citations were assessed to obtain potentially relevant articles. The titles of the citations were reviewed as follows: eligible titles described the relationship between nutrition/diet/fats and cerebrovascular disease/cerebral ischemia risk. Shortlisted articles were then screened by their abstracts and full texts using the same criteria as for the PubMed search. This crosschecking was continued until no further publications were found. After 695 citations were scrutinized, 7 articles remained. As a result, 11 eligible articles were included in the present review. Two investigators (M.S. and S.K.) independently conducted all searches, and disagreements were resolved by discussion.
Quality Assessment and Data Extraction
Overall study quality was assessed according to the reporting quality and the methodological quality. The reporting quality indicates whether the necessary information for observational studies was sufficiently reported. We used the Strengthening the Reporting of Observational Studies in Epidemiology Statement (STROBE) checklist to score the reporting quality [22]. Methodological quality, which determines the reliability of findings, refers to the appropriateness of the methods employed in epidemiological research. The methodological quality was qualitatively assessed based on the following aspects: subject selection, ARA exposure assessment, outcome diagnosis, methods for controlling confounders, and statistical analysis. The studies with reporting quality scores under 13 or with insufficient temporal information between exposure and outcome were considered to be of low quality. The remaining studies were categorized into high/medium/low quality based on their methodological quality. Two reviewers (M.S. and S.K.) independently conducted the quality assessments, and discordant results were resolved by consensus.
The following information was tabulated for each study: author names and publication year, study settings, subject characteristics and matching parameters, ARA exposure assessment, cerebral ischemia diagnosis, adjusted potential confounders, study quality, and main findings from the fully adjusted model. Considering the importance of the temporal relationship between exposure and outcome on the causality assessment, we classified case-control studies into two groups according to the temporal information reporting.
We did not conduct a meta-analysis due to the heterogeneity among the studies included and the limited number of high-quality studies. This review therefore presents a qualitative assessment of ARA exposure and cerebral ischemia risk.
Results
We identified 11 eligible articles [23,24,25,26,27,28,29,30,31,32,33], and their study characteristics are shown in table 1.
Table 1.
Summary of observational studies on the association between ARA and risk of cerebral ischemia
| First author [Ref.], year | Study | Subjects | Exposure assessment | Ischemic stroke assessment (diagnosis) | Adjustment for potential confounders | Quality assessment |
Main findings |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| STROBE score | study quality | intergroup comparison | p or ptrend | |||||||
| Study design: nested case-control study | ||||||||||
| Yaemsiri [23], 2013 | WHI-OS, USA, 1993–1998 (follow-up by 2003) | 964 ischemic stroke patients, 964 controls, aged 50–79 years at baseline, 1 case matched to 1 control by age, race/ethnicity, date of study enrollment, and follow-up time | Serum fatty acids at baseline, fasting blood, GC analysis, masking of case-control status not indicated, precision indicated | Incident ischemic strokes confirmed by self-reports during annual medical history updates plus medical charts, brain imaging and death certificates, adjudication performed by local physicians and central trained neurologists; ischemic stroke classification: TOAST classification | BMI, smoking status, diabetes, aspirin use, systolic blood pressure, antihypertensive medication use, HDL-C/T-CHO ratio, normalized TG | 24 | High |
|
Not shown | |
| Iso [24], 2002 | Survey, Japan, 1984–1993, prospective design (follow-up by 1998) | 197 stroke patients, 591 controls, aged 40–85 years at baseline, 1 case matched to 3 controls by sex, age, community, years of serum storage, and fasting status at serum collection | Serum fatty acids at baseline, nonfasting blood, GC analysis, masking of case-control status not indicated, precision not indicated | Incident strokes confirmed by annual mailing questionnaires, death certificates, medical records, national insurance claims, ambulance records, and reports of local physicians, public health nurses, and health volunteers; stroke definition: a focal neurological disorder persisting for >24 h, confirmed by CT and/or MRI; embolic infarction diagnosis: imaging study and neurology consult when evidence of an embolic source was present in the medical records; other types of stroke diagnosis: imaging study | For ARA analysis, only univariate analysis was performed | 23 | High | Mean serum ARA composition, % | p | |
| Total ischemic stroke: 4.7 | Control:4.9 | n.s. | ||||||||
| Lacunar infarction: 4.7 | Control: 4.9 | n.s. | ||||||||
| Large-artery occlusive infarction: 4.5 | Control: 4.6 | n.s. | ||||||||
| Embolic infarction: 5.2 | Control: 5.3 | n.s. | ||||||||
| Univariate OR per 1-SD increase in serum ARA composition | p | |||||||||
| Total ischemic stroke: 0.86 | n.s. | |||||||||
| Lacunar infarction: 0.86 | n.s. | |||||||||
| Large-artery occlusive infarction: 0.84 | n.s. | |||||||||
| Embolic infarction: 0.89 | n.s. | |||||||||
| Miettinen [25], 1986 | Survey, within a randomized multifactorial primary prevention trial of cardiovascular diseases, Finland, 1974–1975 (5–6 years follow-up) | 9 male patients with stroke caused by cerebral artery thrombosis, 52 male controls, aged 44–56 years at baseline, matched by age, body weight, serum total cholesterol, serum TG, blood pressure, smoking status, and glucose tolerance | Serum CE, serum TG, serum PL, fasting blood, GC analysis, masking of case-control status not indicated, precision not indicated | Cerebral artery thrombosis, not having electrocardiographic evidence of left ventricular hypertrophy and hemorrhage on CSF | None | 10 | Low | Serum ARA composition, % of control | Mean serum ARA composition (SD), % | p |
| CE: case: 98 | CE: control: 2.55 (0.12) | n.s. | ||||||||
| TG: case: 98 | TG: control: 0.44 (0.06) | n.s. | ||||||||
| PL: case: 86 | PL: control: 6.43 (0.23) | n.s. | ||||||||
| Study design: case-control study (temporal relationship among exposure and outcome is demonstrated) | ||||||||||
| Park [26], 2009 | Survey, Korea, 2007–2009 | 40 first-event ischemic stroke cases, 40 controls without history of stroke, hypertension, cancer, hyperlipidemia, or diabetes, matched by age and sex | Erythrocyte fatty acids, GC analysis, masking of case-control status not indicated, precision indicated | Diagnostic procedure of ischemic stroke not shown; ischemic stroke classification: TOAST diagnostic system | For ARA analysis, only univariate analysis was performed | 17 | Medium | Mean erythrocyte membrane ARA composition (SEM), % | p | |
| Total ischemic stroke: 11.12 (0.93) | Control: 13.20 (0.44) | <0.05 | ||||||||
| Large-artery atherosclerosis: 12.99 (1.30) | Control: 13.20 (0.63) | n.s. | ||||||||
| Small artery occlusion: 9.47 (1.17) | <0.05 | |||||||||
| Cardioembolism: 12.09 (2.61) | n.s. | |||||||||
| Other cause: 11.79 (1.13) | n.s. | |||||||||
| Ricci [27], 1997 | Survey, Italy, 1992–1994 | 89 ischemic stroke patients, 89 controls without history of clinical vascular diseases (admission period within 3 days), matched by age and sex | Erythrocyte membrane PC, GC analysis, masking of case-control status indicated, precision indicated | Ischemic stroke confirmed by CT within 15 days; ischemic stroke classification: OCSP method | For ARA analysis, only univariate analysis was performed | 19 | Medium | Mean erythrocyte membrane PC ARA composition, % | p | |
| Case: 7.66 | Control: 7.65 | 0.98 | ||||||||
| Study design: case-control study (temporal relationship among exposure and outcome is unclear) | ||||||||||
| Ricci [28], 1987 | Survey, Italy | 28 male first-event ischemic stroke patients aged 40–75 years, 56 male controls without any acute vascular disease or history of stroke or myocardial infarction, matched by age, smoking status, hypertension, and diabetes | Erythrocyte membrane PC, fasting blood, GC analysis, masking of case-control status not indicated, precision not indicated | Ischemic stroke definition: an acute focal neurologic deficit lasting for >24 h, confirmed by CT within 3 days | None | 16 | Low | Mean erythrocyte membrane PC ARA composition (SD), % | p | |
| Case: 2.41 (2.05) | Control: 3.79 (2.25) | 0.008 (n.s.) | ||||||||
| Ciavatti [29], 1978 | Survey, France | 11 thrombosis patients with an acute cerebrovascular accident within <48 h aged 68 (4.9) years [mean (SD)], 11 controls with no previous thrombosis or deep venous thrombosis aged 63 (3.8) years | Platelet PL, plasma fatty acids, fasting blood, GC analysis, masking of case-control status not indicated, precision not indicated | Not shown | None | 10 | Low | Mean platelet PL ARA composition (SD), % | p | |
| Case: 12.9 (0.6) | Control: 13.3 (0.4) | n.s. | ||||||||
| Mean plasma ARA composition (SD), % | p | |||||||||
| Case: 3.0 (0.3) | Control: 3.4 (0.5) | n.s. | ||||||||
| Cumings [30], 1967 | Survey, UK | 29 cerebral infarction patients with diastolic blood pressure <110 mm Hg aged 38–68 years, 14 cerebral infarction patients with diastolic blood pressure ≥110 mm Hg aged 51–64 years, 11 controls | Serum fatty acids, fasting status not indicated, GC analysis, masking of case-control status not indicated, precision not indicated | Not shown | None | 8 | Low | Mean serum ARA concentration, mg/100 ml Male case, diastolic blood pressure <110 mm Hg: 6.5 Male case, diastolic blood pressure ≥110 mm Hg: 5.9 Female case, diastolic blood pressure <110 mm Hg: 6.8 Female case, diastolic blood pressure >110 mm Hg: 5.4 | p | |
| Control: 7.4 | n.s. | |||||||||
| Study design: cross-sectional study | ||||||||||
| Ikeya [31], 2013 | Survey, Japan, 2009–2011 | 65 first-event ischemic stroke patients aged 70 (10) years [mean (SD)], 65 controls (aged >50 years) without ischemic stroke history and medications containing EPA, DHA, or ARA, matched by age | Plasma fatty acids, fasting blood, GC analysis, masking of case-control status not indicated, precision not indicated | Ischemic stroke diagnosed by medical history, brain magnetic resonance angiographic results, cardiac ultrasonographic results, Doppler ultrasonographic results of the carotid artery, and/or electrocardiographic results; Ischemic stroke subacute phase definition: ≤60 days from the onset of ischemic stroke; ischemic stroke classification: TOAST classification | For ARA analysis, only univariate analysis was performed | 16 | Low | Mean plasma ARA concentration (SD), μg/ml | p | |
| Total ischemic stroke: 166.2 (39.8) | Control (total): 167.2 (35.4) | n.s. | ||||||||
| Cardioembolism: 167.1 (30.8) | Control (with arterial fibrillation): 151.3 (37.2) | n.s. | ||||||||
| Large-artery atherosclerosis: 166.0 (42.4) | Control (without arterial fibrillation): 172.0 (33.8) | n.s. | ||||||||
| Färkkilä [32], 1987 | Survey, Finland | 12 acute brain infarction patients aged 35.3 (9.8) years [mean (SD)], 13 controls aged 37.8 (5.0) years, matched by age | Plasma CE, TG, PL, platelet fatty acids, fasting blood, GC analysis, masking of case-control status not indicated, precision not indicated | Acute brain infarction confirmed with examination of CSF, and CT or/and MRI | None | 11 | Low | Mean plasma CE ARA composition (SD), % | p | |
| Case: 6.2 (1.7) | Control: 6.3 (1.3) | n.s. | ||||||||
| Mean plasma TG ARA composition (SD), % | p | |||||||||
| Case: 1.5 (0.7) | Control: 1.3 (0.3) | n.s. | ||||||||
| Mean plasma PL ARA composition (SD), % | p | |||||||||
| Case: 10.5 (2.2) | Control: 10.3 (1.3) | n.s. | ||||||||
| Mean platelets ARA composition (SD), % | p | |||||||||
| Case: 28.1 (7.6) | Control: 42.0 (3.9] | <0.001 | ||||||||
| Tilvis [33], 1987 | Survey, Finland | 14 ischemic stroke patients aged 34 (1) years [mean (SD)], 27 controls, matched by sex, age, blood pressure, and body weight | Serum PL, platelet fatty acids, analytical method not indicated | Not shown | None | 5 | Low | Mean serum PL ARA composition (SD) (% of the sum of fatty acids from 14:0 to 18:3), approximate numeric value from graph | p | |
| Case: 10 (0.8) | Control: 10 (0.8) | n.s. | ||||||||
| Mean platelet ARA composition (SD) (% of the sum of fatty acids from 14:0 to 18:3), approximate numeric value from graph | p | |||||||||
| Case: 32 (1.5) | Control: 43 (1.5) | <0.001 | ||||||||
BMI = Body mass index; CE = cholesterol ester(s); GC = gas chromatography; DHA = docosahexaenoic acid; EPA = eicosapentaenoic acid; HDL-C = high density lipoprotein cholesterol; n.s. = not significant; OCSP = Oxfordshire Community Stroke Project; PC = phosphatidylcholine(s); PL = phospholipid(s); T-CHO = total cholesterol; TG = triglyceride(s); WHI-OS = Women's Health Initiative Observational Study.
The overall study quality was high in the studies by Yaemsiri et al. [23] and Iso et al. [24] and medium in the studies by Park et al. [26] and Ricci et al. [27]. As to the remaining 7 studies [25,28,29,30,31,32,33], the overall quality was low. Although 1 case-control study [28] and 1 cross-sectional study [31] had moderate reporting quality (STROBE score 16), their temporal information between exposure and outcome, participant characteristics, or study settings were not sufficiently provided. The remaining 5 articles [25,29,30,32,33] were determined to be of low reporting quality (STROBE score range 5-11). No studies were adjusted for well-known potential confounders except for that of Yaemsiri et al. [23]. All eligible studies measured blood ARA levels as an estimate of exposure. ARA levels in serum [23,24,25,30,33], erythrocyte membranes [26,27,28], plasma [29,31,32], and/or platelets [29,32,33] were analyzed. Our literature search did not identify any articles that evaluated dietary ARA intake and tissue ARA as assessments of exposure. Outcome definition, classification, or diagnostic procedure were mentioned in 3 nested case-control studies [23,24,25], 3 case-control studies [26,27,28], and 2 cross-sectional studies [31,32].
Yaemsiri et al. [23] and Iso et al. [24] indicated the serum ARA value of each case-control group in every cerebral ischemia subtype: overall ischemic stroke, lacunar infarction, atherothrombotic stroke or large-artery occlusive infarction, embolic infarction, and ischemic strokes of undetermined etiology. In the study by Yaemsiri et al. [23], ischemic stroke diagnosis was ascertained by physicians and neurologists according to the medical records/histories, brain imaging, and death certificate information. Ischemic stroke was classified according to the Trial of ORG 10172 Acute Stroke Trial Classification (TOAST) system [34]. Iso et al. [24] defined stroke as focal acute neurological disorder that persisted for at least 24 h or until death and was confirmed by computed tomography (CT) or magnetic resonance imaging (MRI). Each stroke subtype was confirmed by imaging studies and/or medical records. In both studies, no significant difference in serum ARA levels and no significant change in the cerebral ischemia risk was found regardless of the cerebral ischemia subtype [23,24].
Miettinen et al. [25] reported the risk of cerebral artery thrombosis as the outcome, which was diagnosed by the absence of left ventricular hypertrophy and hemorrhage in the cerebrospinal fluid (CSF). This report showed no significant differences in serum ARA levels between the case and control groups.
Although Park et al. [26] and Ricci et al. [27] classified ischemic stroke subtypes according to the TOAST system [35] or the Oxfordshire Community Stroke Project method [36], only Park et al. [26] indicated the erythrocyte ARA value of each case-control group in every subtype. For overall ischemic stroke, case patients had significantly lower erythrocyte membrane ARA levels than control subjects. Among the four subtypes of ischemic stroke, only small-artery occlusion patients had significantly decreased erythrocyte membrane ARA levels compared to the control group [26]. Ricci et al. [27] did not find significant differences in erythrocyte membrane phosphatidylcholine ARA levels between the case and control groups.
Ricci et al. [28] defined the outcome as an acute focal neurologic deficit lasting >24 h that was attributed to ischemia on clinical grounds and CT. Ischemic stroke patients had slightly lower erythrocyte membrane ARA levels than control subjects; however, this difference was not significant.
Ikeya et al. [31] confirmed the presence of ischemic stroke based on medical history, imaging studies, and/or electrocardiographic results. Ischemic stroke cases were classified according to the TOAST system, and the plasma ARA concentrations of two ischemic stroke subtypes (cardioembolism and large-artery atherosclerosis) were assessed. No significant difference in plasma ARA levels between the case and control groups was found regardless of the cerebral ischemia subtype.
Färkkilä et al. [32] investigated the risk of acute brain infarction that was confirmed by CSF examination, CT, or MRI. A significantly lower ARA composition was found in the platelet membrane lipids of patients compared to control subjects, whereas plasma ARA levels were not significantly different between patients and controls.
The remaining 3 articles estimated the risk of thrombosis with acute cerebrovascular accident [29], cerebral infarction [30], and ischemic stroke [33]. Ciavatti et al. [29] found no significant differences in plasma and platelet ARA levels between case and control subjects. Cumings et al. [30] classified their case group into four categories by blood pressure and sex. Then they analyzed the serum ARA composition of each case category and the control group. There were no significant differences in serum ARA compositions between any of the groups. Tilvis et al. [33] reported that the serum ARA composition in case subjects was not statistically different from that of control subjects, whereas the platelet ARA composition of the control group was significantly higher than that of the case group.
Discussion
We systematically reviewed observational studies investigating the association between ARA exposure and cerebral ischemia risk in free-living populations. None of the 11 eligible articles indicated a positive association or a dose-response relationship between an increased cerebral ischemia risk and ARA exposure.
Cerebral ischemia is generally classified into four main categories by etiology: atherothrombotic, small-vessel disease, cardioembolic, and other causes [37,38]. According to a previous report, cerebral ischemia has also been classified by various factors such as clinical findings, information from diagnostic imaging, or etiology [39]. However, a universal definition has not been established because advances in basic neuroscience and clinical neurodiagnostic technologies have changed the classifications and nomenclature over time [40]. Therefore, in order to identify eligible articles, we needed to search for studies assessing every type of cerebral ischemia risk during our review process followed by an assessment of each outcome by etiology.
Considering the function of ARA in atherosclerosis, it is reasonable to focus on the atherothrombotic type of cerebral ischemia risk in this review. Nevertheless, only 4 studies [23,24,26,31] reported the serum ARA value of each case-control group in every cerebral ischemia subtype. For large-artery occlusive infarction risk, which represented the atherothrombotic type of cerebral ischemia, the studies indicated that there was no statistically significant difference in serum ARA levels between the case and control groups [23,24,26,31]. The remaining 7 studies did not assess the risk in each subtype of cerebral ischemia separately; therefore, various etiological types of cerebral ischemia risk were included in their results. Consequently, we could not draw any conclusions about the relationship between ARA exposure and the atherothrombotic type of cerebral ischemia risk.
For the overall cerebral ischemia risk, no article indicated a positive association or a dose-response relationship between an increased cerebral ischemia risk and ARA exposure. Three studies [26,32,33] reported that the blood ARA level of the control group was significantly higher than that of the case group. Färkkilä et al. [32] and Tilvis et al. [33] found that the control group had a higher ARA composition in platelet phospholipids than the case group. The authors supposed that low ARA contents in platelets might be associated with an enhanced ARA consumption to lipid mediator production. This consideration suggests that platelet ARA levels may be affected by factors other than dietary intake. Park et al. [26] reported that the erythrocyte ARA levels were significantly lower in the overall case group. According to the ischemic stroke subtype analysis data, this difference may be derived from erythrocyte ARA composition changes in small-artery occlusion, which we did not focus on because of its etiological characteristics.
The biological plausibility of a relationship between ARA intake and cerebral ischemia risk is inconclusive. An intervention study [41] suggested that aspirin intake prevents blood coagulation and reduces the risk of ischemic stroke. This finding indicates that the cyclooxygenase metabolites of ARA might be associated with the risk of blood coagulation or ischemic stroke. Seyberth et al. [42] reported a marked increase in platelet aggregation in healthy men consuming 6 g/day of ARA ethyl ester. In contrast, another human study [43] reported that a 50-day intervention with a high-ARA diet (containing 1.5 g/day of ARA) does not cause any changes in platelet aggregation, prothrombin time, partial thromboplastin time, or antithrombin III levels compared to a control diet (containing 210 mg/day of ARA). Furthermore, our present study could not find a positive association between blood ARA levels and an increased cerebral ischemia risk. The following reasons may explain these controversial findings.
First, blood ARA levels may not always represent dietary intake. Kobayashi et al. [44] and Kawabata et al. [45] reported that correlations between dietary estimates and ARA levels in the serum phospholipid, plasma, or erythrocyte membrane were very low. Several factors may influence blood fatty acid levels. For example, the blood levels of polyunsaturated fatty acids are affected not only by diet, but also by the genetic variants of fatty acid conversion enzymes [46,47]. Second, the increment of blood ARA levels may not directly cause an increase in ARA metabolites. In our previous study, healthy elderly persons ingested 240 or 720 mg of ARA per day for 4 weeks. Urinary thromboxane A2 metabolites did not differ significantly with regard to ARA supplementation or time points, although plasma ARA compositions increased dose-dependently [48]. Third, some ARA metabolites may play a protective role against cerebral ischemia. Previous reports proposed that beneficial ARA metabolites such as prostaglandin I2, epoxyeicosatrienoic acid, and anandamide have potential for managing cerebral ischemia [20,21,49].
All articles included in this review were listed in the PubMed database. Our PubMed search formula identified only 4 eligible articles [23,24,31,33], whereas the remaining 7 articles were identified from a reference search (fig. 1). Therefore, reference searching serves an important role in our comprehensive literature search. Our PubMed search formula did not recognize these 7 articles due to the key words ‘exposure’ [25,26,27,28,29,30], ‘study design’ [29,30,32], and ‘outcome’ terms [25,29,30]. For the ‘exposure’ term, the additional search with the key word ‘fatty’ recognized all 6 articles [25,26,27,28,29,30]. In the case of ‘study designs’, all 3 articles [29,30,32] did not use general study design words, which may be partly because the articles were published before the publication of the STROBE checklist [22]. As to the ‘outcome’ term, the authors used various words to represent cerebral ischemia in their titles or abstracts such as: stroke [25], cerebrovascular accident [29], or cerebral infarction [30]. These reporting characteristics made it difficult to effectively search for intended articles with a focus on ARA and cerebral ischemia on the PubMed database. We therefore employed the following search strategies: first, we conducted a PubMed search using the formula that represented ARA exposure specifically and cerebral ischemia comprehensively; then, we assessed reference titles to include the broad exposure description (e.g. nutrition or diet) to identify articles. We did not exclude articles by study design until we confirmed the study design from the description in the articles.
This systematic review has 3 main limitations. Our search was restricted to publications in English and articles from the PubMed database. We did not set the search terms for tissue ARA levels before the PubMed search, thus we identified those articles only from the reference search. We did not use the search term ‘fatty’ or ‘fatty acid’ in the PubMed search. Considering the reporting characteristics of the eligible studies, this search strategy led to an efficient retrieval according to our previous review [50]. However, these limitations may have seriously influenced the completeness of our literature search.
We did not find a positive association between ARA exposure and cerebral ischemia risk. The eligible studies reported inconsistent findings: cerebral ischemia risk did not change or significantly decreased. We could not draw any conclusions about the relationship between ARA and the atherothrombotic type of cerebral ischemia risk due to the limited number of eligible high-quality studies. Further evidence from well-designed observational studies is required. Simultaneously, in order to develop effective preventive measures against cerebral ischemia, it is imperative to establish standardized definitions, nomenclatures, classifications, and diagnostic procedures.
Disclosure Statement
This study was supported in part by a grant from Suntory Wellness Limited, Japan. M.S., S.K., H.T., T.S., M.K., K.E., H.K., T.R., and H.S. are employees of Suntory Wellness Limited. K.S. works for the group company of Suntory Wellness Limited. S.S. has consultancy relationships with Suntory Wellness Limited.
Appendix
PubMed Search Terms and Strategies
| Number | Items | Terms |
|---|---|---|
| A. Search terms for exposure, outcome, and study design | ||
| Exposure | ||
| #1 | Intake | Humans[mesh] AND (arachidonic[tiab] OR arachidonate[tiab] OR arachidonates[tiab] OR ‘20:4’[tiab] OR ‘C20:4’[tiab] OR eicosatetraenoic[tiab] OR Arachidonic acid[mesh]) AND (dietary[tiab] OR diet[tiab] OR diets[tiab] OR Dietary fats[mesh] OR intake[tiab] OR intakes[tiab] OR consumption[tiab]) |
| #2 | Biomarker | Humans[mesh] AND (arachidonic[tiab] OR arachidonate[tiab] OR arachidonates[tiab] OR ‘20:4’[tiab] OR ‘C20:4’[tiab] OR eicosatetraenoic[tiab] OR Arachidonic acid[mesh]) AND (blood[tiab] OR serum[tiab] OR plasma[tiab] OR erythrocyte[tiab] OR erythrocytes[tiab] OR ‘red cell’[tiab] OR ‘red cells'[tiab] OR Blood[mesh]) AND (composition[tiab] OR compositions[tiab] OR profile[tiab] OR profiles[tiab] OR ratio[tiab] OR ratios[tiab] OR status[tiab] OR statuses[tiab] OR concentration[tiab] OR concentrations[tiab] OR level[tiab] OR levels[tiab]) |
| Outcome | ||
| #3 | Cerebral ischemia | Brain ischemia[mesh] OR brain ischemia[tiab] OR brain ischaemia[tiab] OR cerebral ischemia[tiab] OR cerebral ischaemia[tiab] OR ischemic encephalopathy[tiab] OR ischaemic encephalopathy[tiab] OR brain infarct*[tiab] OR brain venous infarct*[tiab] OR cerebral infarct*[tiab] OR cerebral venous infarct*[tiab] OR cortical infarct*[tiab] OR subcortical infarct*[tiab] OR choroidal artery infarct*[tiab] OR ischemic stroke*[tiab] OR ischaemic stroke*[tiab] OR transient ischemic attack*[tiab] OR transient ischaemic attack*[tiab] |
| Study design | ||
| #4 | Study design | Epidemiolog*[tiab] OR prospective[tiab] OR retrospective[tiab] OR cohort[tiab] OR Cohort studies[mesh] OR ‘case control’[tiab] OR ((case[tiab] OR cases[tiab]) AND (control[tiab] OR controls[tiab])) OR Case-control studies[mesh] OR ‘control subjects'[tiab] OR ‘control group’[tiab] OR ‘cross sectional’[tiab] OR Cross-sectional studies[mesh] |
B. PubMed search strategy Term combination (#1 OR #2) AND #3 AND #4
References
- 1.Truelsen T, Begg S, Mathers C. The Global Burden of Cerebrovascular disease. World Health Organization. Geneva. 2000.
- 2.Kannel WB, Wolf PA, McGee DL, Dawber TR, McNamara P, Castelli WP. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA. 1981;245:1225–1229. [PubMed] [Google Scholar]
- 3.Tanaka H, Ueda Y, Hayashi M, Date C, Baba T, Yamashita H, Shoji H, Tanaka Y, Owada K, Detels R. Risk factors for cerebral hemorrhage and cerebral infarction in a Japanese rural community. Stroke. 1982;13:62–73. doi: 10.1161/01.str.13.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 5.Abbott RD, Donahue RP, MacMahon SW, Reed DM, Yano K. Diabetes and the risk of stroke. The Honolulu Heart Program. JAMA. 1987;257:949–952. [PubMed] [Google Scholar]
- 6.Singer DE, Nathan DM, Anderson KM, Wilson PW, Evans JC. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes. 1992;41:202–208. doi: 10.2337/diab.41.2.202. [DOI] [PubMed] [Google Scholar]
- 7.Boysen G, Nyboe J, Appleyard M, Sørensen PS, Boas J, Somnier F, Jensen G, Schnohr P. Stroke incidence and risk factors for stroke in Copenhagen, Denmark. Stroke. 1988;19:1345–1353. doi: 10.1161/01.str.19.11.1345. [DOI] [PubMed] [Google Scholar]
- 8.Iso H, Jacobs DR, Jr, Wentworth D, Neaton JD, Cohen JD. MRFIT Research Group: Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320:904–910. doi: 10.1056/NEJM198904063201405. [DOI] [PubMed] [Google Scholar]
- 9.Abbott RD, Yin Y, Reed DM, Yano K. Risk of stroke in male cigarette smokers. N Engl J Med. 1986;315:717–720. doi: 10.1056/NEJM198609183151201. [DOI] [PubMed] [Google Scholar]
- 10.Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The Framingham Study. JAMA. 1988;259:1025–1029. [PubMed] [Google Scholar]
- 11.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ. 1989;298:789–794. doi: 10.1136/bmj.298.6676.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xun P, Qin B, Song Y, Nakamura Y, Kurth T, Yaemsiri S, Djousse L, He K. Fish consumption and risk of stroke and its subtypes: accumulative evidence from a meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2012;66:1199–1207. doi: 10.1038/ejcn.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka K, Ishikawa Y, Yokoyama M, Origasa H, Matsuzaki M, Saito Y, Matsuzawa Y, Sasaki J, Oikawa S, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. JELIS Investigators: Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke. 2008;39:2052–2058. doi: 10.1161/STROKEAHA.107.509455. [DOI] [PubMed] [Google Scholar]
- 14.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 15.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capra V, Bäck M, Barbieri SS, Camera M, Tremoli E, Rovati GE. Eicosanoids and their drugs in cardiovascular diseases: focus on atherosclerosis and stroke. Med Res Rev. 2013;33:364–438. doi: 10.1002/med.21251. [DOI] [PubMed] [Google Scholar]
- 17.Back M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc Drugs Ther. 2009;23:41–48. doi: 10.1007/s10557-008-6140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3–11. [PubMed] [Google Scholar]
- 19.Kawabe J, Ushikubi F, Hasebe N. Prostacyclin in vascular diseases. Recent insights and future perspectives. Circ J. 2010;74:836–843. doi: 10.1253/circj.cj-10-0195. [DOI] [PubMed] [Google Scholar]
- 20.Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 21.Imig JD, Simpkins AN, Renic M, Harder DR. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev Mol Med. 2011;13:e7. doi: 10.1017/S1462399411001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative: Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaemsiri S, Sen S, Tinker LF, Robinson WR, Evans RW, Rosamond W, Wassenthiel-Smoller S, He K. Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke. 2013;44:2710–2717. doi: 10.1161/STROKEAHA.111.000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, Imano H, Okamura T, Naito Y, Shimamoto T. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. doi: 10.1161/01.str.0000023890.25066.50. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen TA, Huttunen JK, Naukkarinen V. Cholestanol and fatty acids of serum lipids as risk factors of stroke. Monogr Atheroscler. 1986;14:19–25. [PubMed] [Google Scholar]
- 26.Park Y, Park S, Yi H, Kim HY, Kang SJ, Kim J, Ahn H. Low level of n-3 polyunsaturated fatty acids in erythrocytes is a risk factor for both acute ischemic and hemorrhagic stroke in Koreans. Nutr Res. 2009;29:825–830. doi: 10.1016/j.nutres.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Ricci S, Celani MG, Righetti E, Caruso A, De Medio G, Trovarelli G, Romoli S, Stragliotto E, Spizzichino L. Fatty acid dietary intake and the risk of ischaemic stroke: a multicentre case-control study. J Neurol. 1997;244:360–364. doi: 10.1007/s004150050102. [DOI] [PubMed] [Google Scholar]
- 28.Ricci S, Patoia L, Berrettini M, Binaglia L, Scarcella MG, Bucaneve G, Vecchini A, Carloni I, Agostini L, Parise P, Del Favero A. Fatty acid pattern of red blood cell membranes and risk of ischemic brain infarction: a case-control study. Stroke. 1987;18:575–578. doi: 10.1161/01.str.18.3.575. [DOI] [PubMed] [Google Scholar]
- 29.Ciavatti M, Michel G, Dechavanne M. Platelet phospholipid in stroke. Clin Chim Acta. 1978;84:347–351. doi: 10.1016/0009-8981(78)90251-6. [DOI] [PubMed] [Google Scholar]
- 30.Cumings JN, Grundt IK, Holland JT, Marshall J. Serum-lipids and cerebrovascular disease. Lancet. 1967;2:194–195. doi: 10.1016/s0140-6736(67)90012-8. [DOI] [PubMed] [Google Scholar]
- 31.Ikeya Y, Fukuyama N, Kitajima W, Ogushi Y, Mori H. Comparison of eicosapentaenoic acid concentrations in plasma between patients with ischemic stroke and control subjects. Nutrition. 2013;29:127–131. doi: 10.1016/j.nut.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Färkkilä MA, Rasi V, Tilvis RS, Ikkala E, Viinikka L, Ylikorkala O, Färkkilä AM, Miettinen TA. Low platelet arachidonic acid in young patients with brain infarction. Thromb Res. 1987;48:721–727. doi: 10.1016/0049-3848(87)90437-3. [DOI] [PubMed] [Google Scholar]
- 33.Tilvis RS, Erkinjuntti T, Sulkava R, Färkkilä M, Miettinen TA. Serum lipids and fatty acids in ischemic stroke. Am Heart J. 1987;113:615–619. doi: 10.1016/0002-8703(87)90642-9. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein LB, Jones MR, Matchar DB, Edwards LJ, Hoff J, Chilukuri V, Armstrong SB, Horner RD. Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. Stroke. 2001;32:1091–1097. doi: 10.1161/01.str.32.5.1091. [DOI] [PubMed] [Google Scholar]
- 35.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 36.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 37.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis. 2009;27:502–508. doi: 10.1159/000210433. [DOI] [PubMed] [Google Scholar]
- 38.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Wolf ME, Hennerici MG. The ASCOD phenotyping of ischemic stroke (Updated ASCO Phenotyping) Cerebrovasc Dis. 2013;36:1–5. doi: 10.1159/000352050. [DOI] [PubMed] [Google Scholar]
- 39.Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. Classification of stroke subtypes. Cerebrovasc Dis. 2009;27:493–501. doi: 10.1159/000210432. [DOI] [PubMed] [Google Scholar]
- 40.Saver JL. Proposal for a universal definition of cerebral infarction. Stroke. 2008;39:3110–3115. doi: 10.1161/STROKEAHA.108.518415. [DOI] [PubMed] [Google Scholar]
- 41.Rist PM, Buring JE, Kase CS, Kurth T. Effect of low-dose aspirin on functional outcome from cerebral vascular events in women. Stroke. 2013;44:432–436. doi: 10.1161/STROKEAHA.112.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seyberth HW, Oelz O, Kennedy T, Sweetman BJ, Danon A, Frölich JC, Heimberg M, Oates JA. Increased arachidonate in lipids after administration to man. Clin Pharmacol Ther. 1975;18:521–529. doi: 10.1002/cpt1975185part1521. [DOI] [PubMed] [Google Scholar]
- 43.Nelson GJ, Schmidt PC, Bartolini G, Kelley DS, Kyle D. The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 1997;32:421–425. doi: 10.1007/s11745-997-0055-7. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi M, Sasaki S, Kawabata T, Hasegawa K, Tsugane S. JPHC: Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I to assess fatty acid intake: comparison with dietary records and serum phospholipid level. J Epidemiol. 2003;13:S64–S81. doi: 10.2188/jea.13.1sup_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawabata T, Hirota S, Hirayama T, Adachi N, Kaneko Y, Iwama N, Kamachi K, Araki E, Kawashima H, Kiso Y. Associations between dietary n-6 and n-3 fatty acids and arachidonic acid compositions in plasma and erythrocytes in young and elderly Japanese volunteers. Lipids Health Dis. 2011;10:138. doi: 10.1186/1476-511X-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, Tsai MY, Ferrucci L. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet. 2009;5:e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glaser C, Heinrich J, Koletzko B. Role of FADS1 and FADS2 polymorphisms in polyunsaturated fatty acid metabolism. Metabolism. 2010;59:993–999. doi: 10.1016/j.metabol.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 48.Kakutani S, Ishikura Y, Tateishi N, Horikawa C, Tokuda H, Kontani M, Kawashima H, Sakakibara Y, Kiso Y, Shibata H, Morita I. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: a randomized controlled study. Lipids Health Dis. 2011;10:241. doi: 10.1186/1476-511X-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhopadhyay S, Tulis DA. Endocannabinoid regulation of matrix metalloproteinases: implications in ischemic stroke. Cardiovasc Hematol Agents Med Chem. 2007;5:311–318. doi: 10.2174/187152507782109917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai M, Kakutani S, Horikawa C, Tokuda H, Kawashima H, Shibata H, Okubo H, Sasaki S. Arachidonic acid and cancer risk: a systematic review of observational studies. BMC Cancer. 2012;12:606. doi: 10.1186/1471-2407-12-606. [DOI] [PMC free article] [PubMed] [Google Scholar]