Abstract
Background & Aims
Cathelicidin (LL-37 in human and mCRAMP in mice) represents a family of endogenous antimicrobial peptides with anti-inflammatory effects. LL-37 also suppresses collagen synthesis, an important fibrotic response, in dermal fibroblasts. Here, we determined whether exogenous cathelicidin administration modulates intestinal fibrosis in two animal models of intestinal inflammation and in human colonic fibroblasts.
Methods
C57BL/6J mice (n = 6 per group) were administered intracolonically with a trinitrobenzene sulphonic acid (TNBS) enema to induce chronic (6–7 weeks) colitis with fibrosis. We administered mCRAMP peptide (5 mg/kg every 3 day, week 5–7) or cathelicidin gene (Camp)-expressing lentivirus (107 infectious units week 4) intracolonically or intravenously, respectively. We then infected 129Sv/J mice with Salmonella typhimurium orally to induce cecal inflammation with fibrosis. Camp-expressing lentivirus (107 infectious units day 11) was administered intravenously.
Results
TNBS-induced chronic colitis was associated with increased colonic collagen (col1a2) mRNA expression. Intracolonic cathelicidin (mCRAMP peptide) administration or intravenous delivery of lentivirus-overexpressing cathelicidin gene significantly reduced colonic col1a2 mRNA expression in TNBS-exposed mice compared with vehicle administration. Salmonella infection also caused increased cecal inflammation associated with collagen (col1a2) mRNA expression that was prevented by intravenous delivery of Camp-expressing lentivirus. Exposure of human primary intestinal fibroblasts and human colonic CCD-18Co fibroblasts to transforming growth factor-β1 (TGF-β1) and/or insulin-like growth factor 1 induced collagen protein and mRNA expression, which was reduced by LL-37 (3–5 μM) through a MAP kinase-dependent mechanism.
Conclusions
Cathelicidin can reverse intestinal fibrosis by directly inhibiting collagen synthesis in colonic fibroblasts.
Keywords: Antimicrobial Peptide, Collagen, Inflammatory Bowel Disease
Abbreviations used in this paper: CD, Crohn’s disease; DSS, dextran sulfate; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; HA, hemagglutinin; HEK293T, human embryonic kidney 293T cells; IBD, inflammatory bowel disease; IGF-1, insulin-like growth factor 1; LB, Lysogeny broth; LL-37, cathelicidin; LV, lentivirus; mCRAMP, cathelicidin; MEK, mitogen-activated protein kinase; PD98059, 2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one; RT-PCR, reverse-transcription polymerase chain reaction; TFA, trifluoroacetic acid; TGF-β1, transforming growth factor-β1; TNBS, trinitrobenzene sulphonic acid; TNFα, tumor necrosis factor α; U0126, 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene; UCLA, University of California–Los Angeles
Summary.
Administration of cathelicidin exerts effective antifibrogenic effects in colitis associated intestinal fibrosis. Cathelicidin inhibits transforming growth factor β1-induced collagen expression in fibroblasts. The antifibrogenic effect is extracellular-regulated kinase pathway dependent.
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis, is manifested by chronic inflammation of the gastrointestinal tract with significant morbidity and at times life-threatening complications.1 Patients with chronic CD often develop transmural luminal narrowing and form strictures caused by excessive extracellular matrix deposition.2, 3 The mechanisms of intestinal fibrosis are complex and not well understood. Transforming growth factor-β1 (TGF-β1) levels are often higher in the mucosa overlying CD strictures compared with nonstrictured gut.2 Therapies for fibrosis are ineffective, and surgical resection is often required.2 Approximately 75% of CD patients eventually undergo surgery, most frequently because of intestinal fibrosis.3 Therapy with anti–tumor necrosis factor α (anti-TNFα) monoclonal antibodies can effectively reduce inflammation but may not prevent or reverse fibrosis in CD patients.4 Previous studies also have shown that inhibition of TGF-β1 reduces colonic fibrosis in a mouse chronic colitis model.5
Cathelicidins (LL-37 in humans and mCRAMP in mice) represent a family of endogenous antimicrobial peptides mediating part of the innate immune response that protects the host from infection.5 Several studies support the importance of cathelicidins in the pathogenesis of various disease states. Cathelicidin-deficient (Camp−/−) mice are more susceptible to infections6 and have reduced angiogenesis and wound healing.7, 8 Park et al9 also showed that LL-37 reduces collagen synthesis in dermal fibroblasts, suggesting a potential role for cathelicidins in reducing fibrosis. Intracolonic administration of mCRAMP reduces the severity of dextran sulfate (DSS)-mediated acute colitis in mice,10 and Camp−/− mice have exacerbated acute DSS-induced colitis.11 In addition, cathelicidin effectively reduces Clostridium difficile–mediated colitis and acute C difficile toxin A–induced enteritis in mice.12 Despite its potent anti-inflammatory effects in acute colitis, the potential role of cathelicidin in the development and progress of chronic colitis has not been explored, and the possibility that cathelicidins modulate intestinal fibrosis has never been studied.
Here we used the well-established chronic trinitrobenzene sulphonic acid (TNBS)-induced colitis mouse model as well as the Salmonella-mediated cecal inflammation model to study intestinal fibrosis associated with these models.13, 14 We found that intracolonic peptide or intravenous lentiviral (LV) administration of cathelicidin attenuates colitis-associated intestinal fibrosis and inflammation in vivo. LL-37 reduces collagen expression induced by TGF-β1 and insulin-like growth factor 1 (IGF-1) in human colonic fibroblasts in vitro. Our results also indicate that reduced collagen expression in response to cathelicidin is mediated via extracellular signal-regulated kinase (ERK) activation in these cells.
Materials and Methods
Cell Cultures
Human CCD-18Co colonic fibroblasts (2 × 106 cells/plate) were cultured in minimal essential medium Eagle’s medium (American Type Culture Collection, Manassas, VA) containing 10% fetal bovine serum (Invitrogen/Life Technologies, Carlsbad, CA) and 1% penicillin/streptomycin (Invitrogen).15 Human primary normal intestinal fibroblasts (three normal and two CD patients) were isolated as previously described elsewhere.16 Cells (2 × 106 cells/plate) were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin/streptomycin and were serum starved overnight before experiments. LL-37 peptide was purchased from Biopeptide (San Diego, CA). TGF-β1, IGF-1, proinflammatory cytokine cocktail (TNFα, IL-1β, and interferon-γ, 10 ng/mL each), U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene) and PD98059 (2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one) were purchased from Fisher Scientific (Pittsburgh, PA). LL-37, TGF-β1, IGF-1, and the proinflammatory cytokine cocktail were dissolved in trifluoroacetic acid (TFA) 0.1% as the vehicle solution. PD98059 and U0126 were dissolved in dimethyl sulfoxide. Human primary intestinal fibroblasts from normal subjects and CD patients and cultured human colonic CCD-18Co fibroblasts responded to TGF-β1 and/or IGF-1 differently. Based on preliminary experiments, we used two different conditions (TGF-β1 alone and TGF-β1 + IGF-1) to stimulate collagen expression. IGF-1 alone was not sufficient to induce collagen expression in all the fibroblasts we used (data not shown).
Trinitrobenzene Sulphonic Acid Chronic Colitis Model
Camp−/− mice in C57BL/6J strain were obtained from Dr. Richard Gallo at the University of California–San Diego and were bred and maintained at the University of California–Los Angeles (UCLA) animal facility under standard environmental conditions. The animal studies were approved by the animal research committee of UCLA. Chronic TNBS-associated colitis was induced as previously reported elsewhere.14, 15 Mice (8–9 weeks old) were injected with TNBS solution or 30% ethanol (50 μL) intracolonically for 5 weeks as illustrated in Figure 1 A. Intracolonic administration was achieved by inserting a polyethylene (PE10) catheter 3 cm into rectum under light isoflurane anesthesia. A group of mice were injected with 5 mg/kg of mCRAMP intracolonically every 3 days starting at week 5. Another mouse group were injected with Camp-expressing lentivirus (1 × 107 infectious units/mouse) or hemagglutinin (HA)-expressing lentivirus intravenously once, starting at week 5 as illustrated in Figure 6 A. Colonic tissues were obtained at week 6 or 7 after carbon dioxide euthanasia.
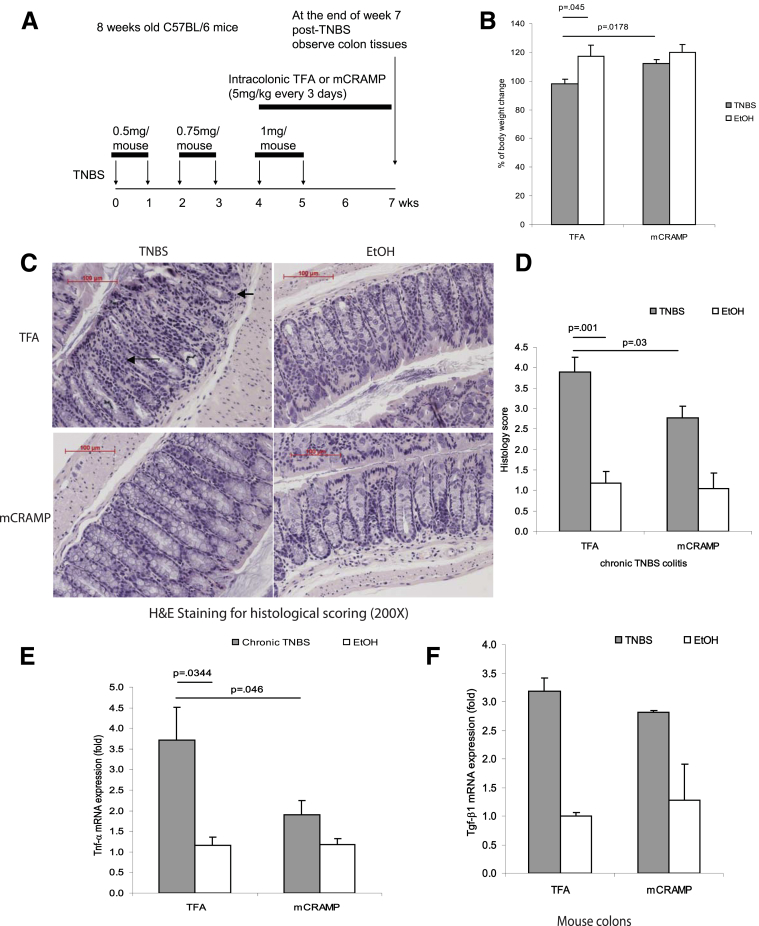
Figure 1.
Cathelicidin peptide administration reduces colonic inflammation in TNBS-mediated chronic colitis in mice. (A) Illustration of experimental plan of TNBS-mediated chronic colitis. Mice were injected with increasing doses of TNBS solution intracolonically once a week for 6 weeks. Some mice received intracolonic injection of mCRAMP peptide or vehicle solution 0.1% of trifluroacetic acid (control) starting from the fifth TNBS injection. Colonic changes were evaluated 2 weeks after the last TNBS injection. (B) Percentage of body weight change of different groups (from week 0 to week 7). All ethanol-treated groups had approximately 20% body weight gain. TNBS-treated wild-type mice had statistically significantly less body weight gain compared with ethanol control (P = .045). Administration of mCRAMP peptide to TNBS-treated wild-type partially restored the body weight gain (P = .0178). (C) Representative H&E images of colonic tissues. Large arrows show immune cell infiltration. Small arrows show collagen deposition and mucosal transformation with altered cryptal architecture. (D) Histology score was based on H&E staining images of colonic tissues. TNBS treatment in mice led to increased tissue damage with a statistically significantly higher histology score (P = .001) compared with the ethanol control. Treatment with mCRAMP statistically significantly reduced the histology score (P = .03). (E) TNBS treatment statistically significantly induced colonic proinflammatory cytokine Tnfα mRNA (P = .0344) expression. Intracolonic mCRAMP administration statistically significantly reduced TNBS-induced mRNA (P = .046) expression in mice. (F) Colonic Tgf-β1 mRNA expression in mice with chronic TNBS colitis. Colonic Tgf-β1 mRNA expression was not affected by intracolonic mCRAMP peptide administration. Per group, n = 6 mice.
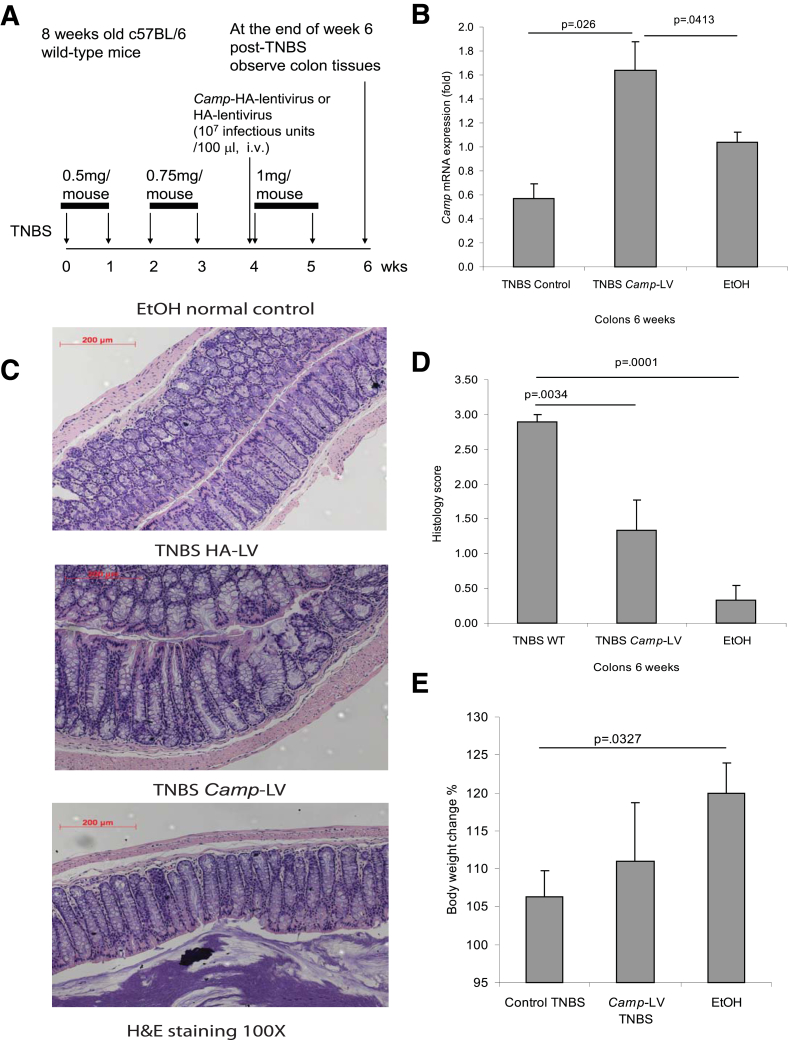
Figure 6.
Lentiviral cathelicidin expression reduces colonic inflammation in TNBS-mediated chronic colitis in mice. (A) Illustration of experimental plan of TNBS-mediated chronic colitis with LV vector protocol. Mice were injected with increasing doses of TNBS solution intracolonically once a week for 6 weeks as described in Methods. Some mice received intravenous injection of mouse cathelicidin Camp-HA-expressing lentivirus or HA-expressing lentivirus (control), starting from the fifth TNBS injection. Colonic changes were evaluated 1 week after the last TNBS injection. (B) Colonic Camp mRNA expression was statistically significantly increased in the Camp-LV group (P = .026 and P = .0413). (C) Representative H&E images of colonic tissues. (D) Histology score based on H&E staining images of colonic tissues. TNBS treatment in mice led to increased tissue damage with a statistically significantly higher histology score (P = .001) compared with the ethanol control. Treatment of Camp-LV statistically significantly reduced the histology score (P = .0034). (E) Percentage of body weight change of different groups (from week 0 to week 6). Cathelicidin-expressing LV infection did not alter mice body weight. Per group, n = 6 mice.
Salmonella Infection Model
Wild-type mice in 129Sv/J strain were obtained from Jackson Laboratory (Bar Harbor, ME) and were maintained at the UCLA animal facility under standard environmental conditions. Each mouse was administered 20 mg of streptomycin by oral gavage. The Salmonella typhimurium SL1344 strain was obtained from Dr. Richard Issacson (University of Minnesota) and was cultured in Lysogeny broth (LB broth) for 48 hours. At 24 hours later, the mice were given Salmonella typhimurium SL1344 strain 1 × 108 cfu or LB broth in 100 μL by oral gavage as previously described elsewhere.13 Some groups were injected with Camp-expressing lentivirus (1 × 107 infectious units/mouse) or HA lentivirus intravenously once starting on day 11, as illustrated in Figure 8A. Cecal tissues were obtained on day 21 after carbon dioxide euthanasia.
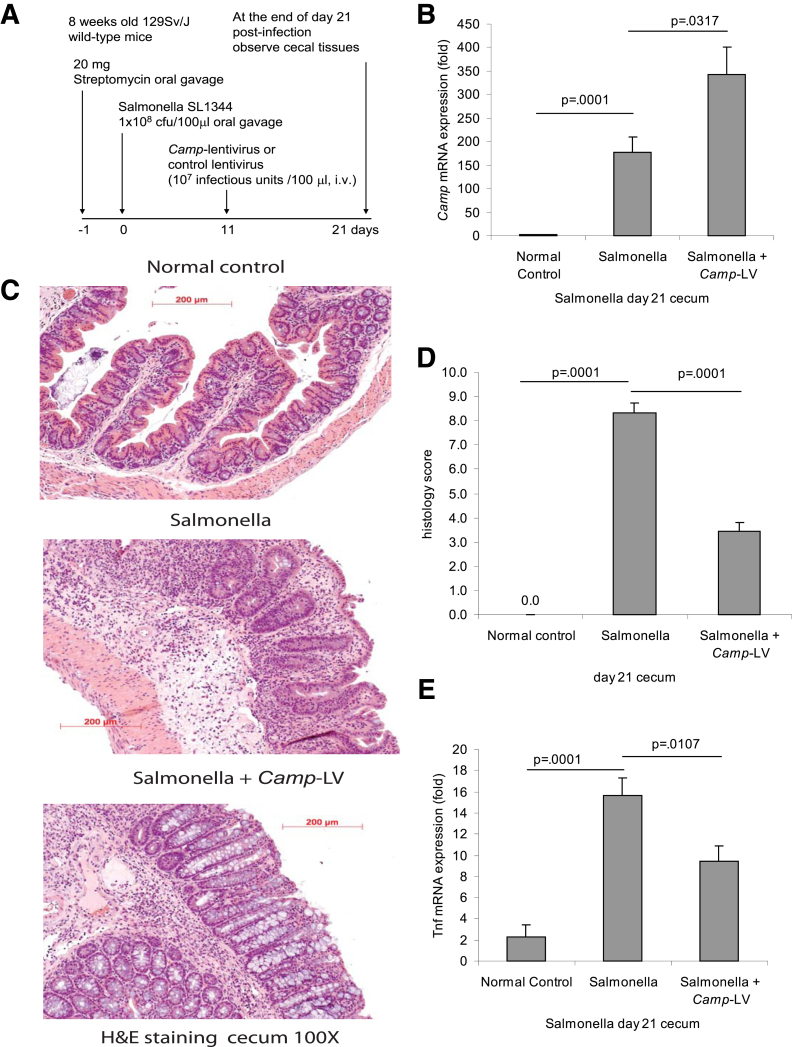
Figure 8.
Lentiviral cathelicidin expression reduces colonic inflammation in Salmonella-induced cecal inflammation in mice. (A) Illustration of the experimental plan of Salmonella-mediated cecal inflammation with LV vector protocol. Mice were fed with streptomycin to reduce the microflora load in the intestine and facilitate infection, and 24 hours later, the mice were inoculated with Salmonella. Some mice received intravenous injection of mouse cathelicidin Camp-HA-expressing lentivirus or control lentivirus on day 11. Cecal changes were evaluated on day 21. (B) Cecal Camp mRNA expression was statistically significantly increased in the Salmonella-infected group and was further augmented in the Camp-LV group (P = .0001 and P = .0317). (C) Representative H&E images of colonic tissues. (D) Histology score was based on H&E staining images of colonic tissues. TNBS treatment in mice led to increased tissue damage with a statistically significantly higher histology score (P = .0001) compared with the ethanol control. Treatment of Camp-LV statistically significantly reduced histology score (P = .0001). (E) Salmonella infection statistically significantly induced cecal Tnfα mRNA (P = .0001) expression. Camp-lentivirus treatment significantly reduced Salmonella induced Tnfα mRNA (P = .0107) expression. Per group, n = 6.
Virus Production and Transduction
The generation of cathelicidin-expressing lentivirus was performed by UCLA vector core facility. Lentivirus-based vectors encoding CRAMP were generated by transient cotransfection of human embryonic kidney 293T (HEK293T) cells with a three-plasmid combination, as described previously elsewhere, with slight modifications.17 Briefly, 8 × T175 flasks of nonconfluent HEK293T cells were cotransfected with 39 μg pMDLg/pRRE, 21 μg of pMDG (encoding the VSV-G envelope), 15 μg of pRSV-REV, and 60 μg pRRL-CMV-CRAMP-Ires green fluorescent protein (GFP) by the CaPi-DNA coprecipitation method.18, 19 The plasmid vectors were kindly provided by Dr. Luigi Naldini (University of Torino, Italy). The next day, the medium was adjusted to make a final concentration of 10 mM sodium butyrate, and the cells were incubated for 5 hours to obtain high-titer virus production as previously described elsewhere.20 After the 5-hour incubation, the cells were washed and incubated in fresh medium without sodium butyrate. Conditioned medium was harvested 16 hours later and passed through 0.45-mm filters. The harvested supernatant was transferred into a centrifuge tube and layered on top of a 10-mL 20% (w/v) sucrose cushion. The tubes were centrifuged using a SW28 rotor (Beckman Coulter, Brea, CA) for 2 hours at 22,000 rpm at 4°C. The virus-containing pellet was resuspended by gentle agitation in an appropriate volume of Dulbecco’s phosphate-buffered saline, followed by a short centrifugation step (5 minutes at 16,000g) to pellet debris. Finally, the opaque pellet was transferred to a new tube. The biological titer of the LVs carrying the enhanced green fluorescent protein (eGFP) reporter was analyzed by transduction of HEK293T cells followed by flow cytometry and infectious particles/mL were calculated as previously described elsewhere.21
Histologic Scoring
Murine colonic tissues were fixed, sectioned, and stained with H&E. Microphotographs at 200× magnification were recorded at multiple locations and analyzed by two investigators in a blinded manner, scoring the specimens on a 0–12 scale for chronic TNBS colitis as previously described elsewhere.22 The fibrosis score for both TNBS and Salmonella models was determined as previously described elsewhere.23 In brief, several mucosal and submucosal locations for each Masson trichrome–stained slide were evaluated on a 0–3 scale (0 = no fibrosis, 1 = mild fibrosis, 2 = moderate fibrosis, and 3 = severe fibrosis).
Mouse TNF-α, Mouse and Human TGF-β1 Enzyme-Linked Immunosorbent Assay
The levels of mouse TNF-α (DY410), mouse TGF-β1 (DY1679) and human TGF-β1 (DY240) of R&D Systems (Minneapolis, MN) were measured by the enzyme-linked immunosorbent assay kits according to manufacturer’s instructions.
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was isolated with the RNeasy kit (Qiagen, Valencia, CA) and reversed transcribed into cDNA by a Superscript III kit (no. 11752; Invitrogen). Quantitative reverse-transcription polymerase chain reaction (RT-PCR) reactions were run with an ABI Prism 7500 Fast sequence detector system (Applied Biosystems, Foster City, CA) as we have previously described elsewhere.15 The levels of mRNA were determined by using cataloged primers (Invitrogen) for human COL1A2 (Hs01028956_m1), CAMP (Hs00189038_m1), mouse vimentin (Mm01333430_m1), 18S (Hs99999901_s1) and mouse Col1a2 (Mm01309565_m1), Camp (Mm00438285_m1), Tgf-β1 (Mm01178820_m1), Tnfα (Mm00443259_g1), and Gapdh (Mm99999915_g1). Results were expressed as relative fold difference.
Masson Trichrome Staining
Staining of collagen deposition of colonic tissue sections was performed by Masson Trichrome staining kit (HT-10; Sigma-Aldrich, St. Louis, MO) and Bouin solution (HT-10132) as previously described elsewhere.15
Immunofluorescence Staining
Frozen mouse colonic tissues were embedded in optimum cutting temperature solution and fixed in acetone. After incubation with blocking buffer, slides were incubated with primary antibodies including rabbit polyclonal anticollagen sc-28655, rabbit polyclonal anti-PCNA (anti-proliferating cell nuclear antigen) sc-7907, or goat polyclonal antivimentin sc-7557 (1:50 dilution) for 2 hours at room temperature, washed with phosphate-buffered saline and incubated with fluorescent-conjugated secondary antibodies, including donkey anti-rabbit Texas Red sc-2784 and bovine anti-goat FITC sc-2348, for 1 hour (1:100 dilution). Slides were then rinsed and mounted with 4,6-diamidino-2-phenylindole (DAPI) mounting solution. Images were analyzed with a AX10 confocal microscope (Carl Zeiss, Thornwood, NY).
Immunohistochemistry
Colon tissues were fixed in 4% paraformaldehyde and embedded in paraffin. After incubation with blocking buffer, the sections were incubated with a goat polyclonal antivimentin antibody (sc-7557, 1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), a rabbit polyclonal anti-F4/80 antibody (sc-26643-R, 1:50 dilution; Santa Cruz Biotechnology), or a rabbit polyclonal anti-phospho-ERK antibody (no. 4370, 1:50 dilution; Cell Signaling Technologies, Danvers, MA) overnight at 4°C. After washing, the sections were incubated with donkey anti-goat IgG or bovine anti-rabbit IgG, and the slides were stained with an ABC kit for color development (sc-2018; Santa Cruz Biotechnology). Immunohistochemistry was assisted by Translational Pathology Core Laboratory (TPCL) of UCLA. Images were analyzed with a Zeiss AX10 microscope at magnification of 200×.
Western Blot Analyses
Cells were lysed in radioimmunoprecipitation assay buffer with proteinase inhibitor cocktail (Cell Signaling Technologies). Equal amounts of cell extracts were fractionated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and proteins were transferred onto nitrocellulose membranes (400 mA for 1.5 hours at 4°C; Bio-Rad Laboratories, Hercules, CA). Membranes were blocked in 5% nonfat milk in TBST (50 mM Tris, pH 7.5, 0.15M NaCl, 0.05% Tween 20), and then were incubated with antibodies against phospho-extracellular signal-regulated kinase (phospho-ERK) (no. 4370), β-tubulin (no. 2146; Cell Signaling Technologies), phospho-epidermal growth factor receptor (phospho-EGFR) (no. sc12351; Santa Cruz Biotechnology), pro-COL1A1 (no. SP1D8; Development Studies Hybridoma Bank, Iowa City, IA). Horseradish peroxidase-labeled antibodies were detected by chemiluminescence (Fisher Scientific, Pittsburgh, PA), and the signals were captured using the luminescent image analyzer LAS4000 (Fujifilm, Tokyo, Japan).
Statistical Analyses
Results were expressed as mean ± standard error of the mean (SEM) and were analyzed by using the Prism professional statistics software program (GraphPad, San Diego, CA). Unpaired Student t tests were used for intergroup comparisons. P values of statistically significant differences are shown in each figure.
Results
Cathelicidin Reduces Chronic, TNBS-Induced Colitis in Mice
We have previously shown that cathelicidins have anti-inflammatory effects against DSS-induced colitis and C difficile infection in mice.11, 12 To investigate the role of cathelicidin in chronic colitis, mice were administered a low dose of TNBS intracolonically every week as previously described elsewhere.14, 15 The experimental design of these experiments is illustrated in Figure 1A. After 7 weeks, the body weight of the TNBS-treated mice was statistically significantly lower (P = .045) compared with that of ethanol-treated (control) mice (Figure 1B). Intracolonic administration of the mCRAMP peptide to TNBS-exposed mice restored their body weight loss to levels similar to those of the control mice (P = .0178, Figure 1B). Moreover, chronic TNBS administration induced colonic tissue damage with statistically significantly higher (P = .001) histologic scores compared with the damage induced in control mice (Figure 1C and D). Intracolonic mCRAMP administration statistically significantly reduced the colonic histology score (P = .03) in TNBS-treated mice (Figures 1D).
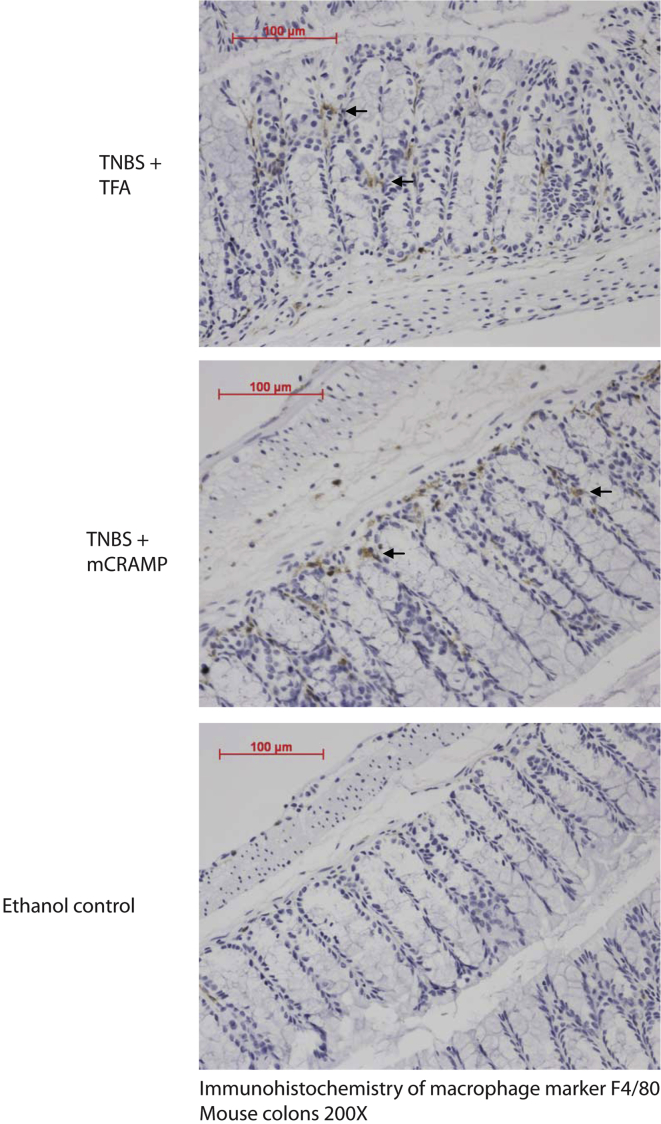
TNBS caused a statistically significant increase of the colonic proinflammatory cytokine tumor necrosis factor α (Tnfα) mRNA levels (P = .0344; Figure 1E), which were statistically significantly reduced by mCRAMP administration (P = .046, Figure 1E). Intracolonic TNBS also increased colonic Tgf-β1 mRNA expression; intracolonic administration of cathelicidin did not alter this response (Figure 1F). However, intracolonic mCRAMP did not change the increased colonic F4/80-positive macrophage infiltration in TNBS-treated mice (Figure 2). Thus, intracolonic administration of cathelicidin reduced chronic experimental colitis in an IBD mouse model, consistent with our results in the C difficile infection model of colitis.12
Figure 2.
Cathelicidin administration does not affect macrophage infiltration in colonic tissues: immunohistochemistry of macrophage marker F4/80 in colonic tissues. Macrophages are identified by brown staining (arrows). TNBS-treated mice had increased F4/80 immunostaining compared with the ethanol control group. Intracolonic mCRAMP treatment did not affect F4/80-expressing macrophage infiltration in TNBS-treated mice.
Cathelicidin Reduces Chronic Colitis-Associated Collagen Expression
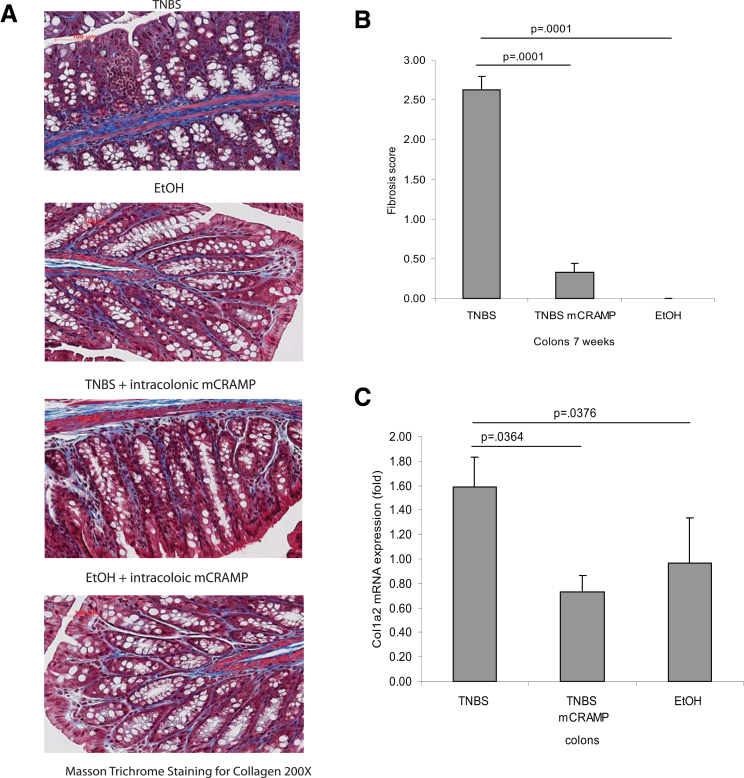
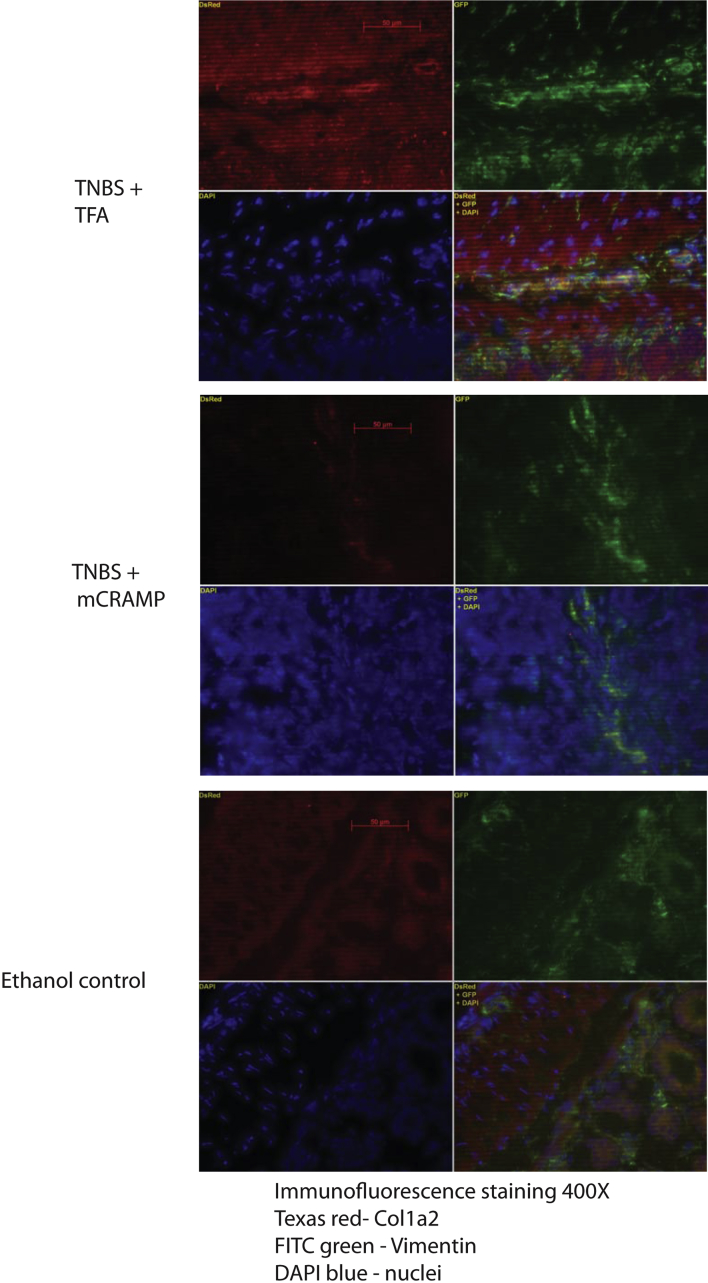
Intestinal fibrosis with stricture formation is an important complication in CD patients.3 We next evaluated the effect of intracolonic cathelicidin in TNBS colitis-associated colonic fibrosis with Masson trichrome staining for collagen formation. We found increased collagen deposition in the colon of TNBS-treated mice, both in the mucosa and submucosa, compared with control mice (Figure 3A), which was in line with previous observations.14, 15 Increased fibrosis was also reflected by an increased fibrosis score (Figure 3B). Moreover, compared with ethanol treatment, chronic TNBS administration increased the collagen Col1a2 mRNA colonic expression (P = .0376, Figure 3C) that was statistically significantly reduced by intracolonic mCRAMP administration (P = .0364, Figure 3C), suggesting antifibrogenic effects. Double immunofluorescence staining showed increased colonic collagen protein expression in and around vimentin-expressing fibroblasts in TNBS-treated mice (Figure 4). Colocalization of collagen and vimentin indicates that fibroblasts are main sources of collagen expression. Intracolonic mCRAMP peptide administration also reduced collagen expression in and around colonic fibroblasts of TNBS-treated mice (Figure 4).
Figure 3.
Cathelicidin peptide administration reduces colonic collagen deposition in mice with chronic TNBS colitis. (A) Masson Trichrome staining for collagen in colonic tissues. Collagen was stained in blue with a red background. Collagen deposited in the mucosal and submucosal layer of TNBS-treated mice was reduced by mCRAMP peptide treatment. (B) Fibrosis score of colonic tissues. The elevated fibrosis score of TNBS-treated mice (P = .0001) was statistically significantly reduced by mCRAMP peptide treatment (P = .0001). (C) Quantitative real-time RT-PCR of collagen col1a2 mRNA expression in colonic tissues of mice. TNBS treatment statistically significantly induced colonic col1a2 mRNA (P = .0376) expression. Intracolonic treatment of mCRAMP reduced colonic col1a2 mRNA expression (P = .0364). Per group, n = 6 mice.
Figure 4.
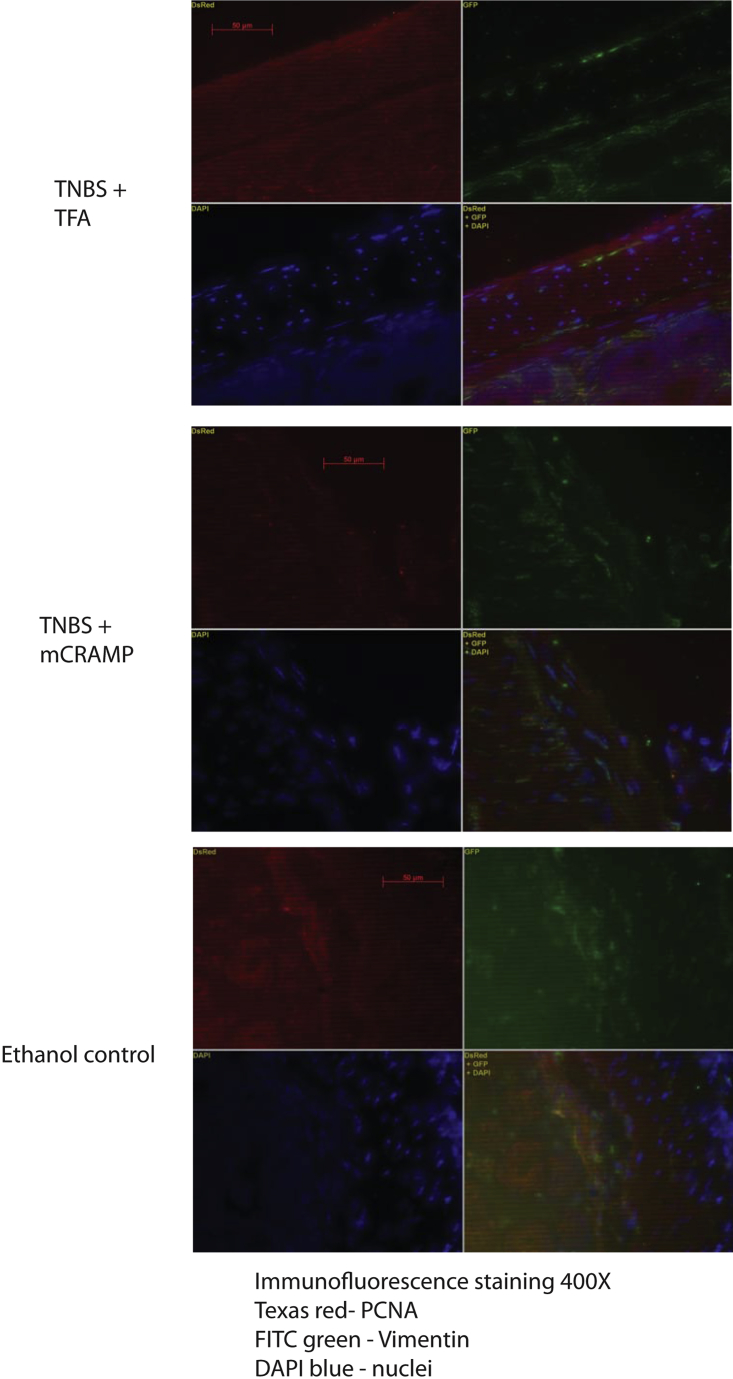
Cathelicidin reduces collagen expression in colonic fibroblasts: immunofluorescence staining of collagen (red), fibroblast marker vimentin (green), and nuclei DAPI (blue). In TNBS + TFA, intense red collagen staining was observed in and around fibroblasts that were identified by green color. Overlapping images of red collagen staining and green vimentin staining resulted in a yellowish color (lower right corner), indicating colocalization of collagen expression in fibroblasts. The TNBS + mCRAMP treatment group and the ethanol group had a low level of collagen staining in and around the vimentin green staining, indicating low collagen expression in fibroblasts.
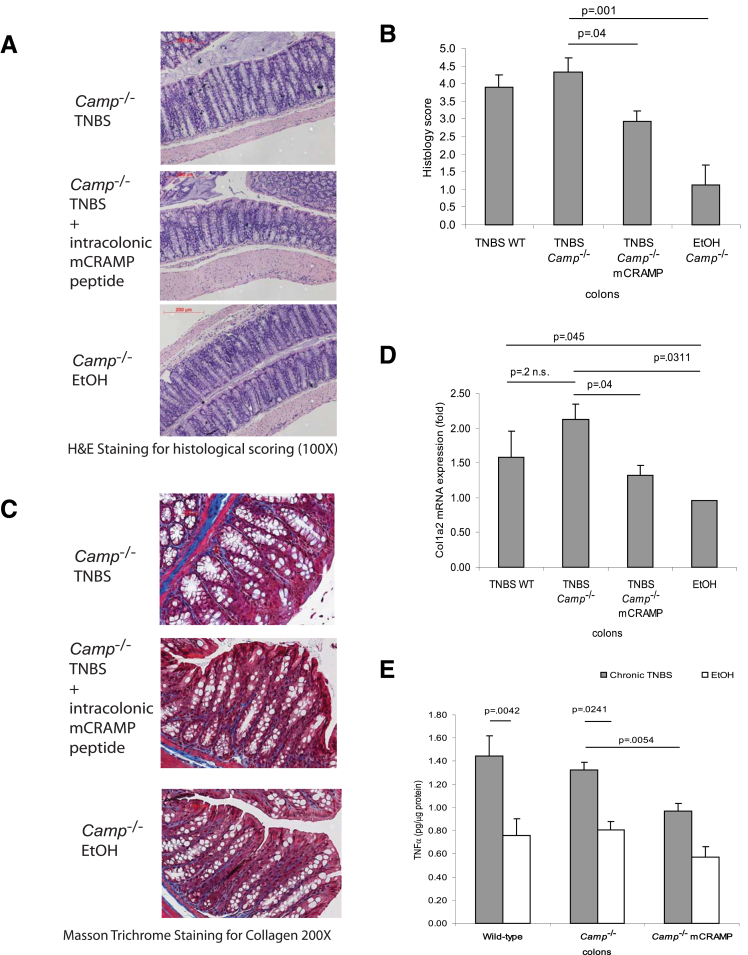
On the other hand, cathelicidin-deficient mice showed similar colonic histologic damage (Figure 5A and B), collagen expression (Figure 5C and D), or TNFα protein levels (Figure 5E) as the wild-type mice in chronic TNBS colitis. Our data also indicate that expression of endogenous colonic cathelicidin in wild-type mice was not induced during chronic TNBS colitis (Figure 6B). However, intracolonic cathelicidin mCRAMP administration reduced colonic tissue damage (Figure 5A and B) and exerted antifibrogenic effects in TNBS-treated Camp−/− mice (Figure 5C and D).
Figure 5.
Intracolonic cathelicidin peptide administration reduces colonic collagen deposition in cathelicidin-deficient mice with chronic TNBS colitis. (A) Representative H&E images of colonic tissues. (B) Histology score based on H&E staining images of colonic tissues. TNBS treatment in Camp−/− mice led to increased tissue damage with a statistically significantly higher histology score (P = .001) compared with the ethanol control. Peptide treatment of mCRAMP statistically significantly reduced the histology score in Camp−/− mice (P = .04). (C) Masson Trichrome staining for collagen in colonic tissues. Collagen was stained in blue. Collagen deposited in mucosal and submucosal layer of TNBS treated Camp−/− mice was reduced by mCRAMP peptide treatment. (D) Quantitative real-time RT-PCR of collagen col1a2 mRNA expression in colonic tissues of mice. TNBS treatment statistically significantly induced colonic col1a2 mRNA (P = .0311) expression. Intracolonic treatment of mCRAMP reduced colonic col1a2 mRNA expression in Camp−/− (P = .04) mice. (E) TNBS treatment statistically significantly induced colonic Tnfα protein (P = .0042 in wild-type; P = .0241 in Camp−/− mice) expression. Intracolonic mCRAMP administration statistically significantly reduced TNBS-induced Tnfα protein expression in Camp−/− (P = .0054) mice. Per group, n = 6 mice.
Lentiviral Expression of Cathelicidin Reduces Colitis-Associated Intestinal Fibrosis
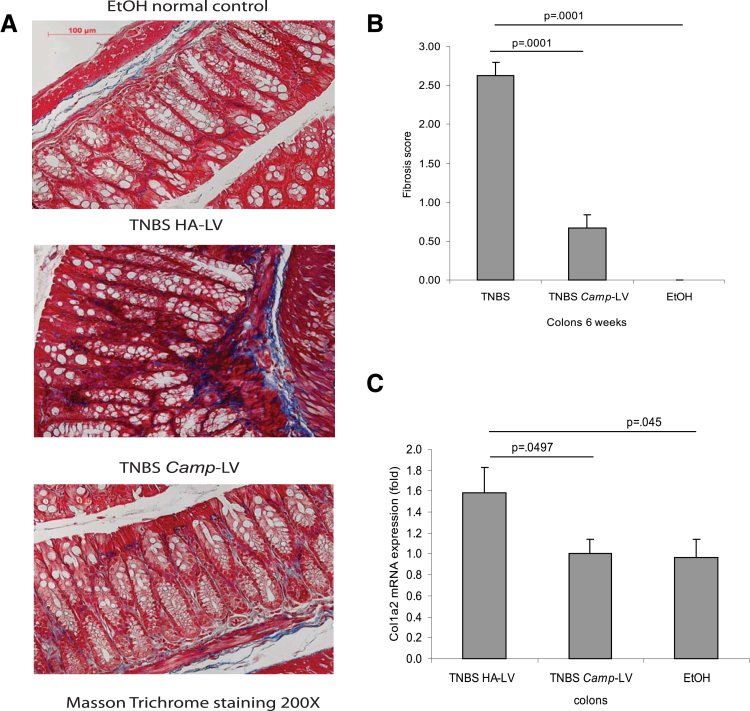
To further examine the effects of cathelicidin in colitis-associated fibrosis, we injected intravenously cathelicidin-overexpressing lentiviruses and determined their effects in the chronic TNBS-associated model of colitis and colonic fibrosis using the experimental approach shown in Figure 6A. We found that intravenous administration of cathelicidin-expressing lentiviruses increased colonic cathelicidin mRNA levels compared with the injection of control lentiviruses (Figure 6B). Moreover, injection of cathelicidin-overexpressing lentiviruses significantly reduced the histologic damage and histology scores in response to chronic TNBS administration (Figure 6C and D) without affecting the body weight changes in response to TNBS (Figure 6E). Lentiviral overexpression of cathelicidin also inhibited TNBS-induced collagen deposition in the colon, as reflected by a statistically significant reduction in the colonic fibrosis score (P = .0001, Figure 7A and B) and reduced colonic collagen Col1a2 mRNA expression (P = .0497, Figure 7C).
Figure 7.
Lentiviral cathelicidin expression reduces colonic collagen deposition in mice with chronic TNBS colitis. (A) Masson Trichrome staining for collagen in colonic tissues. Collagen deposited in the mucosal and submucosal layer of TNBS-treated mice was reduced by Camp-expressing LV treatment. (B) Fibrosis score of colonic tissues. The elevated fibrosis score of TNBS-treated mice was statistically significantly reduced by Camp-expressing lentivirus treatment (P = .0001). (C) Colonic col1a2 mRNA expression was statistically significantly reduced in Camp-lentivirus group (P = .0497). Per group, n = 6 mice.
Lentiviral Expression of Cathelicidin Reduces Cecal Fibrosis during Salmonella Infection
Salmonella induces intestinal inflammation and fibrosis in a TH1 and TH17-dependent manner.13 Thus, we next examined whether injection of LV-overexpressing cathelicidin has any effect on Salmonella-induced fibrosis using the experimental design shown in Figure 8A. Interestingly, Salmonella infection by itself resulted in increased mRNA levels of cecal cathelicidin (P = .0001; Figure 8B), which was further increased by injection of cathelicidin-expressing lentiviruses in the inflamed cecum (P = .0317, Figure 8B). As shown by H&E staining, Salmonella caused severe cecal inflammation and tissue damage that was reduced by lentiviral cathelicidin expression (Figure 8C and D). Cathelicidin overexpressing lentiviruses also statistically significantly reduced Salmonella-induced proinflammatory cytokine Tnfα mRNA expression (P = .0107) (Figure 8E). No change in body weight was observed in any of the Salmonella-infected groups (Figure 9A).
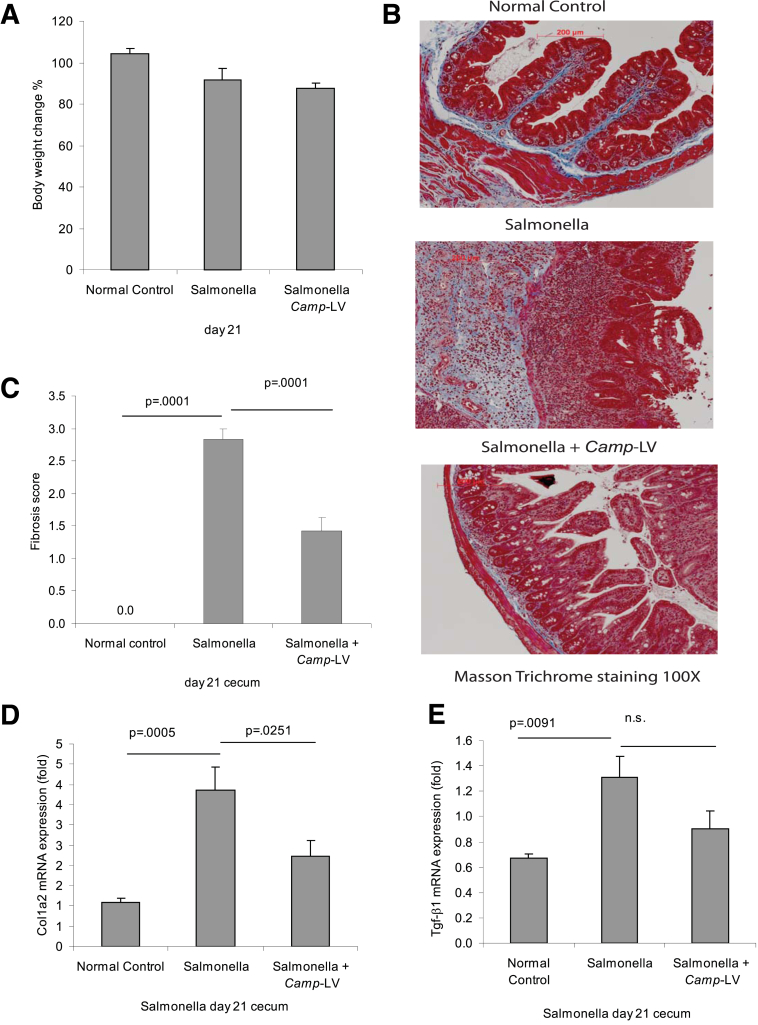
Figure 9.
Lentiviral cathelicidin expression reduces colonic collagen deposition in mice with Salmonella-induced cecal fibrosis. (A) Percentage of body weight change of different groups (from day 0 to day 21). Salmonella- or cathelicidin-expressing LV infection did not alter the body weight of mice. (B) Masson Trichrome staining for collagen in colonic tissues. Collagen deposited in mucosal and submucosal layers of Salmonella-infected mice was reduced by Camp-expressing lentivirus treatment. (C) Fibrosis score of colonic tissues. The elevated fibrosis score of Salmonella-infected mice was statistically significantly reduced by Camp-expressing LV treatment (P = .0001). (D) Colonic col1a2 mRNA expression was statistically significantly reduced in the Camp-lentivirus group (P = .0251). (E) Cecal Tgf-β1 mRNA expression in mice with Salmonella infection. Salmonella infection increased the cecal Tgf-β1 mRNA expression (P = .0091), but the increase was not affected by Camp-lentivirus. Per group, n = 6 mice.
Masson trichrome staining indicated strong cecal collagen deposition in Salmonella-infected mice, which was diminished by LV cathelicidin overexpression (Figure 9B), reflected by a statistically significant reduction in the fibrosis score (P = .0001, Figure 9C) and cecal collagen mRNA expression (P = .0251, Figure 9D). Overexpression of cathelicidin in Salmonella-infected mice did not statistically significantly affect cecal Tgf-β1 mRNA expression (Figure 9E).
Cathelicidin Inhibits Collagen Expression Induced by TGF-β1 and IGF-1 in Human Intestinal Fibroblasts
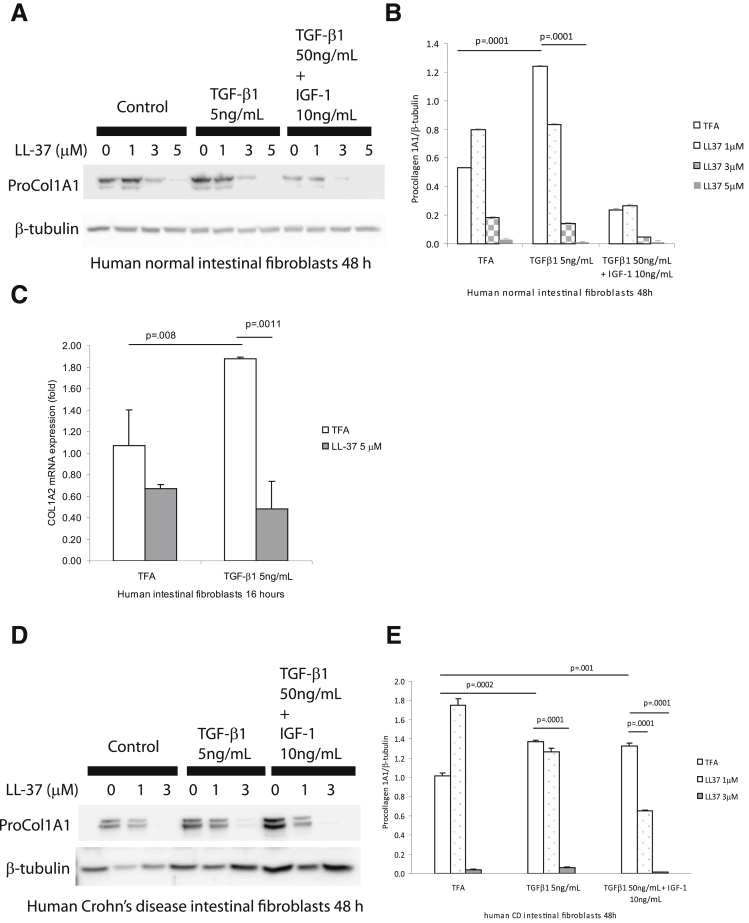
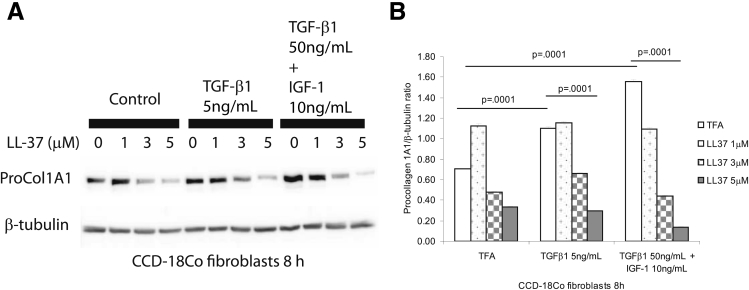
In the mucosa of CD strictures, TGF-β1 and IGF-1 stimulate collagen expression in intestinal fibroblasts, leading to fibrosis.3 Previous studies indicated that TGF-β1 stimulates collagen synthesis in lung and dermal fibroblasts.24 To study the antifibrogenic mechanism of cathelicidin, we coincubated human primary normal intestinal fibroblasts with TGF-β1 (5 ng/mL) together with human cathelicidin (LL-37, 5 μM). TGF-β1 alone stimulated procollagen 1A1 protein expression (P = .0001) in human primary normal intestinal fibroblasts after 48 hours (Figure 10A and B). Coincubation with human cathelicidin statistically significantly reduced TGF-β1-stimulated (P = .0001) procollagen 1A1 protein expression (Figure 10A and B). Similarly, LL-37 statistically significantly inhibited TGF-β1-stimulated COL1A2 mRNA expression in human primary intestinal fibroblasts (Figure 10C) and also reduced procollagen 1A1 protein expression induced by TGF-β1 in human primary intestinal fibroblasts isolated from CD patients (Figure 10D and E). Moreover, in cultured human colonic CCD-18Co fibroblasts, cathelicidin inhibited TGF-β1- and/or IGF-1-induced collagen protein synthesis (Figure 11).
Figure 10.
Cathelicidin reduces collagen expression in human primary intestinal fibroblasts. (A and B) Human primary intestinal fibroblasts isolated from normal subjects were treated with TGF-β1 (50 ng/mL) and IGF-1 (10 ng/mL) or TGF-β1 (5 ng/mL) alone with or without LL-37 for 48 hours. Western blot analyses showed that TGF-β1 (P = .0001) alone induced pro-COL1A1 protein expression. LL-37 (5 μM) statistically significantly suppressed collagen expression in the presence of TGF-β1 alone (P = .0001). (C) Serum-starved human primary normal intestinal fibroblasts were exposed to TFA, LL-37 (5 μM), and/or TGF-β1 (5 ng/mL) for 16 hours. LL-37 statistically significantly inhibited collagen mRNA expression induced by TGF-β1. (D and E) Human primary intestinal fibroblasts from a CD patient were treated with TGF-β1 (50 ng/mL) and IGF-1 (10 ng/mL) together or TGF-β1 (5 ng/mL) alone with or without LL-37 (0–3 μM) for 48 hours. Western blot analyses showed that both TGF-β1 (50 ng/mL) and IGF-1 (10 ng/mL) together and TGF-β1 (5 ng/mL) alone induced pro-COL1A1 protein expression. LL-37 (3 μM) statistically significantly suppressed collagen expression in the presence of TGF-β1 and/or IGF-1 (P = .0001). The results are representative of three healthy individuals and two CD patients.
Figure 11.
Cathelicidin reduces collagen expression in human CCD-18Co colonic fibroblasts. (A and B) Human colonic CCD-18Co fibroblasts were incubated with TGF-β1 (50 ng/mL) and IGF-1 (10 ng/mL) or TGF-β1 (5 ng/mL) alone with or without LL-37 for 8 hours. Western blot analyses showed that TGF-β1 and IGF-1 together (P = .0001) and TGF-β1 (P = .0001) alone induced pro-COL1A1 protein expression. LL-37 (5 μM) statistically significantly suppressed collagen expression in the presence of TGF-β1 and IGF-1 together (P = .0001) or TGF-β1 alone (P = .0001). Results are representative of three independent experiments.
Cathelicidin Reduces Collagen Expression in Human Colonic Fibroblasts via ERK Activation
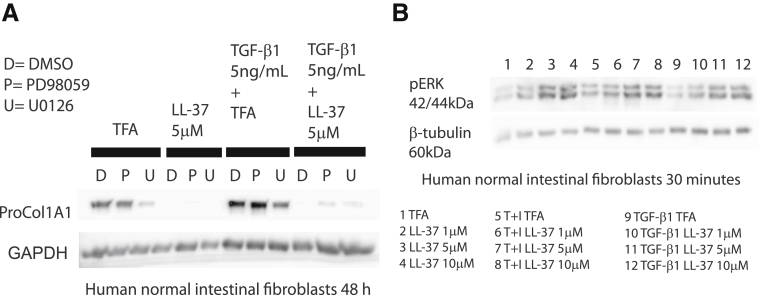
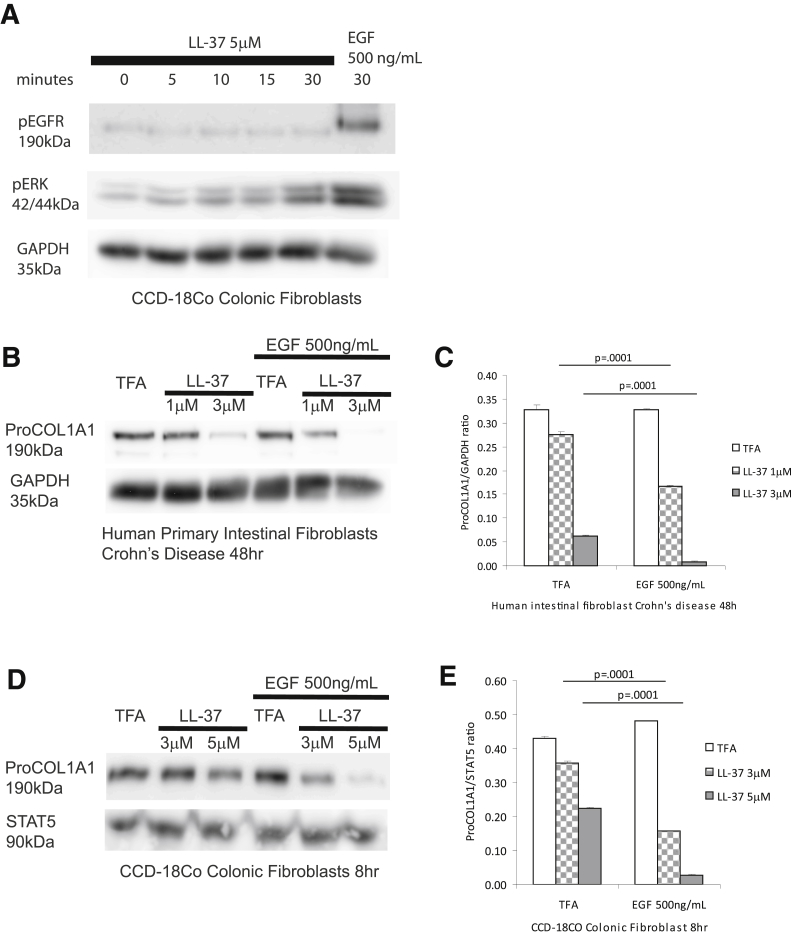
ERK activation has been shown to be involved in the antifibrogenic effects of cathelicidin in dermal fibroblasts.9 We hypothesized that ERK may represent a pathway modulating the antifibrogenic effects of cathelicidin in primary intestinal fibroblasts. We found that LL-37-induced inhibition of TGF-β1-mediated collagen protein expression in human primary normal intestinal fibroblasts was partially reversed by pretreatment of cells with the ERK inhibitors PD98059 and U0126 (Figure 12A). Moreover, cathelicidin induced ERK phosphorylation in a concentration-dependent manner regardless of the presence of TGF-β1 or IGF-1 in human normal primary intestinal fibroblasts (Figure 12B). Consistent with these finding, cathelicidin-induced ERK phosphorylation (addition of EGF serving as a positive control), but not EGFR phosphorylation in human CCD-18Co colonic fibroblasts (Figure 13A). Addition of EGF also augmented the antifibrogenic effects of cathelicidin in both human primary intestinal fibroblasts obtained from CD patients and human CCD-18Co colonic fibroblasts (Figure 13B–E). These results suggest that cathelicidin inhibits fibrogenesis via an ERK-dependent pathway in native human colonic fibroblasts.
Figure 12.
Antifibrogenic effect of cathelicidin is ERK dependent. (A) Human primary normal intestinal fibroblasts were pretreated with dimethyl sulfoxide, MEK1 inhibitor PD98059 (10 μM), or MEK1/2 inhibitor U0126 (10 μM) for 30 minutes, followed by LL-37 (5 μM) or TFA 0.1% and/or TGF-β1 (5 ng/mL) exposure for 48 hours. Pro-COL1A1 and GAPDH protein expression was detected by Western blot. Blockade of ERK partially reversed the antifibrogenic effects of cathelicidin. (B) Human primary normal intestinal fibroblasts were exposed to LL-37 (1–10 μM) or TFA 0.1% and/or TGF-β1 (50 ng/mL) and IGF-1 (10 ng/mL) together or TGF-β1 (5 ng/mL) alone for 30 minutes. Cathelicidin activated ERK phosphorylation in human fibroblasts.
Figure 13.
Epidermal growth factor–mediated MAP kinase activation promotes the antifibrogenic effects of cathelicidin. (A) Serum-starved CCD-18Co fibroblasts were exposed to 5 μM of LL-37 (0–30 minutes) or EGF (500 ng/mL, 30 minutes). Protein lysate was used for Western blot analyses. LL-37 induced the phosphorylation of ERK but not EGFR. EGF (as a positive control) induced both ERK and EGFR phosphorylation. (B) Serum-starved human primary intestinal fibroblasts were exposed to TFA, LL-37 (1–3 μM), or EGF (500 ng/mL) for 48 hours. Collagen protein expression was determined by Western blot analysis. (C) Quantitative analysis of the Western blot. EGF statistically significantly promoted cathelicidin-mediated inhibition of collagen expression in fibroblasts (LL-37 1 μM, P = .0001; LL-37 3 μM, P = .0001). (D) Serum-starved CCD-18Co colonic fibroblasts were exposed to TFA, LL-37 (3–5 μM), or EGF (500 ng/mL) for 8 hours. Collagen protein expression was determined by Western blot analysis. (E) Quantitative analysis of the Western blot. EGF statistically significantly promoted cathelicidin-mediated inhibition of collagen expression in fibroblasts (LL-37 3 μM, P = .0001; LL-37 5 μM, P = .0001). The results are representative of two independent experiments.
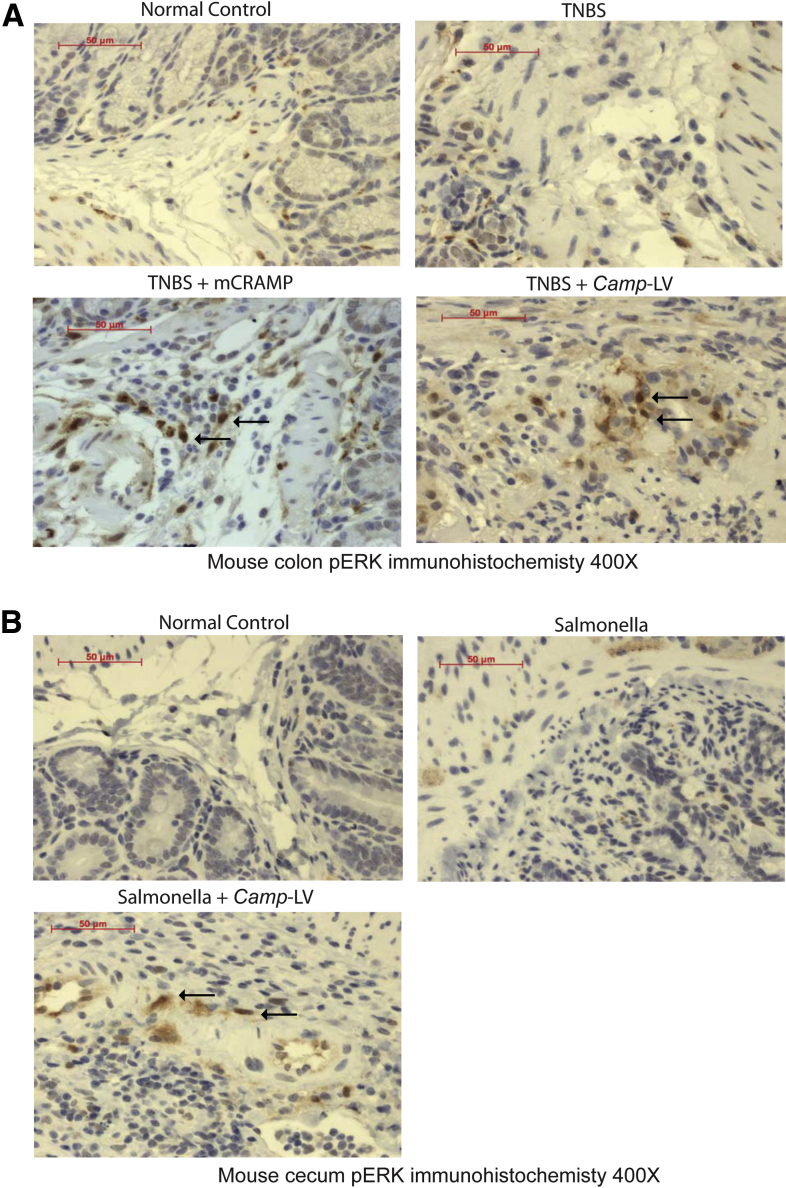
To confirm cathelicidin mediated ERK activation in vivo, we determined ERK phosphorylation in submucosal tissues of colons and cecum where most fibroblasts are populated. We found the submucosal tissues of normal, TNBS-treated, or Salmonella-infected mice had a low expression of phosphorylated ERK (Figure 14A and B). Both intracolonic mCRAMP peptide administration and intravenous Camp-expressing lentivirus administration activated colonic or cecal ERK phosphorylation in the TNBS or Salmonella model of intestinal fibrosis (Figure 14A and B).
Figure 14.
Cathelicidin activates ERK phosphorylation in submucosal tissue in vivo. (A) Immunohistochemistry of phospho-ERK in mouse colons. Both normal control and TNBS-treated groups had low phospho-ERK signal in submucosal tissue. TNBS-treated mice with intracolonic mCRAMP peptide administration or intravenous Camp-expressing lentivirus administration had more phospho-ERK signal. (B) Immunohistochemistry of phospho-ERK in mouse cecum. Both normal control and Salmonella-treated groups had a low phospho-ERK signal in submucosal tissue. Salmonella-treated mice with intravenous Camp-expressing LV administration had more phospho-ERK signal. Per group, n = 6 mice.
Discussion
We and others have previously shown that the antimicrobial peptide cathelicidin possesses potent anti-inflammatory activities against acute intestinal inflammation.10, 11, 12 Consistent with these findings, our results using the models of chronic TNBS colitis and Salmonella infection and two different approaches—intracolonic administration of cathelicidin peptide and intravenous injection of cathelicidin-expressing lentiviruses—demonstrate that cathelicidin exerts significant anti-inflammatory effects, including reduction of mucosal Tnfα protein or mRNA expression (Figures 1E, 5E, and 8E). Both approaches also lead to substantial improvement of histologic evidence of colitis in both animal models (Figures 1D, 5B, 6D, and 8D). Taken together with previous findings,10, 11, 12 our results demonstrate a role for exogenous administration of cathelicidin against both acute and chronic IBD-related colitis as well as infectious colitis of different etiologies. Most importantly, we report here that cathelicidin inhibits colitis-associated colonic fibrosis in chronic TNBS and Salmonella infection models in vivo and in human intestinal fibroblasts in vitro, suggesting that exogenous cathelicidin may represent an effective approach in inhibiting colitis-associated fibrosis.
The role of antimicrobial peptides or proteins, including cathelicidins, in colitis-associated intestinal fibrosis or stricture formation has never been reported. We report here that cathelicidin inhibits colitis-associated colonic fibrosis in vivo and substantially reduces TGF-β1- and/or IGF-1-mediated collagen protein synthesis in human intestinal fibroblasts isolated from three normal subjects and two CD patients (Figure 10A–E) as well as in human colonic CCD-18Co fibroblasts (Figure 11).
In rats, systemic blockade of putative cathelicidin receptor P2X7 by A740003 [N-(1-([(cyanoimino)(5-quinolinylamino) methyl] amino)-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide] and brilliant blue G prevented TNBS-induced colitis25 while A438079 [3-[[5-(2,3-dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine], another P2X7 inhibitor, reduced mouse liver fibrosis.26 Another putative cathelicidin receptor formyl peptide receptor-like 1 (FPRL1) was found to mediate LL-37-induced collagen production in human lung fibroblasts.27 However, the antifibrogenic effect of LL-37 in human intestinal fibroblasts was not altered by pretreatment with the FPRL1 antagonist WRW4 or the P2X7 antagonist KN62 (data not shown), suggesting that the antifibrogenic effects of cathelicidin do not depend on these two putative cathelicidin receptors.
This antifibrogenic effect likely involves an ERK-dependent mechanism as it can be blocked by the mitogen-activated protein kinase 1 (MEK1) inhibitor PD98059 and the MEK1/2 inhibitor U0126 (Figure 12A). Cathelicidin induces ERK phosphorylation in human normal intestinal fibroblasts and human CCD-18Co colonic fibroblasts (Figures 12B and 13A). Specifically, the effects of cathelicidin in collagen synthesis appear to be mediated by MEK1, as the MEK1 inhibitor PD98059 was sufficient to reverse LL-37-mediated suppression of TGF-β1-induced collagen synthesis (Figure 12A). These results confirm that the antifibrogenic effects of cathelicidin in intestinal fibroblasts are ERK-dependent and are consistent with a previous report demonstrating that the antifibrogenic mechanism of cathelicidin in dermal fibroblasts involves ERK activation.9
Cathelicidin did not affect protein expression of the fibrogenic TGF-β1 in CCD-18Co colonic fibroblasts, even in the presence of a proinflammatory cytokine cocktail (TNFα, IL-1β, and interferon-γ) (Figure 15A and B). Moreover, intracolonic cathelicidin administration or injection of cathelicidin-overexpressing lentiviruses did not affect intestinal TGF-β1 mRNA expression in both TNBS and Salmonella fibrosis mouse models (Figures 1F and 9E). These results suggest that the antifibrogenic effects of cathelicidin are independent of the classic TGF-β1-mediated profibrogenic events.
Figure 15.
Cathelicidin does not affect TGF-β1 expression in fibroblasts. (A) CCD-18Co fibroblasts were treated with proinflammatory cytokine cocktail with or without LL-37 for 48 hours. TGF-β1 protein secretion from CCD-18Co was not affected by cathelicidin. (B) TGF-β1 protein remaining in the cell lysate was not affected by cathelicidin. The results are representative of three independent experiments.
On the other hand, cathelicidin treatment did not induce fibroblast cell proliferation in vitro, and did not affect colonic or cecal expression of the fibroblast marker vimentin expression (data not shown) and proliferating cell nuclear antigen staining (Supplementary Figure 1) in our in vivo models. We did not observe colonic apoptosis (terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling assay) after intracolonic or intravenous cathelicidin administration (data not shown), suggesting that cathelicidin is unlikely to cause tissue damage in the intestine.
Despite the positive antifibrogenic effects of cathelicidin administration, TNBS-treated cathelicidin-deficient mice did not show additional colonic fibrosis or tissue damage compared with TNBS-treated wild-type mice (Figure 5). The lack of increased endogenous cathelicidin expression (Figure 6B) may be related to low levels of inflammation seen in this chronic TNBS-induced model as reflected by low colonic TNFα protein level (Figure 5E). Colonic TNFα levels were similar among TNBS treated wild-type and Camp−/− mice (Figure 5E). Although colonic cathelicidin mRNA expression is increased in acute DSS-induced colitis,11 intestinal cathelicidin mRNA expression is not altered in CD patients.28
Several studies have underlined the importance of the bacterial composition of the intestinal flora in IBD pathophysiology and epithelial barrier integrity. Along these lines, the ability of cathelicidin to alter the gut microflora may be associated with the antifibrogenic effects reported here.10, 11 Because the microflora composition associated with intestinal fibrosis has not been identified in animal or human colon, future studies are necessary to explore this possibility.
In summary, our report provides novel evidence for an antifibrogenic effect of cathelicidin in vivo and in vitro. Our results strongly suggest that cathelicidin administration may be a novel approach to prevent or treat IBD, and IBD-related colonic fibrosis.
Acknowledgments
The cathelicidin-expressing lentivirus was produced by the UCLA Vector Core, supported by National Institutes of Health National Cancer Institute JCCC/P30 [Grant CA016042] and National Institute of Diabetes and Digestive and Kidney Diseases CURE/P30 [Grant DK041301].
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by a Pilot and Feasibility Study grant from the UCLA-CURE Center, Crohn’s and Colitis Foundation of America Research Fellowship [Grant 1406], Career Development Award [Grant 2691], the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant K01 DK084256] (to H.W.K.), a Crohn’s and Colitis Foundation of America student research fellowship [Grants 287244, 3831, and 324000] (to M.C., S.H., and D.H.-Y.T.) [DK50984] (to C.F.) Support was also provided by the Blinder Research Foundation for Crohn’s Disease, the Eli and Edythe Broad Chair (to C.P.), and the United States Public Health Service [Grant DK072471] (to C.P.).
Supplementary Material
Supplementary Figure 1.
Cathelicidin did not affect cell proliferation in colonic tissues. Immunofluorescence staining of cell proliferation marker PCNA (red), fibroblast marker vimentin (green) and nuclei DAPI (blue). In TNBS+TFA, weak red PCNA staining was observed in and around fibroblasts that were identified by green color. Overlapping images of red PCNA staining, green vimentin staining and blue nuclear staining are shown in lower right corner. All groups had low level of PCNA staining in and around vimentin green staining, indicating low cell proliferation in colonic fibroblasts.
References
- 1.Fiocchi C. Genes and ‘in-vironment’: how will our concepts on the pathophysiology of inflammatory bowel disease develop in the future? Dig Dis. 2012;30(Suppl 3):2–11. doi: 10.1159/000342585. [DOI] [PubMed] [Google Scholar]
- 2.Wettergren A., Christiansen J. Risk of recurrence and reoperation after resection for ileocolic Crohn’s disease. Scand J Gastroenterol. 1991;26:1319–1322. doi: 10.3109/00365529108998629. [DOI] [PubMed] [Google Scholar]
- 3.Spinelli A., Correale C., Szabo H., Montorsi M. Intestinal fibrosis in Crohn’s disease: medical treatment or surgery? Curr Drug Targets. 2009;11:242–248. doi: 10.2174/138945010790309984. [DOI] [PubMed] [Google Scholar]
- 4.Sorrentino D., Avellini C., Beltrami C.A. Selective effect of infliximab on the inflammatory component of a colonic stricture in Crohn’s disease. Int J Colorectal Dis. 2006;21:276–281. doi: 10.1007/s00384-005-0739-0. [DOI] [PubMed] [Google Scholar]
- 5.Ho S., Pothoulakis C., Koon H.W. Antimicrobial peptides and colitis. Curr Pharm Des. 2013;19:40–47. doi: 10.2174/13816128130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman P., Johansson L., Wan H. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect Immun. 2006;74:6982–6991. doi: 10.1128/IAI.01043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koczulla R., von Degenfeld G., Kupatt C. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaykhiev R., Beisswenger C., Kandler K. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L842–848. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 9.Park H.J., Cho D.H., Kim H.J. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843–850. doi: 10.1038/jid.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tai E.K., Wu W.K., Wong H.P. A new role for cathelicidin in ulcerative colitis in mice. Exp Biol Med (Maywood) 2007;232:799–808. [PubMed] [Google Scholar]
- 11.Koon H.W., Shih D.Q., Chen J. Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology. 2011;141:1852–1863. doi: 10.1053/j.gastro.2011.06.079. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hing T.C., Ho S., Shih D.Q. The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut. 2013;62:1295–1305. doi: 10.1136/gutjnl-2012-302180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassl G.A., Valdez Y., Bergstrom K.S. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134:768–780. doi: 10.1053/j.gastro.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Lawrance I.C., Wu F., Leite A.Z. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Koon H.W., Shih D., Karagiannides I. Substance P modulates colitis-associated fibrosis. Am J Pathol. 2010;177:2300–2309. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strong S.A., Pizarro T.T., Klein J.S. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology. 1998;114:1244–1256. doi: 10.1016/s0016-5085(98)70431-7. [DOI] [PubMed] [Google Scholar]
- 17.Naldini L., Blomer U., Gage F.H. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakoda T., Kaibuchi K., Kishi K. smg/rap1/Krev-1 p21s inhibit the signal pathway to the c-fos promoter/enhancer from c-Ki-ras p21 but not from c-raf-1 kinase in NIH3T3 cells. Oncogene. 1992;7:1705–1711. [PubMed] [Google Scholar]
- 20.Sakoda T., Kasahara N., Hamamori Y., Kedes L. A high-titer lentiviral production system mediates efficient transduction of differentiated cells including beating cardiac myocytes. J Mol Cell Cardiol. 1999;31:2037–2047. doi: 10.1006/jmcc.1999.1035. [DOI] [PubMed] [Google Scholar]
- 21.Pfeifer A., Hofmann A. Lentiviral transgenesis. Methods Mol Biol. 2009;530:391–405. doi: 10.1007/978-1-59745-471-1_21. [DOI] [PubMed] [Google Scholar]
- 22.Kruschewski M., Foitzik T., Perez-Canto A. Changes of colonic mucosal microcirculation and histology in two colitis models: an experimental study using intravital microscopy and a new histological scoring system. Dig Dis Sci. 2001;46:2336–2343. doi: 10.1023/a:1012334727509. [DOI] [PubMed] [Google Scholar]
- 23.Johnson L.A., Luke A., Sauder K. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “top-down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis. 2011;18:460–471. doi: 10.1002/ibd.21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mia M.M., Boersema M., Bank R.A. Interleukin-1β attenuates myofibroblast formation and extracellular matrix production in dermal and lung fibroblasts exposed to transforming growth factor-β1. PLoS One. 2014;9:e91559. doi: 10.1371/journal.pone.0091559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marques C.C., Castelo-Branco M.T., Pacheco R.G. Prophylactic systemic P2X7 receptor blockade prevents experimental colitis. Biochim Biophys Acta. 2013;1842:65–78. doi: 10.1016/j.bbadis.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Huang C., Yu W., Cui H. P2X7 blockade attenuates mouse liver fibrosis. Mol Med Rep. 2013;9:57–62. doi: 10.3892/mmr.2013.1807. [DOI] [PubMed] [Google Scholar]
- 27.Sun C., Zhu M., Yang Z. LL-37 secreted by epithelium promotes fibroblast collagen production: a potential mechanism of small airway remodeling in chronic obstructive pulmonary disease. Lab Invest. 2014;94:991–1002. doi: 10.1038/labinvest.2014.86. [DOI] [PubMed] [Google Scholar]
- 28.Schauber J., Rieger D., Weiler F. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006;18:615–621. doi: 10.1097/00042737-200606000-00007. [DOI] [PubMed] [Google Scholar]