Abstract
Phosphatidylcholines, sphingomyelins, cholesterol, and cholesterol esters were enriched with 13C by chemical synthesis in specific positions of their hydrophilic groups and aliphatic chains. Their spin-lattice relaxation times were determined in organic solvents. The substances were organized as liposomes and recombined with total human high density apolipoproteins and the two separated main components, apolipoprotein A-I (apoLp-Gln-I) and apolipoprotein A-II (apoLp-Gln-II).
These 13C nuclear magnetic resonance data established that in reassembled high density lipoproteins the phospholipid molecules bind to the apoprotein moieties with their hydrophobic fatty acid chains and not with their hydrophilic zwitterionic groups. Apolipoprotein A-I preferentially binds phosphatidylcholine, although its lipid-binding capacity is smaller than that of apolipoprotein A-II. Apolipoprotein A-II avidly reassembles with sphingomyelin by hydrophobic interactions. A model of the molecular organization of the high density lipoportein particle has been derived.
Keywords: reassembling of [13C]lipids and high density apolipoproteins, spin lattice relaxation
Full text
PDF
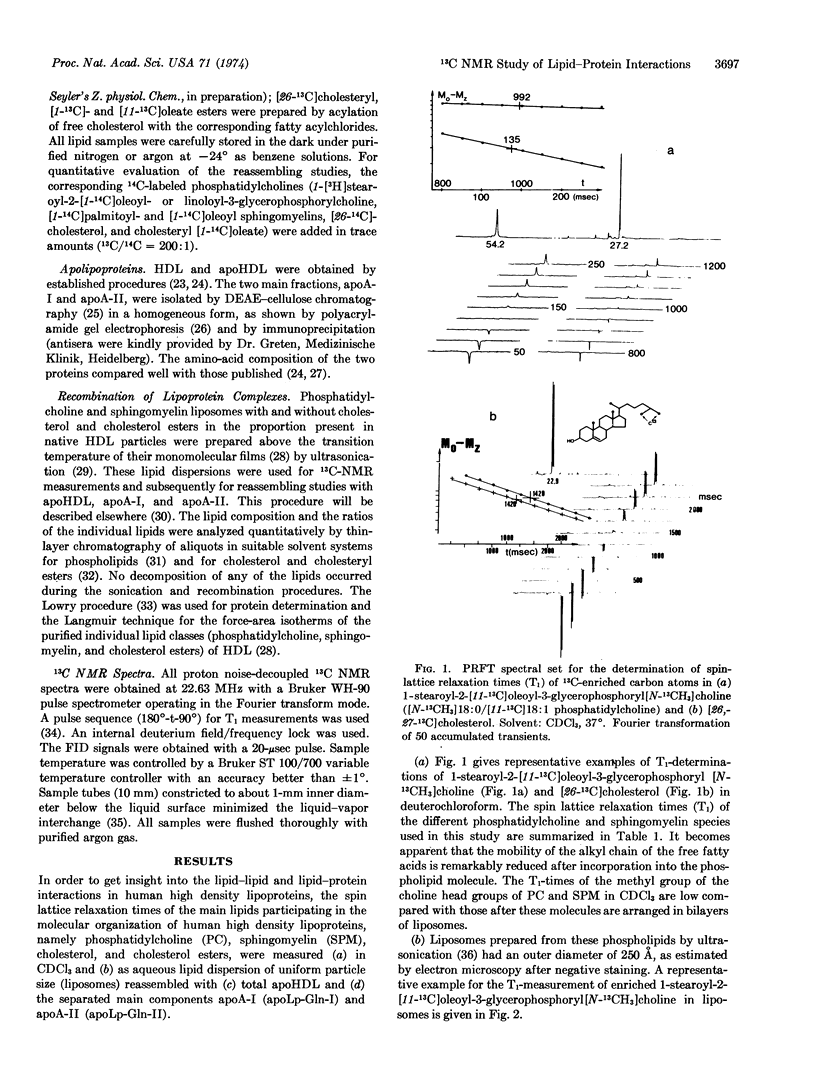
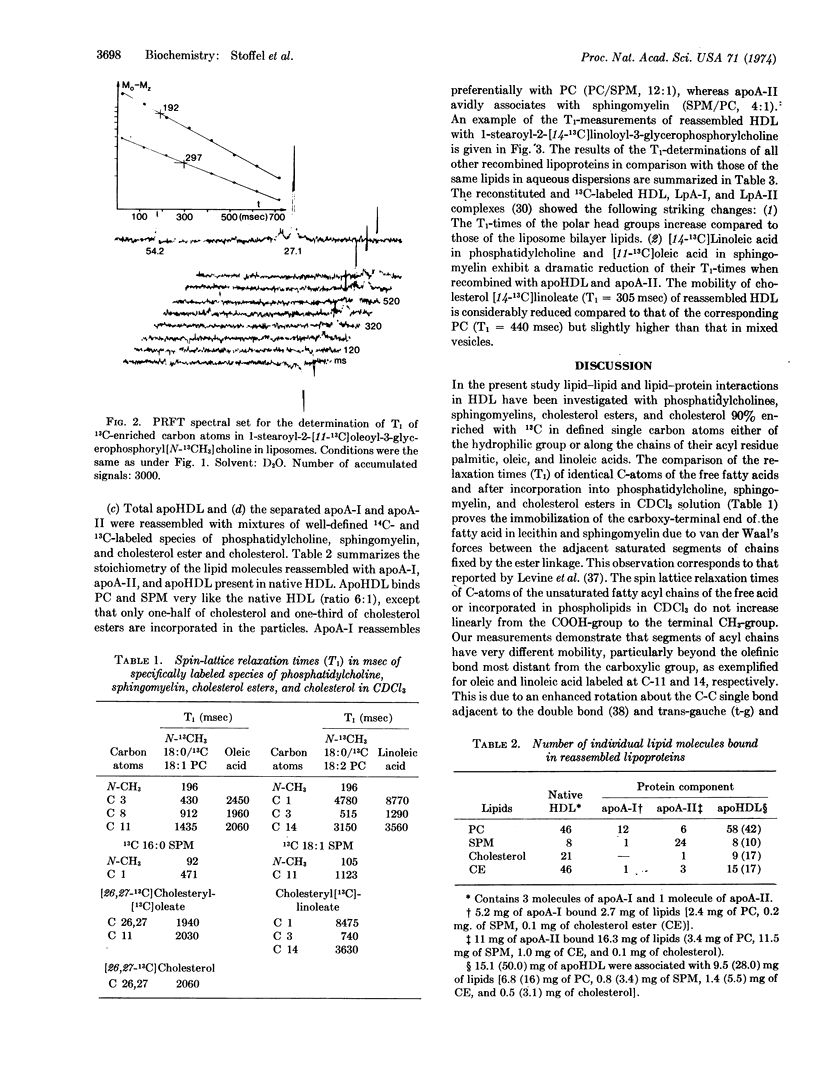
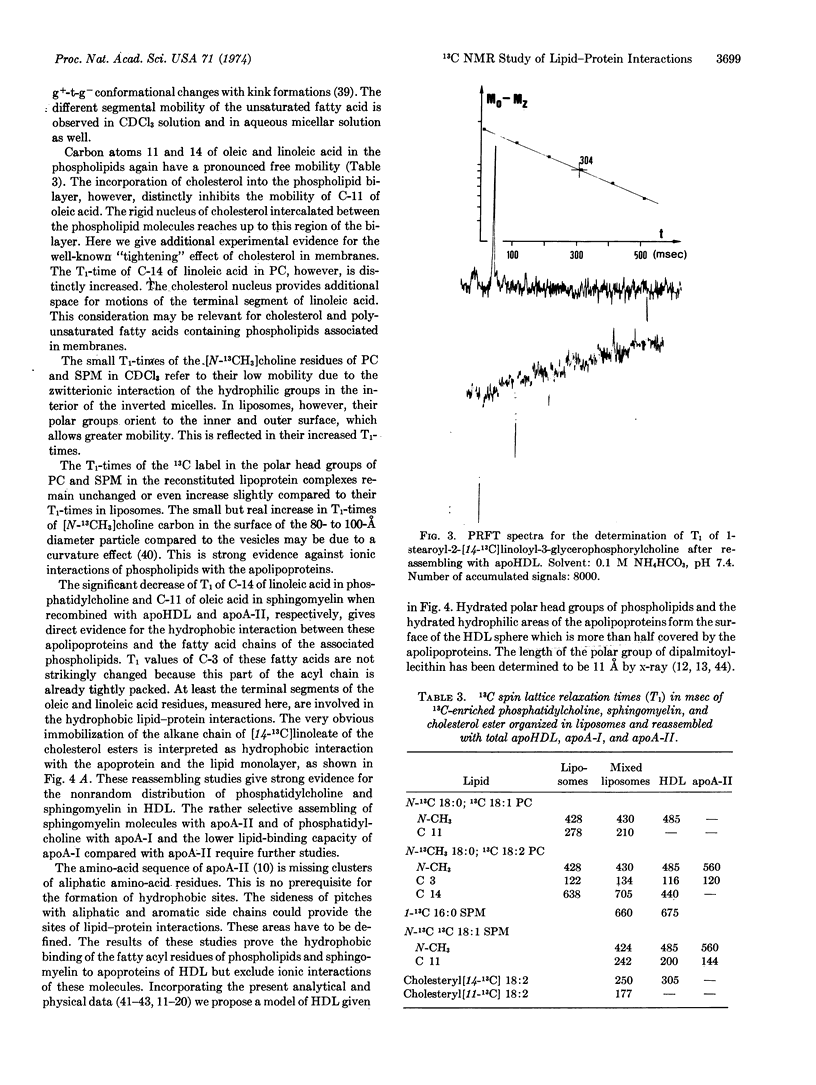
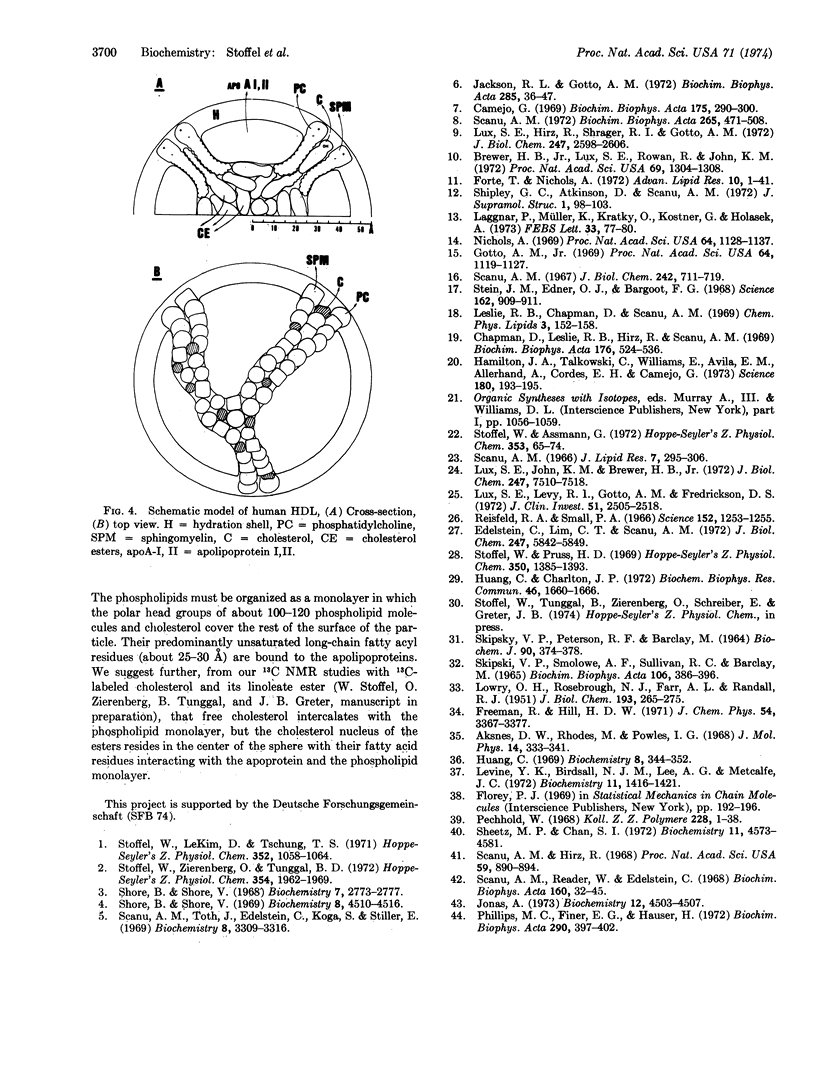
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer H. B., Jr, Lux S. E., Ronan R., John K. M. Amino acid sequence of human apoLp-Gln-II (apoA-II), an apolipoprotein isolated from the high-density lipoprotein complex. Proc Natl Acad Sci U S A. 1972 May;69(5):1304–1308. doi: 10.1073/pnas.69.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camejo G. The structure of human density lipoprotein: a study of the effect of phospholipase A and trypsin on its components and of the behavior of the lipid and protein moieties at the air-water interphase. Biochim Biophys Acta. 1969 Mar;175(2):290–300. doi: 10.1016/0005-2795(69)90007-5. [DOI] [PubMed] [Google Scholar]
- Chapman D., Leslie R. B., Hirz R., Scanu A. M. High-resolution NMR spectra of high-density serum lipoproteins. Biochim Biophys Acta. 1969 Apr 29;176(3):524–536. doi: 10.1016/0005-2760(69)90219-7. [DOI] [PubMed] [Google Scholar]
- Edelstein C., Lim C. T., Scanu A. M. On the subunit structure of the protein of human serum high density lipoprotein. I. A study of its major polypeptide component (Sephadex, fraction 3). J Biol Chem. 1972 Sep 25;247(18):5842–5849. [PubMed] [Google Scholar]
- Forte T., Nichols A. V. Application of electron microscopy to the study of plasma lipoprotein structure. Adv Lipid Res. 1972;10:1–41. [PubMed] [Google Scholar]
- Gotto A. M., Jr Recent studies on the structure of human serum low-and high-density lipoproteins. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1119–1127. doi: 10.1073/pnas.64.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Talkowski C., Williams E., Avila E. M., Allerhand A., Cordes E. H., Camejo G. Natural abundance carbon-13 nuclear magnetic resonance spectra of human serum lipoproteins. Science. 1973 Apr 15;180(4082):193–195. doi: 10.1126/science.180.4082.193. [DOI] [PubMed] [Google Scholar]
- Huang C., Charlton J. P. Studies on the state of phosphatidylcholine molecules before and after ultrasonic and gel-filtration treatments. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1660–1666. doi: 10.1016/0006-291x(72)90800-5. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Gotto A. M. A study of the cystine-containing apolipoprotein of human plasma high density lipoproteins: characterization of cyanogen bromide and tryptic fragments. Biochim Biophys Acta. 1972 Nov 28;285(1):36–47. doi: 10.1016/0005-2795(72)90178-x. [DOI] [PubMed] [Google Scholar]
- Jonas A. Location of aromatic amino acid residues in bovine serum high-density lipoprotein. Biochemistry. 1973 Oct 23;12(22):4503–4507. doi: 10.1021/bi00746a031. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laggner P., Müller K., Kratky O., Kostner G., Holasek A. Studies on the structure of lipoprotein A of human high density lipoprotein HDL3: the spherically averaged electron density distribution. FEBS Lett. 1973 Jun 15;33(1):77–80. doi: 10.1016/0014-5793(73)80163-2. [DOI] [PubMed] [Google Scholar]
- Leslie R. B., Chapman D., Scanu A. M. Nuclear magnetic resonance studies on serum low density lipoproteins (LDL2). Chem Phys Lipids. 1969 Apr;3(2):152–158. doi: 10.1016/0009-3084(69)90006-1. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C nuclear magnetic resonance relaxation measurements of synthetic lecithins and the effect of spin-labeled lipids. Biochemistry. 1972 Apr 11;11(8):1416–1421. doi: 10.1021/bi00758a014. [DOI] [PubMed] [Google Scholar]
- Lux S. E., Hirz R., Shrager R. I., Gotto A. M. The influence of lipid on the conformation of human plasma high density apolipoproteins. J Biol Chem. 1972 Apr 25;247(8):2598–2606. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Brewer H. B., Jr Isolation and characterization of apoLp-Gln-II (apoA-II), a plasma high density apolipoprotein containing two identical polypeptide chains. J Biol Chem. 1972 Dec 10;247(23):7510–7518. [PubMed] [Google Scholar]
- Lux S. E., Levy R. I., Gotto A. M., Fredrickson D. S. Studies on the protein defect in Tangier disease. Isolation and characterization of an abnormal high density lipoprotein. J Clin Invest. 1972 Oct;51(10):2505–2519. doi: 10.1172/JCI107066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols A. V. Functions and interrelationships of different classes of plasma lipoproteins. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1128–1137. doi: 10.1073/pnas.64.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Finer E. G., Hauser H. Differences between conformations of lecithin and phosphatidylethanolamine polar groups and their effects on interactions of phospholipid bilayer membranes. Biochim Biophys Acta. 1972 Dec 1;290(1):397–402. doi: 10.1016/0005-2736(72)90084-3. [DOI] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Scanu A. Binding of human serum high density lipoprotein apoprotein with aqueous dispersions of phospholipids. J Biol Chem. 1967 Feb 25;242(4):711–719. [PubMed] [Google Scholar]
- Scanu A. Forms of human serum high density lipoprotein protein. J Lipid Res. 1966 Mar;7(2):295–306. [PubMed] [Google Scholar]
- Scanu A., Hirz R. On the structure of human serum high-density lipoprotein: studies by the technique of circular dichroism. Proc Natl Acad Sci U S A. 1968 Mar;59(3):890–894. doi: 10.1073/pnas.59.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanu A., Reader W., Edelstein C. Molecular weight and subunit structure of human serum high density lipoproteinafter chemical modifications by succinic anhydride. Biochim Biophys Acta. 1968 May 6;160(1):32–45. doi: 10.1016/0005-2795(68)90061-5. [DOI] [PubMed] [Google Scholar]
- Scanu A., Toth J., Edelstein C., Koga S., Stiller E. Fractionation of human serum high density lipoprotein in urea solutions. Evidence for polypeptide heterogeneity. Biochemistry. 1969 Aug;8(8):3309–3316. doi: 10.1021/bi00836a027. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Shipley G. G., Atkinson D., Scanu A. M. Small-angle x-ray scattering of human serum high-density lipoproteins. J Supramol Struct. 1972;1(2):98–104. doi: 10.1002/jss.400010203. [DOI] [PubMed] [Google Scholar]
- Shore B., Shore V. Heterogeneity in protein subunits of human serum high-density lipoproteins. Biochemistry. 1968 Aug;7(8):2773–2777. doi: 10.1021/bi00848a011. [DOI] [PubMed] [Google Scholar]
- Shore B., Shore V. Isolation and characterization of polypeptides of human serum lipoproteins. Biochemistry. 1969 Nov;8(11):4510–4516. doi: 10.1021/bi00839a043. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipski V. P., Smolowe A. F., Sullivan R. C., Barclay M. Separation of lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1965 Oct 4;106(2):386–396. doi: 10.1016/0005-2760(65)90047-0. [DOI] [PubMed] [Google Scholar]
- Steim J. M., Edner O. J., Bargoot F. G. Structure of human serum lipoproteins: nuclear magnetic resonance supports a micellar model. Science. 1968 Nov 22;162(3856):909–911. doi: 10.1126/science.162.3856.909. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Assmann G. On the metabolism of sphinganyl- and sphingenyl-1-phosphorylcholine. Hoppe Seylers Z Physiol Chem. 1972 Jan;353(1):65–74. doi: 10.1515/bchm2.1972.353.1.65. [DOI] [PubMed] [Google Scholar]
- Stoffel W., LeKim D., Tschung T. S. A simple chemical method for labelling phosphatidylcholine and sphingomyelin in the choline moiety. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1058–1064. doi: 10.1515/bchm2.1971.352.2.1058. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Pruss H. D. Monolayer studies with synthetic saturated, mono- and polyunsaturated mixed 1,2-diglycerides, 1,2-diacylphosphatidylethanolamines and phosphatidylcholines at the air-water-interface. Hoppe Seylers Z Physiol Chem. 1969 Nov;350(11):1385–1393. doi: 10.1515/bchm2.1969.350.2.1385. [DOI] [PubMed] [Google Scholar]
- Stoffel W., Zierenberg O., Tunggal B. D. 13 C-nuclear magnetic resonance spectroscopic studies on saturated, mono-, di- and polyunsaturated fatty acids, phospho- and sphingolipids. Hoppe Seylers Z Physiol Chem. 1972 Dec;353(12):1962–1969. doi: 10.1515/bchm2.1972.353.2.1962. [DOI] [PubMed] [Google Scholar]



