Summary
The displacement loop (D-loop) is the DNA strand invasion product formed during homologous recombination. Disruption of nascent D-loops represents a mechanism of anti-recombination. During Synthesis-Dependent Strand Annealing D-loop disruption after extension of the invading strand is an integral step of the pathway and ensures a non-crossover outcome. The proteins implicated in D-loop disruption are DNA motor proteins/helicases acting by migrating DNA junctions. Here we report an unanticipated mechanism of D-loop dissolution mediated by DNA topoisomerase 3 (Top3) and dependent on its catalytic activity. D-loop dissolution catalyzed by yeast Top3 is highly specific for yeast Rad51/Rad54-mediated D-loops, whereas protein-free D-loops or D-loop mediated by bacterial RecA protein or human RAD51/RAD54 resist dissolution. Also the human Topoisomerase IIIα-RMI1–RMI2 complex is capable of dissolving D-loops. Consistent with genetic data, we suggest that the extreme growth defect and hyper-recombination phenotype of Top3-deficient yeast cells is in part a result of unprocessed D-loops.
Introduction
Homologous recombination (HR) is a highly conserved and ubiquitous mechanism for the repair or tolerance of complex DNA damage such as double-stranded breaks or interstrand crosslinks (Li and Heyer, 2008). HR is essential for meiotic chromosome segregation and crossover formation involving the formation and resolution of double Holliday junctions (dHJ) (Hunter, 2007). In addition, HR is required for the recovery of blocked or broken replication forks. Filaments of the Rad51 protein on ssDNA perform the signature reactions of HR: homology search and DNA strand invasion (Heyer et al., 2010). The product of strand invasion is the displacement loop (D-loop), a joint molecule in which the invading strand primes DNA synthesis on a donor template. In yeast, the Rad54 protein is required for D-loop formation and displaces Rad51 from the heteroduplex (hDNA) giving the DNA polymerase access to the invading 3’-end (Li and Heyer, 2009). In somatic cells, HR is heavily skewed towards using the sister chromatid as a template and favors a non-crossover (NCO) outcome (Johnson and Jasin, 2000; Kadyk and Hartwell, 1992). This avoids the potential for loss-of heterozygosity, a process known to be involved in tumorigenesis (LaRocque et al., 2011). To ensure an NCO outcome, the D-loop is disrupted after DNA polymerase extension and the extended strand is annealed to the second end of the original DSB in a process termed Synthesis-Dependent Strand Annealing (SDSA).
D-loops constitute reversible, metastable intermediates of the HR pathway (Heyer et al., 2010). The nascent D-loop, i.e. the D-loop before extension by DNA polymerase, can be reversed to its component DNA molecules to abort HR. This mechanism of anti-recombination has been implicated in a process termed hDNA rejection, where mismatches between invading strand and the donor template trigger abortion of HR (Hombauer et al., 2011). Disruption of extended D-loops, i.e. D-loops after extension by DNA polymerase, is an integral part of SDSA and a mechanism of anti-crossover.. The mechanisms involved in D-loop disruption are not fully understood. A number of genes/proteins have been implicated in this process either by genetic, biochemical, or cell biological evidence. These include Saccharomyces cerevisiae Srs2 and Mph1 as well as the Mph1 homologs, FANCM and Fml1 in plants and fission yeast, respectively (Crismani et al., 2012; Ira et al., 2003; Lorenz et al., 2012; Prakash et al., 2009; Robert et al., 2006). In addition, the human RecQ-like helicases BLM and RECQ1, as well as the helicase RTEL1 have been implicated in D-loop dissociation (Bachrati et al., 2006; Barber et al., 2008; Bugreev et al., 2008; van Brabant et al., 2000). Specifically, Srs2, Mph1/FANCM/Fml1, and RTEL1 have been implicated in crossover avoidance. RECQ1, instead, has been implicated in the disruption of dead-end D-loops, where the 5’-end has invaded a donor template. Also Rad54 protein, which is required for D-loop formation by yeast Rad51, disrupts D-loops depending on the specific structure of the joint molecule (Bugreev et al., 2007a; Wright and Heyer, 2014). Common to all reported mechanisms of D-loop disruption is the involvement of DNA helicase/motor proteins that disrupt D-loops by an ATP-driven mechanism involving translocation on ssDNA or dsDNA.
Sgs1 is the single RecQ helicase in the budding yeast S. cerevisiae and represents the homolog to human BLM, one of five RecQ helicases in mammals (Bernstein et al., 2010; Chu and Hickson, 2009). Sgs1 is a 3’–5’ DNA helicase that associates with a topoisomerase (Top3) and an OB-fold protein (Rmi1). The yeast Sgs1-Top3-Rmi1 complex is considered homologous to the human BLM-TOPOIIIα-RMI1–RMI2 complex. Sgs1/BLM is a potent DNA helicase active on a variety of substrates. Top3 and its human homolog TOPOIIIα are type IA DNA topoisomerases that introduce a transient nick in one DNA strand (cut strand or C-strand), allowing a second unbroken ssDNA (transfer strand or T strand) to be transferred reversibly through the nick. The C-strand is cut in a reversible transesterification mechanism involving the formation of a covalent linkage between the 5’-end of the C-strand and the active site tyrosine, Y356, of Top3. In order to act, Top3 needs access to ssDNA; in order to relax dsDNA high temperature and specific reactions conditions such as high glycerol concentrations are required (Chen and Brill, 2007). Rmi1 projects as a loop into the Top3/TOPOIIIα gate stabilizing the open conformation to favor decatenation over relaxation (Bocquet et al., 2014). As a result, Rmi1 enhances the decatenation activity of Top3 while slows DNA relaxation (Cejka et al., 2012). The function of Top3 as an ssDNA decatenase is consistent with genetic data in combination with mutations in Top1 and Top2 that led to the conclusion that Top3 does not act as a relaxase of negatively supercoiled DNA in vivo (Kim and Wang, 1992).
The phenotypes of Sgs1/BLM-deficient cells are exceedingly complex and reflect an involvement in several aspects of DNA metabolism, including DNA replication, DNA checkpoint signaling, and HR (Bernstein et al., 2010; Chu and Hickson, 2009). Both yeast Sgs1-Top3-Rmi1 and human BLM-TOPOIIIα-RMI1–RMI2 complexes are involved at various steps throughout HR. In addition, they also process structures generated during replication fork stalling or collapse, and have been implicated in the resolution of late replication intermediates (Bernstein et al., 2009; Chan et al., 2009; Liberi et al., 2005; Wang, 1991). The specific DNA structures and mechanisms involved are only partly understood, but may be the consequence of a single mechanistic defect in the decatenation of DNA (Cejka et al., 2012; Hickson and Mankouri, 2011). During HR, Sgs1 and its catalytic activity are required for long-range DSB resection to initiate HR (Cejka et al., 2010a; Mimitou and Symington, 2008; Niu et al., 2010; Zhu et al., 2008). Interestingly, while Top3 protein is required for this function, the Top3 catalytic activity is not (Niu et al., 2010). Seminal work on the human BLM-TOPOIIIα-RMI1/2 established a mechanism to process dHJs, a late HR intermediate, into non-crossover products, which had been termed dissolution to distinguish the process from endonucleolytic resolution (Wu and Hickson, 2003). Both the human and yeast complexes collapse the dHJ into a hemi-catenane intermediate by joint catalytic action of BLM/Sgs1 and Top3/TOPOIIIα, such that Top3/TOPOIIIα can dissolve the final hemi-catenane to separate the two parent molecules into a non-crossover outcome (Cejka et al., 2010b; Wu and Hickson, 2003). Both end resection and dHJ dissolution require Sgs1 catalytic activity, but genetic data indicate that Sgs1 also performs helicase-independent functions, which have not been defined yet (Lo et al., 2006; Mullen et al., 2000). Top3 catalytic activity has been demonstrated to be required for dHJ dissolution, but surprisingly the slow growth phenotype of Top3-deficient cells is significantly more pronounced than the phenotype of Sgs1-deficient cells (Mullen et al., 2000; Onodera et al., 2002; Shor et al., 2002; Wallis et al., 1989). The phenotype of Rmi1-deficient cells appears to be indistinguishable from Top3-deficiency and strongly suggests that Top3-Rmi1 form an obligatory functional complex in cells (Mullen et al., 2005). Current models cannot provide a mechanistic explanation for the differential phenotype of sgs1 and top3/rmi1 mutants. It has been suggested that Sgs1 generates DNA intermediates whose resolution requires Top3 (Wallis et al., 1989). However, it is also possible, that in the absence of Top3, DNA intermediates accumulate that are then processed by Sgs1 in a pathological manner. Both models are consistent with the observed partial suppression of the top3 growth defect by sgs1 (Wallis et al., 1989).
In this study we set out to evaluate the role of Sgs1 and the Sgs1-Top3-Rmi1 complex in reversing the D-loop intermediate in HR. As expected based on experiments with purified human BLM protein (Bachrati et al., 2006; van Brabant et al., 2000), Sgs1 was found to dissociate protein-free D-loops in a manner that was dependent on its helicase activity. Surprisingly, Sgs1 was unable to dissociate D-loops in a reconstituted D-loop reaction with the cognate Rad51, Rad54, and RPA proteins. Unexpectedly, we found that yeast Sgs1-Top3-Rmi1 as well as human TOPOIIIα-RMI1–RMI2 dissolve D-loops in such reconstituted reactions. Specifically, Top3 and its catalytic activity were required for D-loop dissolution dependent on the presence of a single-stranded DNA binding protein. This reaction proceeds with significant specificity and does not occur on protein-free D-loops or D-loops generated by bacterial RecA protein or human RAD51/RAD54. Results from several control experiments suggest that Top3 does not act by relaxing the negatively supercoiled duplex substrate consistent with previous biochemical and genetic results that Top3 is inefficient as a DNA relaxase. Sgs1 moderates the activity of Top3 whereas Rmi1 stimulates Top3 in D-loop dissolution. Taken together, we show a novel mechanism of D-loop reversal by a Top3-based mechanism that may share mechanistic similarities with dHJ dissolution catalyzed by the Sgs1-Top3-Rmi1/BLM-TOPO3α-RMI1–RMI2 complexes. We discuss genetic data that are consistent with a specific role of Top3 in reversing HR intermediates ensuring a non-crossover outcome in addition to its known HR roles in DSB end resection and dHJ dissolution.
Results
Sgs1 disrupts protein-free D-loops but fails to disrupt D-loops in reconstituted reactions with Rad51–Rad54
Disruption of nascent D-loops is a potential mechanism of anti-recombination and disruption of extended D-loops is an integral part of the SDSA pathway of HR leading to an NCO outcome. The BLM helicase has been implicated in D-loop disruption, and biochemical experiments have shown that purified BLM disrupts D-loops assembled from oligonucleotide substrates or D-loops produced by bacterial RecA protein from an invading oligonucleotide and a supercoiled target duplex DNA after deproteinization of the substrate (Bachrati et al., 2006; van Brabant et al., 2000). The yeast BLM homolog Sgs1 is a potent DNA helicase active at sub-nanomolar concentrations (Cejka and Kowalczykowski, 2010), but its activity on D-loops has never been tested. Following the approach used with BLM (Bachrati et al., 2006) we tested the activity of yeast Sgs1 on deproteinized D-loops produced by the bacterial RecA protein (Fig. 1A). Using near equimolar amounts of Sgs1 (0.5 nM) and D-loop substrate (~ 1 nM), we show that Sgs1, like human BLM, efficiently disrupts protein-free D-loops (Fig. 1B, C). While it has been assumed that the BLM helicase activity is responsible for D-loop disruption, this had not been formally demonstrated. Consistent with this expectation, Sgs1hd, the helicase-deficient Sgs1-K706A protein, is completely deficient in disrupting protein-free D-loops (Fig. 1B, C). The data show that Sgs1 disrupts D-loops by a mechanism that depends on its ATPase activity that is required for its helicase function.
Figure 1. Sgs1 disrupts protein-free but not Rad51-mediated D-loops.
A, Reaction scheme for deproteinized purified D-loops. B, Purified protein-free D-loops (~1 nM) containing a 5’-end labeled 95-mer were incubated with 0.5 nM Sgs1 or Sgs1hd (Sgs1-K706A), or reaction buffer for 10 min and the reaction products resolved on agarose gels. C, Quantitation of D-loops. Shown are means ± standard deviations of three independent experiments. D, Scheme for Rad51/Rad54-mediated D-loop reaction. E, Representative gel of products from reactions containing 20 nM 5’-end labeled 95-mer, 0.67 µM Rad51 (1 Rad51: 3 nts), 100 nM RPA, 112 nM Rad54, 20 nM supercoiled plasmid DNA, and Sgs1 (0, 1, 5, 10, 20, 50, 100 nM). F, Quantitation of D-loops. Shown are means ± standard deviations of three independent experiments.
In cells, D-loops are unlikely to be protein-free and rather represent different species of protein-DNA complexes. Nascent D-loops likely still have proteins bound to the substrate that performed homology search and strand invasion (e.g. RPA, Rad51, Rad54) (Solinger et al., 2002). To test whether Sgs1 can disrupt nascent D-loops, we reconstituted D-loop formation with the yeast RPA, Rad51, and Rad54 proteins. After an initial 2 min incubation, about 15% D-loops were formed, which represents about 3 nM substrate (input 20 nM dsDNA). Then Sgs1 was added and the amount of D-loops was determined after an additional 10 min of incubation (Fig. 1D), which was sufficient for complete disruption of protein-free D-loops (Fig. 1B, C). A titration of up to 100 nM of Sgs1, representing 30-fold excess of protein over substrate, failed to show any D-loop disruption activity in this assay (Fig.1E, F).
As a member of the RecQ family of helicases Sgs1 translocates along ssDNA with 3’ to 5’ polarity. During the D-loop reaction, Rad54 stimulates formation of the D-loop and removes Rad51 exposing the 3’ end of the heteroduplex (Li and Heyer, 2009). To determine if exposing the 3’ end of the heteroduplex DNA was required for its removal by Sgs1, we added the helicase at different times after initiation of D-loop formation. Sgs1 does not dissolve D-loops even when added up to twenty minutes post-D-loop initiation (data not shown), a time at which the 3’ end is accessible to extension by Polδ (Li and Heyer, 2009). To determine if Sgs1 blocks formation of D-loops by interfering with the Rad51 filament stability or prevents Rad54-mediated joint molecule formation, we added Sgs1 to the reaction with RPA, which is prior to D-loop initiation or with Rad54 at the time of D-loop initiation (Fig. S1A). We found that Sgs1 or Sgs1hd were unable to block formation of D-loops when added at early times during their formation (Fig. S1B, C). Our standard D-loop is formed with 95 base pairs of fully homologous hDNA (Fig. 1A). As Sgs1 is a 3’ to 5’ helicase, it may require a portion of unpaired filament to recognize the D-loop as a substrate (Cejka and Kowalczykowski, 2010). To evaluate such substrate requirements that more closely emulate invasion of ssDNA into a dsDNA molecule, we formed D-loops containing 25 nucleotides of heterology 5’ of the 95 nucleotides of homology. However, such 5’-tailed substrates were also refractory to disruption by Sgs1 in the reconstituted D-loop reaction (Fig. S1D, E). It has been proposed that Sgs1 acts to remove erroneous joint molecules such as those formed by 5’ strand invasion events (Bernstein et al., 2010). BLM was found to disrupt such protein-free 3’-tailed D-loops faster than any other D-loop substrate (Bachrati et al., 2006). We formed D-loops with 25 nucleotides of 3’-heterology emulating a 5’ invasion and found that similar to the 3’ invasions, neither wild type Sgs1 nor Sgs1hd were able to disrupt such joint molecules (Fig. S1D, E).
We conclude that yeast Sgs1, like human BLM, efficiently disrupts protein-free D-loops, but cannot directly act on the formation or turnover of D-loops in reconstituted reactions with yeast RPA, Rad51, and Rad54. Human BLM was reported to disrupt D-loop in reactions reconstituted with human RPA and RAD51 (Bugreev et al., 2007b). This activity depended on activating the RAD51 ATPase activity by chelation of the Ca++ ions present in the reaction to inhibit the RAD51 ATPase. No D-loop disruption by BLM was evident when RAD51 was maintained in the active ATP-bound form (Bugreev et al., 2007b; Nimonkar et al., 2008). Activation of the RAD51 ATPase activity lowers its affinity to DNA (Ristic et al., 2005; van Mameren et al., 2009). Hence, it is possible that D-loop disruption by BLM after Ca++ chelation reflects activity on protein-free substrates.
Top3 dissolves Rad51–Rad54 mediated D-loop with a topoisomerase-dependent mechanism
Sgs1 forms a conserved complex with Top3 and Rmi1 and acts together with these proteins during HR in DSB end resection and dHJ dissolution (Chu and Hickson, 2009; Symington and Gautier, 2011). The availability of purified Sgs1-Top3-Rmi1 (STR) complex (Cejka and Kowalczykowski, 2010; Cejka et al., 2010b; Cejka et al., 2012) afforded us the opportunity to test the entire STR complex in our reconstituted D-loop system (Fig. 2A). Unlike Sgs1 (Fig. 1D–F, Fig. 2C), the STR complex efficiently removed Rad51–Rad54 mediated D-loops (Fig. 2B, C). In reactions containing 2 nM D-loops (20 nM dsDNA input), up to 80% of the D-loops were eliminated (Fig. 2B, C). Unexpectedly, this activity by the STR complex was independent of the Sgs1 ATPase activity, as the complex of Sgs1hd-Top3-Rmi1 (DTR) was as efficient as the wild type complex (Fig. 2B, C). This suggests that the mechanism active in the reconstituted reaction is fundamentally different from the Sgs1-mediated disruption of protein-free D-loops observed in Figure 1. In fact, Top3 alone efficiently eliminated Rad51–Rad54-mediated D-loops (Fig. 2D, E). This activity depended on the topoisomerase activity of Top3, as the Top3 catalytic mutant (Top3cd) affecting the active site tyrosine (Y356F) (Fig. S2) was completely devoid of this activity even at up to 12-fold excess protein over substrate. We term this novel Top3 activity ‘D-loop dissolution’ to acknowledge the similarity to dHJ dissolution by Sgs1-Top3-Rmi1 and BLM-TOPOIII α-RMI1/2 (Cejka et al., 2010b; Wu and Hickson, 2003). In these reactions, we observed not only an overall decrease in the D-loop signal but also indication of topological activity leading to slower migration of D-loops labeled as topoisomers in Fig. 2B, D. This topological activity is specific and not seen with the negatively supercoiled substrate DNA or with D-loop formed by human RAD51/RAD54 (see below).
Figure 2. Topoisomerase activity is necessary and sufficient for dissolution of Rad51–Rad54 reconstituted D-loops by Sgs1-Top3-Rmi1.
A, Reaction scheme and proteins. B, D-loop dissolution by Sgs1, Sgs1-Top3-Rmi1 (STR), Sgs1-K706A-Top3-Rmi1 (DTR), Top3-Rmi1 (TR) (0, 2, 5, 10, 20, 50 nM). C, Quantitation of D-loops. Shown are normalized means ± standard deviations of three independent experiments. The absolute values corresponding to maximal D-loop levels are Sgs1: 14%, DTR: 12%, STR: 11%, and TR: 18%. The Sgs1 data were taken from Figure 1. D, D-loop dissolution by Top3 and Top3cd (Top3-Y356F) (0, 5, 10, 20, 50 nM). E, Quantitation of D-loops. Shown are normalized means ± standard deviations of three independent experiments. The absolute values corresponding to maximal D-loop levels are Top3: 22%, Top3cd: 19%.
Top3 is a ssDNA-specific topoisomerase (Kim and Wang, 1992) that is stimulated by its cognate ssDNA binding protein, RPA, but also by non-cognate ones such as E. coli SSB (Cejka et al., 2012). We tested the role of RPA in the D-loop dissolution reaction and found a mild stimulation of Top3 or Top3-Rmi1-mediated D-loop dissolution with no apparent preference for yeast RPA over human RPA or bacterial SSB (Fig. S2B–D).
Top3 dissolves Rad51–Rad54 reconstituted D-loops in a species-specific manner
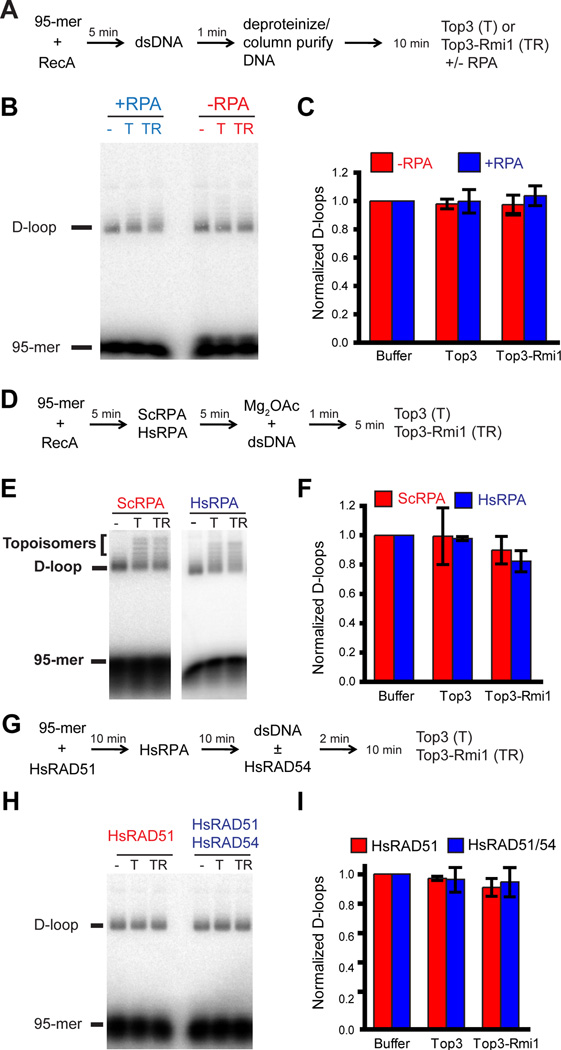
The key steps in HR and their catalysts are well conserved in evolution. Specifically, the central reactions of homology search and DNA strand invasion are catalyzed by a highly conserved nuclear protein filament composed of a RecA protein family homolog bound to ssDNA and ATP. While these proteins, archaeal RadA, bacterial RecA, or eukaryotic Rad51, form structurally and functionally highly similar filaments, they engage in species-specific protein interactions (Heyer, 2007). These characteristics allow testing of the specificity of Top3-mediated D-loop dissolution. First, we employed protein-free D-loops (Fig. 3A) that can readily be disrupted by yeast Sgs1 (Fig. 1A–C). To not confound the analysis with Sgs1, we only tested Top3 and the Top3-Rmi1 complex, but not the Sgs1-Top3-Rmi1 heterotrimer. Neither Top3 nor Top3-Rmi1 in the presence or absence of RPA was able to dissolve protein-free D-loops (Fig. 3B, C). In reactions with yeast Rad51–Rad54, topological isoforms of the D-loops (Fig. 2B, D) were generated during D-loop dissolution that indicate that Top3 was able to topologically relax the D-loop leading to slower migration on agarose gels. Protein-free D-loops, however, showed no evidence of Top3 topological activity (Fig. 3B). This behavior of yeast Top3 is in contrast to Drosophila TopIIIβ, which has been shown to act on deproteinized D-loops by nicking the displaced strand, which leads to accumulation of nicked product on a gel (Wilson-Sali and Hsieh, 2002). Next, we reconstituted the D-loop reaction with bacterial RecA protein using either yeast or human RPA as the ssDNA binding protein (Fig. 3D). RecA-mediated D-loops were also refractory to Top3-mediated dissolution (Fig. 3E, F). However, unlike with protein-free D-loops, there was some evidence of topological activity to relax D-loops in the reaction (Fig. 3E), which depended on the presence of RPA (not shown). The total amount of D-loops did not change significantly. Finally, we reconstituted the D-loop reaction with human RAD51 and human RPA in the presence and absence of human RAD54 (Fig. 3G, see also Fig. 5A, B). Human RAD51/Rad54-mediated D-loops were refractory to Top3-mediated D-loop dissolution and Top3-mediated relaxation of D-loops. We conclude that D-loop dissolution by yeast Top3 is highly species-specific, which indicates that this activity is likely to be of biological significance.
Figure 3. Top3-mediated D-loop dissolution is highly specific.
Top3 does not dissolve protein-free D-loops. A, Reaction scheme for deproteinized D-loops. B, Deproteinized D-loops (~1 nM) were incubated with 0.5 nM Top3 (T) or Top3-Rmi1 (TR) in the presence or absence of 100 nM RPA. C, Quantitation of D-loops. Shown are normalized means ± standard deviations of three independent experiments. The absolute values corresponding to maximal D-loop levels were buffer 11.9%, Top3 12.6%, and Top3-Rmi1 12%. Top3 does not dissolve RecA-mediated D-loops. D, Reaction scheme for RecA-mediated D-loops. E, RecA D-loop reactions were incubated with 0.5 nM Top3 (T) or Top3-Rmi1 (TR) in the presence or absence of 100 nM yeast RPA (ScRPA) or human RPA (HsRPA). F, Quantitation of D-loops. Shown are normalized means ± standard deviations of three independent experiments. The absolute values corresponding to maximal D-loop levels were buffer (ScRPA 5.8%, HsRPA 7.4%), Top3 (ScRPA 5.5%, HSRPA 6%), and Top3-Rmi1 (ScRPA 5.1%, HsRPA 5.7%). Top3 does not dissolve human RAD51-mediated D-loops. G, Reaction scheme for human RAD51- or RAD51/RAD54-mediated D-loops. H, RAD51 D-loop reactions were incubated with 2 nM Top3 (T) or Top3-Rmi1 (TR) in the presence or absence of 100 nM human RPA (HsRPA). I, Quantitation of D-loops. Shown are normalized means ± standard deviations of three independent experiments. The absolute values corresponding to maximal D-loop levels were buffer (RAD51 7.8%, RAD51/RAD54 7.5%), Top3 (RAD51 7.6%, RAD51/RAD54 7.2%, and Top3-Rmi1 (RAD51 7.1%, RAD51/RAD54 7.1%).
Figure 5. Human TOPOIIIα-RMI1–RMI2 dissolves D-loops.
A, Reaction scheme for human RAD51/RAD54-mediated D-loops. B, Quantitation of D-loops. The absolute values corresponding to maximal D-loop levels were TR 8 % and TRR 8 %. C, Reaction scheme for yeast Rad51/Rad54-mediated D-loops. D, Quantitation of D-loops. The absolute values corresponding to maximal D-loop levels were TR 12 % and TRR14 %. E, Reaction scheme for deproteinized D-loops. Deproteinized D-loops (~1 nM) were incubated with TR and TRR. F, Quantitation of D-loops. The absolute values corresponding to starting D-loop levels were TR 39 % and TRR 37 %. Shown are normalized means ± standard deviations of three independent experiments.
The stability of D-loops with an invading 95-mer depends on negative supercoiling of the duplex DNA (Wright and Heyer, 2014). A possible mechanism of D-loop disruption would the relaxation of the negative supercoils. To confirm that ScRad51–Rad54-reconstituted D-loops are a specific template for Top3 activity rather than D-loop dissolution by topoisomerase-mediated relaxation of the negatively supercoiled template, we directly determined the ability of Top3 to relax the negatively supercoiled duplex substrate under our experimental conditions. Bacterial Top1 readily relaxed negatively supercoiled dsDNA, as expected (Fig. S3A). However, 500 nM Top3 did not relax negatively supercoiled DNA under the same reaction conditions used in D-loop assays. This compares to dissolution of 70% of the D-loops by 100-fold less Top3-Rmi1 (5 nM; Fig. 2C). This finding is consistent with Top3 being a single strand-specific DNA topoisomerase with poor activity on negatively supercoiled duplex DNA (Kim and Wang, 1992; Wang, 1996). Top3 readily relaxes hyper-negatively supercoiled DNA or supercoiled DNA containing a single-stranded bubble (Chen et al., 2013). The negatively supercoiled duplex DNA used in our experiments has been prepared to avoid potential denaturation by alkali and is not hyper-negatively supercoiled, which explains why Top3 does not relax this substrate. Second, testing Sgs1, Top3, and Rmi1 as assemblies or as individual components under D-loop reaction conditions showed no evidence for relaxation of negatively supercoiled DNA (Fig. S3B). Importantly, these control experiments support our proposal that D-loop dissolution by yeast Top3 is a distinct mechanism that does not involve relaxation of the negatively supercoiled substrate DNA.
Top3-mediated D-loop dissolution is moderated by Sgs1, stimulated by Rmi1, and unlikely mediated by Rad54
Top3-Rmi1 acts in a complex with Sgs1, and we noted that the presence of Sgs1 consistently mitigated the activity Top3-Rmi1-mediated D-loop dissolution (Fig. 2C). This effect was independent of the Sgs1 ATPase activity, as the helicase-dead Sgs1hd protein exerted a near identical effect as wild type Sgs1 (Fig. 2C). These data suggest that Top3 activity on D-loops is controlled by Sgs1. A structural role for Sgs1 has been also reported for Top3-Rmi1 mediated catenation of bubbled dsDNA (Cejka et al., 2012). RMI1 provides the decatenation loop for the TOPIIIα gate (Bocquet et al., 2014) and stimulates decatenation while inhibiting the relaxation activity of Top3 by stabilizing the nicked intermediate (Cejka et al., 2012). We found that Rmi1 significantly stimulates D-loop dissolution by Top3-mediated D-loop (Fig. 4A–C).
Figure 4. Rmi1 stimulates D-loop dissolution by Top3.
A, Reaction scheme for Rmi1-stimulated reactions. B, D-loop dissolution by Top3 or Top3-Rmi1 (0, 2, 5, 10, 20 nM). C, Quantitation of D-loops. Shown are normalized means ± standard deviations of three independent experiments. The absolute values corresponding to maximal D-loop levels are Top3: 21.1%, Top3-Rmi1: 18.7%. D, Reaction scheme with tailed 95-mer. E, D-loop dissolution time course by 2 nM Top3-Rmi1. F, Quantitation of D-loops. Shown are means ± standard deviations of three independent experiments.
Finally, we sought to exclude the possibility that Top3-mediated D-loop dissolution involves the dsDNA motor protein Rad54. Rad54 has been found to dissociate D-loops in vitro (Bugreev et al., 2007a), and we have recently shown that this activity depends on the specific structure and length of the invading ssDNA (Wright and Heyer, 2014). While Rad54 easily displaces a perfectly homologous oligonucleotide after D-loop formation, a heterologous extension at either end endows such D-loop with some stability against disruption by Rad54 (Wright and Heyer, 2014). Using the same 95-mer but tailed with 25 bp heterology at its 5’ end, we show in a time course experiment that the resulting D-loops are essentially stable over the reaction time against Rad54-mediated dissociation (Fig. 4D–F). As Rad54 is a potent ATPase, the D-loop assays conditions include an ATP regeneration system to ensure ample supply of ATP during the course of the reaction. Addition of Top3-Rmi1 to such 5’-tailed D-loop resulted in robust dissolution eliminating over 70% of the initial D-loop in the first 10 min of the reaction. The results suggest that Top3-mediated D-loop dissolution differs from Rad54-mediated D-loop dissociation. In concordance with these data, Top3-Rmi1 dissolves D-loops with 3’ or 5’-tailed invading strands in the presence or absence of wild type or helicase-dead Sgs1 (Fig. S1E).
Human TopoIIIα-RMI1–RMI2 is more promiscuous in dissolving D-loops
The human TopoIIIα-RMI1–RMI2 is homologous to the yeast Top3-Rmi1 complex. Human TopoIIIα-RMI1–RMI2 also was able to dissolve D-loops in the reconstituted D-loop reaction with human RAD51, RAD54, and RPA (Figs. 5A, B, S4). Surprisingly, human TopoIIIα-RMI1–RMI2 was found to be much more promiscuous than the yeast Top3-Rmi1 complex and able to efficiently dissolve D-loops made by yeast Rad51, Rad54, and RPA or protein-free D-loops (Figs. 5C–F, S4). Side-by-side titrations with yeast Top3-Rmi1 confirmed the previously determined specificity of the yeast complex (Figs. 3, 5). Control experiments showed that like Top3-Rmi1, human TopoIIIα-RMI1–RMI2 also does not relax negatively supercoiled DNA under D-loop reaction conditions (Fig. S5). We conclude that also human TopoIIIα-RMI1–RMI2 is endowed with the ability of dissolving D-loops. The data suggest that in the human system there may exist additional factors that impart specificity to the human complex. This could be BLM, which is known to interact with RAD51, or additional novel factors (Braybrooke et al., 2003).
Discussion
Here we report an unexpected activity of Top3 in specifically dissolving D-loops generated by DNA strand invasion with the cognate Rad51 and Rad54 proteins in reconstituted in vitro reactions. We term this reaction D-loop dissolution, because it shares essential features with other dissolution reactions performed by Top3, including the dissolution of dHJs, which depend on Top3 catalytic activity (Fig. 2). Compared to Sgs1 (Fig. 1), which dissociates protein-free D-loops, and Mph1 (Prakash et al., 2009) which dissociates protein-free D-loops and D-loops generated by yeast or human Rad51, Top3 exerts surprising specificity in D-loop dissolution. Indeed, neither Top3 alone nor Top3-Rmi1 can dissolve protein-free D-loops or D-loops generated by RecA or human RAD51 (Fig. 3). Sgs1 moderates Top3-mediated D-loop dissolution in a way that is independent of the Sgs1 ATPase activity. This may provide a potential explanation for a structural role of Sgs1 in functions independent of its ATPase activity (Cejka et al., 2012; Lo et al., 2006; Mullen et al., 2000). Finally, our control experiments eliminate a simple mechanism, by which Top3 relaxes the duplex substrate. Instead, it appears that the yeast Rad51-mediated D-loop is specifically targeted for topological unlinking by Top3. Deciphering the mechanism and regulation of Top3 activity in this reaction requires additional mechanistic work, but D-loops fit nicely into the range of substrates for ssDNA-specific decatenation reactions previously identified for Top3 (Cejka et al., 2012; Hickson and Mankouri, 2011).
The specificity of Top3-mediated D-loop dissolution prompted us to examine specific protein interactions between Top3 and Rad51 or Rad54. Despite intensive efforts, we were unable to demonstrate significant interactions by immunoprecipitation experiments from yeast whole cell extracts (data not shown). Likewise, despite numerous approaches we were unable to see species-specific interactions between the purified proteins in vitro, although we detected consistent above background association between Top3 with both yeast and human Rad51 (data not shown). We believe that the suspected interactions may occur between the DNA bound forms of the proteins.
The key question is whether the Top3 D-loop dissolution activity is of biological relevance? Significant genetic data with top3 and sgs1 single and double mutants are consistent with a Top3-based mechanism of anti-recombination targeting an early HR intermediate such as nascent D-loops. Top3 plays established roles in HR in long-range end resection and dHJ dissolution in conjunction with Sgs1 and Rmi1. However, these two roles cannot explain the much stronger phenotypes of top3 mutants compared to sgs1 mutants for slow growth and hyperrecombination (Onodera et al., 2002; Shor et al., 2002; Wallis et al., 1989). The observation that expression of the Sgs1-hd protein suppresses the top3 slow growth phenotype only partially, suggests that the Sgs1 helicase activity is not entirely responsible for the slow growth of top3 mutants (Mullen et al., 2000). Moreover, top3 mutants enhance the frequency of crossover 177-fold and non-crossover 69-fold in the SUP4-o system which led to the original genetic discovery of TOP3 (Shor et al., 2002; Wallis et al., 1989). This increase is entirely dependent on HR and eliminated in rad51, rad52, or rad54 mutants (Shor et al., 2002). This result is unexpected for a defect affecting only dHJ dissolution. Moreover, there is additional evidence for Sgs1-independent roles of Top3. Recent analysis in sgs1 cells demonstrated a role of Top3 in eliminating recombination-dependent template switch intermediates accumulating during replication (Glineburg et al., 2013). Consistent with the biological relevance of this observation, Top3 expression suppressed some of the MMS sensitivity of sgs1 top3 double mutants dependent on Top3 catalytic activity (Glineburg et al., 2013; Onodera et al., 2002). Finally, recent results from detailed analyses of meiotic recombination demonstrate Sgs1-independent roles for Top3 in meiotic recombination (Kaur et al., 2014; Tang et al., 2014) that are very consistent with a role of Top3 in dissolving D-loops in vivo. Specifically, the Top3 catalytic activity is required late in meiosis at the exit of pachytene to process Spo11-dependent HR intermediates that impede meiotic chromosome segregation (Tang et al., 2014. Absence of Top3 leads to loss of about 25% of the non-crossover products and persistence of single end invasions, previously identified as D-loop intermediates (Hunter and Kleckner, 2001), and other types of joint molecules. It is unclear whether all accumulating joint molecules represent various forms of D-loops. These phenotypes are not present in Sgs1-deficient cells, and uncover a novel role for Top3 in meiotic recombination that is consistent with Top3-mediated dissolution of D-loops and potentially other recombination-dependent joint molecules.
It is presently unclear whether Top3-Rmi1 acts independent of Sgs1 in a different protein pool or in a manner that is not dependent on Sgs1 protein/activity but still in the same complex. Sgs1 and Top3 are largely stable in cells lacking either binding partner, suggesting that Top3-Rmi1 may exist outside a complex with Sgs1 (Mullen et al., 2005). In sum, the existing and new emerging genetic evidence points to a role of Top3 in dissolving HR-dependent intermediates in addition to the established roles in DSB end resection and dHJ dissolution. Our discovery of a Top3-based mechanism of D-loop dissolution provides a satisfying biochemical mechanism for these genetic observations.
Considering the multitude of enzymes implicated in D-loop disruption (see Introduction), it is important to realize that each enzyme may have overlapping substrate specificity for a variety of different D-loop substrates. In Figure 6, we sketched several different types of nascent and extended D-loop based on known characteristics of DSB or gap repair. The D-loops differ not only in structure and length of the hDNA, but also in the type and extent of bound proteins. Anti-crossover enzymes, such as Srs2, RTEL and Mph1, are likely targeting extended D-loops, whereas anti-recombinases are expected to target the nascent D-loops, which has the DNA strand invasion machinery still bound to it. Top3 showed exquisite specificity for such nascent D-loops, strongly suggesting that it acts as an anti-recombinase in addition to its well-established anti-crossover function in dHJ dissolution. This is consistent with the genetic data showing a strong hyper-rec phenotype for Top3-deficient cells in gene conversion events not associated with crossovers (Bailis et al., 1992; Shor et al., 2002). The observation that Top3-deficiency specifically (Top1 or Top2 defects had no effect) enhances homeologous gene conversion between ectopic SAM genes with a resultant increase in hDNA length spanning many mismatches (Bailis et al., 1992), may lead to the speculation that Top3-mediated D-loop dissolution is connected to hDNA rejection triggered by Msh2–Msh6.
Figure 6.
Different D-loops species during HR-mediated DSB and gap repair. D-loops are a collection of different recombination joint molecules with different DNA junction architecture (3’-end, length, gap invasion) and different HR proteins bound to the individual DNA intermediates. D-loops can form during DSB repair (left) or replication fork-associated gap repair (right) and include nascent D-loops (before extension by DNA polymerase: 3’-end not incorporated or ± branch migration), where proteins involved in strand invasion (e.g. Rad51, Rad51 paralogs, Rad54, RPA, Rad52?, others?) are likely still bound to at least parts of the D-loop (top) and extended D-loops (bottom), where instead or in addition to HR proteins replication proteins (PCNA, RFC, DNA polymerase, RPA) will be present in the D-loop.
In summary, we demonstrate that nascent D-loops are a novel substrate for dissolution by yeast Top3-Rmi1 and human TopoIIIα-RMI1–RMI2 consistent with an in vivo role as a anti-recombinases targeting the nascent D-loop to abort attempted HR events.
Experimental Procedures
DNA substrates
olWDH566 was used as the standard invading oligonucleotide in D-loops and is referred to as 95-mer (see Table S1). The heterologous 95mer (olWDH1613) is referred to as het 95-mer. The 120-mer consisting of the 95-mer olWDH566 sequence with 25 nt 5’ heterology (olWDH1614) is referred to as 5’-het 120-mer. The 120mer consisting of the 95mer olWDH566 sequence with 25 nt 3’ heterology (olWDH1615) is referred to as 3’-het 120-mer. The 25mer complementary to the 3’-and 5’-heterologous region (olWDH1616) was used to create the double-strand tailed substrates referred as 5’-tailed or 3’-tailed 95-mers. All oligonucleotides were purchased from Sigma. The dsDNA is a derivative plasmid (pBSder, 3,000 bp) with a pBSK backbone and 1,200 bp of phiX174 replacing 1,200 bp of pBSK (Wright and Heyer, 2014).
Proteins
Proteins were purified to apparent homogeneity and the absence of relevant contaminating activities was experimentally established as described in the Supplement.
D-loops assays
D-loop reactions were performed as previously described (Li et al., 2009) and detailed conditions are described in the Supplement.
Supplementary Material
Acknowledgments
We thank Michael Lichten and Neil Hunter for communicating unpublished data and stimulating discussions. We are indebted to Ian HIckson and Kata Sarlós for providing human TRR and helpful discussion. We are grateful to Jody Plank, William Wright, Megan Brinkmeyer, Sucheta Mukherjee, and Jie Liu for helpful discussion and comments on the manuscript, Andrew Burch for initial bacmid and virus production, and William Wright, Jachen Solinger, Jie Liu, and Kirk Ehmsen for purifying proteins used in this study. CLF was partially supported by a Ruth Kirschstein National Research Service Award (F32 GM83509). PC was partially supported by a Swiss National Science Foundation Fellowship (PA00A-115375). This work was supported by grants the National Institutes of Health to WDH (GM58015, CA92276, CA154920) and SCK (CA154920, GM41347, GM62653).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachrati CZ, Borts RH, Hickson ID. Mobile D-loops are a preferred substrate for the Bloom's syndrome helicase. Nucleic Acids Res. 2006;34:2269–2279. doi: 10.1093/nar/gkl258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailis AM, Arthur L, Rothstein R. Genome Rearrangement in top3 Mutants of Saccharomyces cerevisiae Requires a Functional RAD1 Excision Repair Gene. Mol Cell Biol. 1992;12:4988–4993. doi: 10.1128/mcb.12.11.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber LJ, Youds JL, Ward JD, McIlwraith MJ, O'Neil NJ, Petalcorin MIR, Martin JS, Collis SJ, Cantor SB, Auclair M, et al. RTEL1 maintains genomic stability by suppressing homologous recombination. Cell. 2008;135:261–271. doi: 10.1016/j.cell.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein KA, Gangloff S, Rothstein R. The RecQ DNA Helicases in DNA Repair. Annu Rev Genet. 2010;44:393–417. doi: 10.1146/annurev-genet-102209-163602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein KA, Shor E, Sunjevaric I, Fumasoni M, Burgess RC, Foiani M, Branzei D, Rothstein R. Sgs1 function in the repair of DNA replication intermediates is separable from its role in homologous recombinational repair. EMBO J. 2009;28:915–925. doi: 10.1038/emboj.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Bizard AH, Abdulrahman W, Larsen NB, Faty M, Cavadini S, Bunker RD, Kowalczykowski SC, Cejka P, Hickson ID, et al. Structural and mechanistic insight into Holliday-junction dissolution by Topoisomerase IIIalpha and RMI1. Nature Struct Mol Biol. 2014;21:261–268. doi: 10.1038/nsmb.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrooke JP, Li JL, Wu L, Caple F, Benson FE, Hickson ID. Functional interaction between the Bloom's syndrome helicase and the RAD51 paralog, RAD51L3 (RAD51D) J Biol Chem. 2003;278:48357–48366. doi: 10.1074/jbc.M308838200. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Brosh RM, Mazin AV. RECQ1 possesses DNA branch migration activity. J Biol Chem. 2008;283:20231–20242. doi: 10.1074/jbc.M801582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugreev DV, Hanaoka F, Mazin AV. Rad54 dissociates homologous recombination intermediates by branch migration. Nature Struct Mol Biol. 2007a;14:746–753. doi: 10.1038/nsmb1268. [DOI] [PubMed] [Google Scholar]
- Bugreev DV, Yu X, Egelman EH, Mazin AV. Novel pro- and anti-recombination activities of the Bloom's syndrome helicase. Genes Dev. 2007b;21:3085–3094. doi: 10.1101/gad.1609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature. 2010a;467:U112–U149. doi: 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Kowalczykowski SC. The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds Holliday junctions. J Biol Chem. 2010;285:8290–8301. doi: 10.1074/jbc.M109.083196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Plank JL, Bachrati CZ, Hickson ID, Kowalczykowski SC. Rmi1 stimulates decatenation of double Holliday junctions during dissolution by Sgs1-Top3. Nature Structl Mol Biol. 2010b;17:1377–1382. doi: 10.1038/nsmb.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Plank JL, Dombrowski CC, Kowalczykowski SC. Decatenation of DNA by the S. cerevisiae Sgs1-Top3-Rmi1 and RPA Complex: A Mechanism for Disentangling Chromosomes. Mol Cell. 2012;47:886–896. doi: 10.1016/j.molcel.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, Palmai-Pallag T, Ying SM, Hickson ID. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nature Cell Biol. 2009;11:753–760. doi: 10.1038/ncb1882. [DOI] [PubMed] [Google Scholar]
- Chen CF, Brill SJ. Binding and activation of DNA topoisomeraseIII by the Rmi1 subunit. J Biol Chem. 2007;282:28971–28979. doi: 10.1074/jbc.M705427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Chan NL, Hsieh TS. New Mechanistic and Functional Insights into DNA Topoisomerases. Annu Rev Biochem. 2013;82:139–170. doi: 10.1146/annurev-biochem-061809-100002. [DOI] [PubMed] [Google Scholar]
- Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nature Rev Cancer. 2009;9:644–654. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- Crismani W, Girard C, Froger N, Pradillo M, Santos JL, Chelysheva L, Copenhaver GP, Horlow C, Mercier R. FANCM limits meiotic crossovers. Science. 2012;336:1588–1590. doi: 10.1126/science.1220381. [DOI] [PubMed] [Google Scholar]
- Glineburg MR, Chavez A, Agrawal V, Brill SJ, Johnson FB. Resolution by Unassisted Top3 Points to Template Switch Recombination Intermediates during DNA Replication. J Biol Chem. 2013;288:33193–33204. doi: 10.1074/jbc.M113.496133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer WD. Biochemistry of eukaryotic homologous recombination. In: Aguilera A, Rothstein R, editors. Molecular Genetics of Recombination. Berlin-Heidelberg: Springer-Verlag; 2007. pp. 95–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer WD, Ehmsen KT, Liu J. Regulation of homologous recombination in eukaryotes. Annu Rev Genet. 2010;44:113–139. doi: 10.1146/annurev-genet-051710-150955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson ID, Mankouri HW. Processing of homologous recombination repair intermediates by the Sgs1-Top3-Rmi1 and Mus81-Mms4 complexes. Cell Cycle. 2011;10 doi: 10.4161/cc.10.18.16919. [DOI] [PubMed] [Google Scholar]
- Hombauer H, Srivatsan A, Putnam CD, Kolodner RD. Mismatch Repair, But Not Heteroduplex Rejection, Is Temporally Coupled to DNA Replication. Science. 2011;334:1713–1716. doi: 10.1126/science.1210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. Meiotic recombination. In: Aguilera A, Rothstein R, editors. Homologous Recombination. Berlin-Heidelberg: Springer-Verlag; 2007. pp. 381–441. [Google Scholar]
- Hunter N, Kleckner N. The single-end invasion: an asymmetric intermediate at the double strand break to double Holliday junction transition of meiotic recombination. Cell. 2001;106:59–70. doi: 10.1016/s0092-8674(01)00430-5. [DOI] [PubMed] [Google Scholar]
- Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RD, Jasin M. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. Embo J. 2000;19:3398–3407. doi: 10.1093/emboj/19.13.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyk LC, Hartwell LH. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992;132:387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, De Muyt A, Lichten M. Top3-Rmi1 DNA single-strand decatenase is integral to the formation and resolution of meiotic recombination intermediates. Mol Cell. 2014 doi: 10.1016/j.molcel.2015.01.020. co-submitted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RA, Wang JC. Identification of the Yeast TOP3 Gene Product as a Single Strand-Specific DNA Topoisomerase. J Biol Chem. 1992;267:17178–17185. [PubMed] [Google Scholar]
- LaRocque JR, Stark JM, Oh J, Bojilova E, Yusa K, Horie K, Takeda J, Jasin M. Interhomolog recombination and loss of heterozygosity in wild-type and Bloom syndrome helicase (BLM)-deficient mammalian cells. Proc Natl Acad Sci USA. 2011;108:11971–11976. doi: 10.1073/pnas.1104421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Heyer WD. RAD54 controls access to the invading 3-OH end after RAD51-mediated DNA strand invasion in homologous recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:638–646. doi: 10.1093/nar/gkn980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Stith CM, Burgers PM, Heyer W-D. PCNA is required for initiating recombination-associated DNA synthesis by DNA polymerase δ. Mol Cell. 2009;36:704–713. doi: 10.1016/j.molcel.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberi G, Maffioletti G, Lucca C, Chiolo I, Baryshnikova A, Cotta-Ramusino C, Lopes M, Pellicioli A, Haber JE, Foiani M. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 2005;19:339–350. doi: 10.1101/gad.322605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YC, Paffett KS, Amit O, Clikernan JA, Sterk R, Brenneman MA, Nickoloff JA. Sgs1 regulates gene conversion tract lengths and crossovers independently of its helicase activity. Mol Cell Biol. 2006;26:4086–4094. doi: 10.1128/MCB.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A, Osman F, Sun W, Nandi S, Steinacher R, Whitby MC. The fission yeast FANCM ortholog directs non-crossover recombination during meiosis. Science. 2012;336:1585–1588. doi: 10.1126/science.1220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Brill SJ. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2000;154:1101–1114. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Nallaseth FS, Lan YQ, Slagle CE, Brill SJ. Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex. Mol Cell Biol. 2005;25:4476–4487. doi: 10.1128/MCB.25.11.4476-4487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc Natl Acad Sci U S A. 2008;105:16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu HY, Chung WH, Zhu Z, Kwon Y, Zhao WX, Chi P, Prakash R, Seong CH, Liu DQ, Lu L, et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature. 2010;467:U108–U143. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera R, Seki M, Ui A, Satoh Y, Miyajima A, Onoda F, Enomoto T. Functional and physical interaction between Sgs1 and Top3 and Sgs1-independent function of Top3 in DNA recombination repair. Genes Genet Sys. 2002;77:11–21. doi: 10.1266/ggs.77.11. [DOI] [PubMed] [Google Scholar]
- Prakash R, Satory D, Dray E, Papusha A, Scheller J, Kramer W, Krejci L, Klein H, Haber JE, Sung P, et al. Yeast Mph1 helicase dissociates Rad51-made D-loops: implications for crossover control in mitotic recombination. Genes Dev. 2009;23:67–79. doi: 10.1101/gad.1737809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic D, Modesti M, van der Heijden T, van Noort J, Dekker C, Kanaar R, Wyman C. Human Rad51 filaments on double- and single-stranded DNA: correlating regular and irregular forms with recombination function. Nucleic Acids Res. 2005;33:3292–3302. doi: 10.1093/nar/gki640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert T, Dervins D, Fabre F, Gangloff S. Mrc1 and Srs2 are major actors in the regulation of spontaneous crossover. EMBO J. 2006;25:2837–2846. doi: 10.1038/sj.emboj.7601158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor E, Gangloff S, Wagner M, Weinstein J, Price G, Rothstein R. Mutations in homologous recombination genes rescue top3 slow growth in Saccharomyces cerevisiae. Genetics. 2002;162:647–662. doi: 10.1093/genetics/162.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger JA, Kiianitsa K, Heyer W-D. Rad54, a Swi2/Snf2-like recombinational repair protein, disassembles Rad51:dsDNA filaments. Mol Cell. 2002;10:1175–1188. doi: 10.1016/s1097-2765(02)00743-8. [DOI] [PubMed] [Google Scholar]
- Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- Tang S, Wu MKY, Zhang R, Hunter N. Pervasive and essential roles of Topoisomerase 3 decatenase in meiosis orchestrate homologous recombination and facilitate chromosome segregation. Mol Cell. 2014 doi: 10.1016/j.molcel.2015.01.021. co-submitted manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brabant AJ, Ye T, Sanz M, German JL, Ellis NA, Holloman WK. Binding and melting of D-loops by the Bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- van Mameren J, Modesti M, Kanaar R, Wyman C, Peterman EJG, Wuite GJL. Counting RAD51 proteins disassembling from nucleoprotein filaments under tension. Nature. 2009;457:745–748. doi: 10.1038/nature07581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- Wang JC. DNA Topoisomerases: Why so many? J Biol Chem. 1991;266:6659–6662. [PubMed] [Google Scholar]
- Wang JC. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- Wilson-Sali T, Hsieh TS. Preferential cleavage of plasmid-based R-loops and D-loops by Drosophila topoisomerase IIIbeta. Proc Natl Acad Sci USA. 2002;99:7974–7979. doi: 10.1073/pnas.122007999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WD, Heyer WD. Rad54 Functions as a Heteroduplex DNA Pump Modulated by Its DNA Substrates and Rad51 during D Loop Formation. Mol Cell. 2014;53:420–432. doi: 10.1016/j.molcel.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Hickson ID. The Bloom's syndrome helicase suppresses crossing-over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.