Abstract
Background:
A majority of the studies done on the western population have shown that Pseudomonas aeruginosa causes many severe infections in patients with bronchiectasis as compared to other pathogens. There is scarcity of similar data from the Asian population.
Materials and Methods:
A prospective study was undertaken to identify the various pathogens isolated from the respiratory samples of 117 patients with bronchiectasis from south India and to compare the clinicomicrobiological profile of infections caused by P. aeruginosa and other respiratory pathogens.
Results:
The respiratory pathogens were isolated from 63 (53.8%) patients. P. aeruginosa was the most common isolate (46.0%) followed by Klebsiella pneumoniae (14.3%) and other pathogenic bacteria. Patients included in the P. aeruginosa group had a higher number of exacerbations (p: 0.008), greater number of hospital admissions (p: 0.007), a prolonged hospital stay (p: 0.03), and poor lung function, compared to the patients infected with the non-Pseudomonas group.
Conclusion:
It is necessary to investigate the etiology of respiratory tract infections among bronchiectasis patients followed by the prompt management of cases diagnosed with P. aeruginosa infections, so as to lower the morbidity and have a better prognosis.
Keywords: Bronchiectasis, Morbidity, Pseudomonas aeruginosa
INTRODUCTION
Bronchiectasis is a persistent or progressive condition characterized by dilated, thick-walled bronchi.[1] It is accompanied by chronic productive cough, airway obstruction, and recurrent infections.[2] A vicious cycle of transmural recurrent infection and subsequent inflammation causes damage primarily to the bronchi and bronchioles. The damaged airways are susceptible to infection usually with colonizing, but severely damaging bacterial and fungal microbes.[3] The symptoms of bronchiectasis vary from intermittent episodes of expectoration to persistent daily expectoration often of large volumes of purulent sputum and may be associated with other non-specific respiratory symptoms including dyspnea, chest pain, and hemoptysis, and may progress to respiratory failure and corpulmonale.[1]
The main bacterial pathogens that are commonly isolated in bronchiectasis are Haemophilus influenzae and Pseudomonas aeruginosa. Other microorganisms encountered include Streptococcus pneumoniae, Haemophilus parainfluenzae, Staphylococcus aureus, Moraxella catarrhalis, nontuberculous mycobacteria, and Aspergillus spp.[4] About one-third of the patients with bronchiectasis are chronically colonized with P. aeruginosa. Patients with P. aeruginosa experience an accelerated decline in lung function and more frequent exacerbations. Patients with no pathogens isolated from their sputum have the mildest disease.[5] It is commonly recommended that microbiological identification of the pathogen and characterization of its antimicrobial susceptibility pattern would aid in decisions regarding antibiotic therapy, should it become needed.[4] Considering that infections with P. aeruginosa have been associated with higher morbidity compared with other bacterial infections, and also the paucity of microbiological studies on bronchiectasis patients in the Asian population, a prospective study was performed to analyze the clinicomicrobiological profile in patients with bronchiectasis.
MATERIALS AND METHODS
A prospective study was conducted for a period of one year from January to December 2012. This study was approved by our Institutional Ethics Committee. Following informed consent at enrollment, patients clinically diagnosed with acute exacerbation of bronchiectasis were included. Those patients with prior antibiotic therapy in the previous two weeks were excluded from the study. An infective exacerbation was defined as a change in one or more of the common symptoms of bronchiectasis (increasing sputum volume or purulence, worsening dyspnea, increased cough, declining lung function, increased fatigue/malaise) or the appearance of new symptoms (fever, pleurisy, hemoptysis, requirement for antibiotic treatment).[1] Lower respiratory tract specimens including sputum and bronchoalveolar lavage fluid were collected at the time of an infective exacerbation, prior to the commencement of antibiotic treatment. The specimens were transported within two hours after collection to the Microbiology Laboratory for further processing. The quality of specimens was evaluated based on gram-stain findings, followed by culture and susceptibility testing. All sputum gram stains were read under an oil immersion objective (x100) and evaluated according to the Bartlett criteria. The specimens were scored 0, +1, or +2 according to the number of leukocytes seen per field and 0, -1, and -2 according to the number of squamous epithelial cells seen per field. Specimens with total scores of zero or less were considered inadequate and heavily contaminated with oropharyngeal flora. Those containing greater than 25 leucocytes and fewer than 10 squamous epithelial cells per field were optimal specimens and processed further.[6] The bronchoalveolar lavage fluid was processed by a semi-quantitative culture with a positive threshold of 104 CFU/mL.[7] The specimens were cultured on Blood agar, Chocolate agar, and MacConkey agar plates and incubated at 37°C for 18-24 hours. The Blood agar and Chocolate agar plates were incubated in 5% CO2 (capnophilic atmosphere). Identification of the bacterial isolates was done following standard bacteriological techniques.[8] The antibiotic sensitivity of the isolates was determined by the Kirby Bauer's disk diffusion method on Mueller-Hinton agar (BD) following the Clinical Laboratory Standard Institute (CLSI) guidelines.[9] These strains were then checked for extended spectrum beta-lactamase production (ESBL) using the double disk approximation method.[10] For quality control, E. coli ATCC 25922, P. aeruginosa ATCC 27853, and K. pneumoniae ATCC 700603 were used. Duplicate isolates from the same patient were excluded from the analysis.
The isolates were grouped as Pseudomonas and non-Pseudomonas groups for analysis of the demographic characteristics; clinical severity in terms of the number of infective exacerbations in the previous year; high resolution computed tomography findings (HRCT); lung function tests, and antibiotic susceptibility profile. To assess lung function, the spirometry values of forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and FEV1/FVC ratio was obtained for all patients. The HRCT thorax was assessed for the number of lobes involved (the lingula was considered as a separate lobe). Each lobe of both lungs was graded for bronchiectatic changes on a 0-3 scale, giving a maximum of 18 points: 0: No bronchiectasis; 1: one or less than one bronchopulmonary segment involved; 2: more than one bronchopulmonary segment involved; 3: gross cystic bronchiectasis.[11]
RESULTS
A total of 117 patients with bronchiectasis were studied. The mean age of the study group was 52.9 years and the range was 7-86 years. Sixty-six were female (56.4%) and 51 (43.6%) were male. Normal oropharyngeal flora was grown in culture from 54 (46.2%) lower respiratory tract specimens. The respiratory pathogens were isolated from the rest of the 63 (53.8%) patients. P. aeruginosa was the most common isolate (46.0%) followed by K. pneumoniae and other pathogenic bacteria, accounting for the rest of the isolates (54%) [Table 1].
Table 1.
Frequency of isolation of pathogens from Bronchiectasis patients

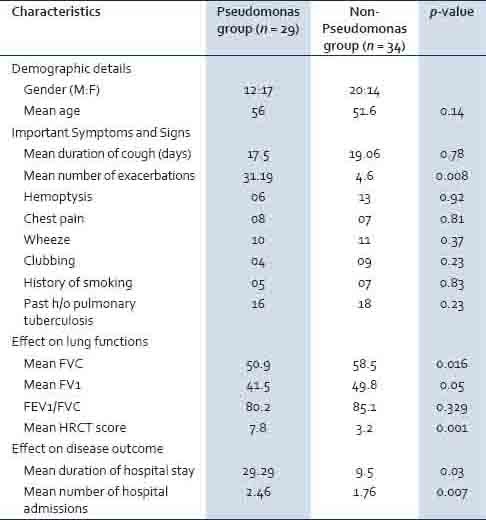
The clinical details of the studied patients [Table 2] showed that cough with expectoration was the presenting symptom in all the patients (100%) followed by hemoptysis in 26.5% of the cases. A past history of pulmonary tuberculosis was present in 34 (29%) patients, whereas, diabetes was noticed in 16 (13.7%) cases. Wheeze and crackles were present in 27.4 and 20.5% of the patients, respectively. Patients infected with P. aeruginosa had a significantly higher number of exacerbations (p: 0.008), greater number of hospital admissions (p: 0.007), a prolonged hospital stay (p: 0.03), poor lung function (p: < 0.05), and increased severity of the disease (p: 0.001) compared to the patients infected with other pathogenic bacteria [Table 3].
Table 2.
Frequency of clinical signs and symptoms among the study group
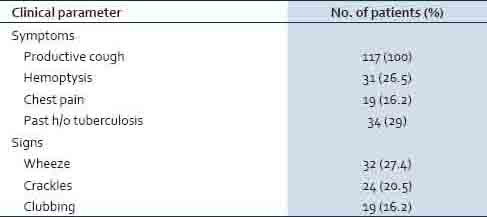
Table 3.
Comparison of demographic factors and the clinical profile of bronchiectasis patients infected with Pseudomonas aeruginosa versus non-Pseudomonas aeruginosa bacteria
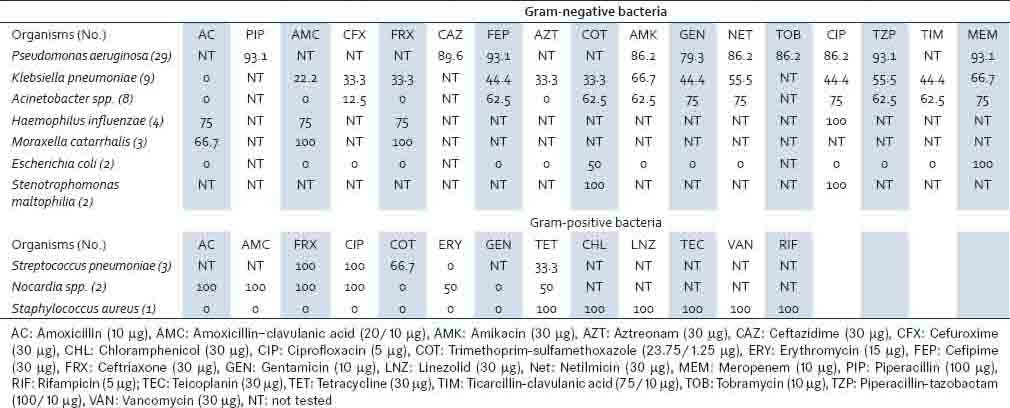
The P. aeruginosa isolates were largely sensitive to most of the antibiotics tested, with 93.1% of them being sensitive to meropenem, piperacillin, and cefepime [Table 4]. Higher rates of drug resistance were noted among the Enterobacteriaceae isolates and Acinetobacter spp. Meropenem was found to be the most effective antibiotic against K. pneumoniae (66.7%). All isolates of K. pneumoniae, E. coli, and Acinetobacter spp. were found to be sensitive to colistin and tigecycline (100%). Among the gram-positive bacterial isolates, S. pneumoniae and Nocardia sp. were the sensitive strains, whereas, the isolate of S. aureus was found to be methicillin-resistant S. aureus.
Table 4.
Antimicrobial susceptibility profile of pathogens from bronchiectasis patients (in percentage)
DISCUSSION
Correlating with the earlier evidence,[12] the present study has also observed a higher incidence of bronchiectasis in females as compared to males. Bronchiectasis often occurs in patients who have systemic diseases or other underlying associated conditions. The two basic pathogenic factors are airway obstruction and bacterial infection in the bronchial tree, leading to bacterial colonization of the bronchial mucosa and subsequent progressive lung damage.[13] A history of previous severe lower respiratory tract infections due to bacterial and viral pneumonia, pertussis or tuberculosis should be sought in all patients with bronchiectasis. Where possible, the temporal relationship of the identified infections with the onset of chronic respiratory symptoms should be determined.[1] Thirty-four (29%) subjects in the present study had a past history of pulmonary tuberculosis.
It has been suggested that all children and adults with bronchiectasis should have an assessment of lower respiratory tract microbiology.[1] Understanding the local spectrum of lower respiratory bacteriology among patients with bronchiectasis will help in choosing the appropriate empirical therapy, pending culture results. P. aeruginosa was the most common isolate (46%) in our study, a finding also noted in other studies.[11,13,14] Other studies have shown H. influenzae to be the most commonly isolated pathogen.[15,16] The differences could be due to the varied distribution of organisms in different geographical locations. Patients infected with P. aeruginosa are known to experience a more accelerated decline in lung function and more frequent exacerbations than those infected with other organisms.[5] A similar observation was made in the present study with patients infected with P. aeruginosa having a higher number of exacerbations (p: 0.008) and a prolonged hospital stay (p: 0.03). Spirometry (FVC and FEV1) also demonstrated a significant difference (p: < 0.05) in the Pseudomonas versus non-pseudomonas group. HRCT is considered to be the best investigation for bronchiectasis patients to determine the involvement of different lobes of the lung with precision. In the present study, HRCT has revealed the increased severity of the disease based on the lobes of the lung involved in patients having P. aeruginosa infection (p: 0.001), as compared to the non-pseudomonas group.
Although the isolates of P. aeruginosa were found to be sensitive to the antibiotics tested, most of them were mucoid strains. Once acquired, P. aeruginosa (especially the mucoid type) is difficult to eradicate from bronchiectasis and cystic fibrosis patients.[14] Prompt eradication treatment at the very onset of infection prior to its transition to a mucoid variant would seem advantageous.[17] Adequate antibiotic therapy, including a combination dosage, modes of delivery, and duration of therapy should be given due consideration. Addition of macrolide antibiotics to the treatment regimen are effective in reducing the number of exacerbations in patients with bronchiectasis, by modulation of the inflammatory response, and their ability to impede biofilm formation.[18]
Even though P. aeruginosa and H. influenzae are the most common bacteria isolated from non-cystic fibrosis bronchiectasis patients, other pathogens including K. pneumoniae, Acinetobacter spp, and S. maltophilia were isolated. These gram-negative bacilli were found to be multidrug-resistant strains. Previous exposure to anti-microbial agents and repeated contact with the healthcare system could lead to colonization and infection with these multidrug-resistant bacteria. In this study, only one isolate of S. aureus was obtained. Persistent isolation of S. aureus should lead to consideration of underlying allergic bronchopulmonary aspergillosis or cystic fibrosis.[1,18]
The study stresses the need to investigate the etiology of respiratory tract infections among bronchiectasis patients and prompt management of cases diagnosed with P. aeruginosa infections so as to lower the morbidity and have a better prognosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65:i1–58. doi: 10.1136/thx.2010.142778. [DOI] [PubMed] [Google Scholar]
- 2.Baydarian M, Walter RN. Bronchiectasis: Introduction, Etiology, and Clinical Features. Dis Mon. 2008;54:516–26. doi: 10.1016/j.disamonth.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Moulton BC, Barker AF. Pathogenesis of Bronchiectasis. Clin Chest Med. 2012;33:211–7. doi: 10.1016/j.ccm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Feldman C. Bronchiectasis: New Approaches to Diagnosis and Management. Clin Chest Med. 2011;32:535–46. doi: 10.1016/j.ccm.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell AE. Bronchiectasis. Chest. 2008;134:815–23. doi: 10.1378/chest.08-0776. [DOI] [PubMed] [Google Scholar]
- 6.Lentino JR, Lucks DA. Nonvalue of Sputum Culture in the Management of Lower Respiratory Tract Infections. J Clin Microbiol. 1987;25:758–62. doi: 10.1128/jcm.25.5.758-762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chastre J, Combes A, Luyt CE. The Invasive (Quantitative) Diagnosis of Ventilator-Associated Pneumonia. Respir Care. 2005;50:797–807. [PubMed] [Google Scholar]
- 8.Collee JG, Miles RS, Watt B. Tests for identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. pp. 131–49. [Google Scholar]
- 9.CLSI document M100-S21. Wayne, Pennsylvania USA: Clinical and Laboratory Standards Institute; 2011. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement. [Google Scholar]
- 10.Tofteland S, Haldorsen B, Dahl KH, Simonsen GS, Steinbakk M, Walsh TR, et al. Effects of phenotype and genotype on methods for detection of extended-spectrum-beta-lactamase-producing clinical isolates of Escherichia coli and Klebsiella pneumoniae in Norway. J Clin Microbiol. 2007;45:199–205. doi: 10.1128/JCM.01319-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson CB, Jones PW, O’Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. Eur Respir J. 1997;10:1754–60. doi: 10.1183/09031936.97.10081754. [DOI] [PubMed] [Google Scholar]
- 12.Morrissey BM, Harper RW. Bronchiectasis: Sex and gender considerations. Clin Chest Med. 2004;25:361–72. doi: 10.1016/j.ccm.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Palwatwichai A, Chaoprasong C, Vattanathum A, Wongsa A, Jatakanon A. Clinical, laboratory findings and microbiologic characterization of bronchiectasis in Thai patients. Respirology. 2002;7:63–6. doi: 10.1046/j.1440-1843.2002.00367.x. [DOI] [PubMed] [Google Scholar]
- 14.Hla SW, Hui KP, Tan WC, Ho B. Genome macro restriction analysis of sequential Pseudomonas aeruginosa isolates from bronchiectasis patients without cystic fibrosis. J Clin Microbiol. 1996;34:575–8. doi: 10.1128/jcm.34.3.575-578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li AM, Sonnappa S, Lex C, Wong E, Zacharasiewicz A, Bush A, et al. Non-CF bronchiectasis: Does knowing the aetiology lead to changes in management? Eur Respir J. 2005;26:8–14. doi: 10.1183/09031936.05.00127704. [DOI] [PubMed] [Google Scholar]
- 16.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respir Med. 2007;101:1633–8. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Jones AM. Eradication therapy for early Pseudomonas aeruginosa infection in CF: Many questions still unanswered. Eur Respir J. 2005;26:373–5. doi: 10.1183/09031936.05.00069705. [DOI] [PubMed] [Google Scholar]
- 18.Amorima A, Gamboab F, Azevedoc P. New advances in the therapy of non-cystic fibrosis bronchiectasis. Rev Port Pneumol. 2013;19:266–75. doi: 10.1016/j.rppneu.2013.03.006. [DOI] [PubMed] [Google Scholar]


