Abstract
Background:
Platelet-rich plasma (PRP) is defined as an autologous concentration of plasma with a greater count of platelets than that of whole blood. Its action depends on the released growth factors from platelets. It has been investigated and used in numerous fields of medicine. Recently, PRP has received growing attention as a potential therapeutic tool for hair loss.
Aims:
To evaluate the efficacy and safety of PRP injections in the scalp of patients with androgenetic alopecia.
Settings and Design:
Prospective cohort study.
Materials and Methods:
20 patients, 18 males and 2 females, with androgenetic alopecia were enrolled in the study. PRP was prepared using a single spin method (Regenlab SA). Upon activation, it was injected in the androgen-related areas of scalp. Three treatment sessions were performed with an interval of 21 days and a booster session at 6 months following the onset of therapy.
Statistical Analysis:
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM, NY, USA).
Results:
Hair loss reduced and at 3 months it reached normal levels. Hair density reached a peak at 3 months (170.70 ± 37.81, P < 0.001). At 6 months and at 1 year, it was significantly increased, 156.25 ± 37.75 (P < 0.001) and 153.70 ± 39.92 (P < 0.001) respectively, comparing to baseline. Patients were satisfied with a mean result rating of 7.1 on a scale of 1-10. No remarkable adverse effects were noted.
Conclusions:
Our data suggest that PRP injections may have a positive therapeutic effect on male and female pattern hair loss without remarkable major side effects. Further studies are needed to confirm its efficacy.
KEYWORDS: Androgenetic alopecia, hair loss, platelet-rich plasma

INTRODUCTION
Androgenetic alopecia (AGA) is a common chronic hair loss disorder. It is characterised by progressive hair loss, affecting both sexes. It affects up to 80% Caucasian men and 40% women. Its frequency increases with age, despite the fact that it may start at puberty.[1,2] Independent of age and gender, patients diagnosed with AGA may undergo significant impairment of quality of life, since hair is considered to be an important feature of self-image.[3] Hair loss affects self-esteem, personal attractiveness and may lead even to depression and other negative effects of life, especially in women.[2,3,4]
A number of products have been proposed as hair loss therapies. Drug therapies specifically approved by Food and Drug Administration (FDA) for treating AGA are limited to minoxidil and finasteride. Both can be used alone or combined.[5] Despite the therapeutic options available, low patient compliance and satisfaction rate as well as the plethora of topical and often important systematic adverse effects lead to the search of new treatment options for AGA.[1,5]
PRP is defined as a volume of the plasma fraction of autologous blood with an above baseline platelet concentration.[6] The regenerative potential of PRP depends on the levels of growth factors released upon activation.[7,8] Main growth factors (GFs) involved in androgenetic alopecia are platelet-derived growth factor (PDGF), transforming growth factor (TGF),vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) with their isoforms.[9,10,11,12] GFs appear to act in the bulge area of the follicle, where they bind to their respective receptors located in stem cells. In the bulge area, primitive stem cells of ectodermal origin are found, giving origin to epidermal cells and sebaceous glands. In matrix, germinative cells of mesenchymal origin are found at the dermal papilla (DP). Interactions between these two kind of cells as well as with binding growth factors (PDGF, TGF-b and VΕGF) activate the proliferative phase of the hair, giving rise to the future follicular unit.[13] Therefore, PRP could serve as a potential treatment of AGA.
Aim of our study was to investigate the clinical efficacy and safety of PRP injections in the scalp of patients with AGA.
MATERIALS AND METHODS
From October 2012 to September 2013, 22 volunteers (20 men, 2 women) enrolled our study. All participants were ≥18, who had not received any topical or systematic treatment for their hair loss during the last 6 months. Patients with present or a history of immunosuppression (malignancy, chemotherapy, steroid therapy), dermatological diseases affecting the scalp, autoimmune disorders, hematologic disorders, platelet dysfunction syndrome and on anticoagulation therapy were excluded from our study. Patients with a tendency for keloids were also excluded. Patients taking aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) discontinued their use 7 days before treatment.
Diagnosis of AGA was made in all patients based on a detailed medical history (any drugs causing hair loss), clinical examination and laboratory tests. Laboratory tests included:
CBC;
Serum iron, serum ferritin, TIBC (Total Iron-Binding capacity);
Folic acid;
T3, T4, TSH, fT3, fT4, anti-TPO;
VDRL and
For women, female hormone profile (DHEAS, testosterone, and rostenedione, prolactin, follicle stimulating and leutinising hormone).
Laboratory tests were assessed in order to exclude other hair loss causes, such as anaemia, poor nutrition, thyroid dysfunction, syphilis or polycystic ovary syndrome.
The stage of alopecia was evaluated according to the Hamilton-Norwood scale for men and Ludwig scale for women.
For the preparation of PRP, RegenKit BCT-3 (Regenlab®) was used. Initially, whole blood was collected from the antecubital vein of the patient (16 ml). The blood was then introduced into two tubes (RegenBCT) and centrifugated for 5 min at 1500 g using the laboratory centrifuge RegenA-PRPCentri. Each tube contained a thixotropic gel composed of a mixture of polymers for plasma separation (elimination of red blood cells) and sodium citrate solution, as an anticoagulant, located above the separator gel. Therefore, after centrifugation, the blood was fractionated with the red blood cells being trapped under the gel and cellular elements settling on the surface of the gel. After removal of 2 ml of upper supernatant plasma (PPP, platelet-poor plasma) from each tube, 3 ml of PRP was yielded, which was resuspended by gentle inversions of the tube. Total amount of yielded PRP was about 6 ml and it was loaded in 1 ml syringes, ready for injections. The activation process included the addition of calcium gluconate in a 1:9 ratio (0.1 ml calcium gluconate per 0.9 ml of PRP). The platelets in whole blood and PRP were microscopically counted and mean platelet counts were 1.9 × 105 and 1.102 × 106 platelets/μl, respectively. The concentration of platelets in PRP was approximately 5.8 times as great as that in whole blood.
Before the PRP applications, all patients were informed about the process and its potent adverse effects and they signed a consent form. They did not have their hair washed two days prior to the treatment. Local anaesthesia was applied in the treatment area and scalp was cleaned using 0.1% octenidine hydrochloride spray. None of the patients received any other treatment for hair loss during PRP treatment.
PRP (0,05-0,1 ml/cm2) was injected, with a 27-G needle, into the androgen-related areas (frontal, parietal, occipital) of the scalp in men and into the problematic areas in women, using BD-Luer LokTM 1 ml syringes. Nappage technique was performed in a depth of 1.5-2.5 mm [Figure 1]. Our protocol proposed three treatment sessions with an interval of 3 weeks. At 6 months from the beginning of the treatment, a booster session was also performed.
Figure 1.

Injecting activated PRP
In our study, we evaluated hair loss, hair density (hair/cm2) and patients’ satisfaction. We also noted any reported adverse effects. The evaluation of results was performed by two independent evaluators, who were not involved in the administration of PRP treatment. Evaluation methods included hair pull test, dermoscopic photomicrographs, macroscopic photographs and a satisfaction questionnaire. All patients were evaluated at six time points: T1, beginning of study; T2, 3 weeks; T3, 6 weeks; T4, 3 months; T5, 6 months; and T6, 1 year.
In order to check the same area at all time points, we used ‘V’ (Kang's point), as proposed by Lee et al.[14] ‘V’ is the point of intersection between the midsagittal line and the coronal line connecting the tips of the tragus. By using a plastic headband and a tapeline, ‘V’ can be measured conveniently because the headband presents the coronal line connecting the roots of the ear vagus, and the tapeline easily shows the midsagittal line [Figure 2]. We measured ‘V’, which is located roughly 1 to 1.3 cm in front of the anterior margin of the headband and record the distance from the headband to the midpoint of the line connecting the lower margins of the eyebrows for reproducibility.
Figure 2.

Defining V point using a headband and a tapeline
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM, NY, USA). The normality of quantitative variables was tests by Kolmogorov-Smirnov test. Hair density was expressed as mean ± standard deviation (SD). Hair density differences between the different time points were assessed by one-way repeated measures ANOVA (rmANOVA); post hoc analysis was performed using the Sidak test. All tests were two tailed and statistical significance was considered for P values less than 0.05.
RESULTS
A total of 22 patients enrolled in the study. Nevertheless, there was a dropout of two patients, who did not complete the therapy protocol and were not included in the statistical analysis. Therefore, 20 patients (18 men, 2 women) were finally included in the study. In Table 1, a summary of patients’ characteristics is presented. The mean age of patients was 34 years (24-72). Among men, according to the Hamilton-Norwood scale, five patients suffered from type II androgenetic alopecia, eight from type III, four from type IV and one from type V alopecia. Both women suffered from I-3 androgenetic alopecia, according to the Ludwig scale. Six patients had previously soughtb medical help for their hair loss and they had been treated with topical minoxidil lotion 5%. During the last 6 months since the onset of PRP therapy, they had not received any medication. None had undergone hair transplantation. Two male patients had been taking NSAIDs due to musculoskeletal problems and they stopped those 8 days before PRP injections.
Table 1.
Patients’ characteristics

In Figure 3, we present the number of hairs taken at the hair pull test. It was performed in a standardised manner by the two evaluators. Despite the fact that it is not an objective evaluation method, it gives a satisfactory general image of hair loss. At T1, the mean number of hairs pulled was eight while at T3 and at T4 reached normal levels, since less than three hairs pulled are considered to constitute normal hair loss. At T5 and at T6, the mean hair loss increased with a number of five and six hairs respectively pulled during hair pull test.
Figure 3.

Number of hairs pulled during hair pull test
Hair density (hair/cm2) significantly increased at T3 (154.80 ± 34.39), at T4 (170.70 ± 37.81) at T5 (156.23 ± 37.75) and at T6 (153.70 ± 39.92) (P < 0.001) compared to the onset of therapy (T1). The highest hair density recorded was at T4 [Table 2, Figure 4]. The % increase rate from baseline (T1) was 0.45%, 8.18%, 19.9%, 9.19% and 7.41% at T2, T3, T4, T5 and at T6, respectively. Regarding failure rate, at T3 and T4, none of the patients presented decreased hair density compared to that of baseline and only one of them presented no change (5%). At T5, one patient (5%) presented a decrease of 1 hair/cm2 in hair density comparing to that of T1, while at T6 30% presented a mean decrease of 2 hair/cm2.
Table 2.
Hair density (mean ± SD) in different time points

Figure 4.

The effect of activated platelet-rich plasma injections on hair density through a 1-year period. Statistical significance is considered for Pvalues less than 0.05
Macroscopic photographs showed an overall improvement in hair density and quality, as laguno-like hair became thicker, normal hair [Figures 5–8].
Figure 5.

Male, 29-year-old, before treatment
Figure 8.

The same patient at three months after treatment
Figure 6.

The same patient at 6 months following the onset of PRP treatment
Figure 7.

Male, 26 years old, before treatment
Patients filled in the patients’ satisfaction questionnaire and reported any adverse effects. They were satisfied with a mean result rating of 7.1 on a linear analogue scale of 1-10 (1 = no result, 10 = best result). 85% of patients reported an improvement in hair quality and thickness flash»), while 65% reported an increase in hair density. At T5, all patients (100%) claimed that they want and/or need the booster session, while, at T6, 75% of needed them.
Regarding PRP›s safety profile, in the satisfaction questionnaire patients reported any adverse effects. During application, 100% of them felt mild pain, despite local anaesthesia. After application, 25% of them felt a mild pain feeling, which subsided after 4 hours, while 60% had scalp sensitivity during first hair wash after treatment injections. None reported any worsened hair shedding, infection or ecchymosis.
DISCUSSION
AGA remains the most common hair disorder without satisfactory treatment. Since androgenetic alopecia is characterised by a shortened anagen phase and miniaturisation of terminal to vellus hair,[5] current therapeutic strategies target cellular proliferation and differentiation during the hair cycle.
Except scalp surgery, which is a surgical treatment option, FDA-approved drug therapies include finasteride and minoxidil.[1,5,15] Minoxidil appears to prolong anagen phase and to promote survival of dermal papilla cells and increase in hair follicle size.[16,17] Finasteride also promotes hair growth of anagen hairs leading to gradual increase in hair diameter and hair elongation[18] and appears to activate anagen hair growth.[19,20]
Presently, there are limited published data regarding PRP's potential effect on hair. Activated PRP seems to promote differentiation of stem cells into hair follicle cells through the upregulation of transcriptional activity of β-catenin. It also induces in vitro proliferation of dermal papilla cells, and increases dermal papilla cell growth by activating ERK signalling. PRP also appears to prolong anagen phase of hair growth cycle through increased expression of FGF-7 and to increase cell survival by inhibiting apoptosis (associated with increased Bcl-2 protein levels as well as activated Akt signalling).[21] It also appears to increase the perifollicular vascular plexus, through the increase of VEGF and PDGF levels, which have an angiogenic potential. Therefore, it constitutes a potent useful tool for androgenetic alopecia treatment.[12]
In our study using non-invasive evaluation methods, such as dermoscopic photomicrographs, [Figures 9 and 10] a significant increase rate in hair density of 19,29% and 9,19% was noted at 3 and 6 months respectively with large variability in results (from no improvement to significant improvement). Hair density followed an upward curve, reached a peak at 3 months, decreased at 6 months but its value was significant higher than that of baseline (T1). At 1 year (T6), hair density continued its downward trend although remaining significantly higher than that of T1. Hair loss after the second treatment session (T3) decreased and at T4 reached normal levels. Nevertheless, at 6 months (T5), number of hairs pulled during hair pull test were increased to five, without, however, reaching the number of eight hairs of T1. Taking these in account, the booster session at 6 months was necessary, in order to maintain and improve the results achieved. Finally, at 1 year (T6), number of hairs pulled increased to six, less than eight (T1).
Figure 9.
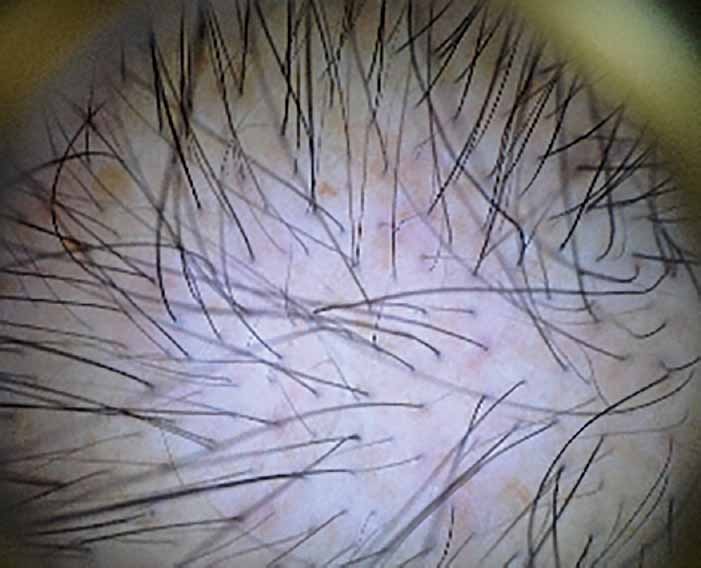
Dermoscopic photomicrographs from a male, 32-year-old, with grade III androgenetic alopecia, before the onset of treatment (x10 magnification)
Figure 10.

Dermoscopic photomicrographs, from the same 32-year-old patient, at 3 months following treatment (x10 magnification)
Patients with grade II-III alopecia according to the Norwood-Hamilton scale had better results compared to patients with more advanced alopecia. Both women were satisfied with the results. Furthermore, patients with vellus hair had better results compared to those who had few but normal hair, as PRP appeared to act on hair diameter causing thin hair to become thicker.
Advantage of our study was that we used a standardised approved PRP preparation kit (Regenkit-BCT-3), since automated kits appear to give better results as mentioned in the literature. Furthermore, we described in detail our protocol of PRP preparation and application, making our results reproducible. Our follow-up, also, was longer (1 year) compared to most other studies.
Nevertheless, our study was not a randomised, double-blind, controlled trial and potent bias might be present. In our case, for instance, most of our patients had vellus hair and were non-smokers. Smoking is mentioned since PRP promotes and improves hair follicle vascularisation. Therefore, smoking as well as other factors affecting microcirculation may play a role in trial results.
Apart from these, hair evaluation methods were not objective. Hair pull test was performed in a standardised manner by the two evaluators, but it remains a subjective evaluation method. Macroscopic photographs showed an overall image of hair growth and hair density. Dermoscopic photomicrographs showed an increase in hair density as number of hairs were counted manually by the evaluators twice and was the most objective method. Phototrichogram, a more objective evaluation method was not performed as it needs to be performed on a shaven part of the patient's scalp which is not accepted by most patients, particularly women. Nevertheless, an adequate means of measuring hair growth over time in a reproducible, economical and non-invasive manner is not available and all the above methods give a relatively fair assessment of the results after treatment.
Finally, sample size was small, despite the fact that we had statistical significant results.
CONCLUSION
In conclusion, PRP injections appeared to be effective in the treatment of androgenetic alopecia in both males and females, without remarkable adverse effects, while they were accompanied by a high patients’ satisfaction rate. Nevertheless, more randomised, controlled, double-blind studies with approved devices for PRP preparation, details in PRP preparation and application, with larger sample size, longer follow-up and objective evaluation methods are needed. The questions are whether PRP will provide as much benefit as existing treatment options, such as minoxidil and whether it could be used as monotherapy or in combination with other treatments. Nevertheless, it seems to be a good choice especially for women of childbearing age, who cannot take oral medication such as finasteride, and for patients with adverse effects and/or no results from finasteride or minoxidil.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.McElwee KJ, Shapiro JS. Promising therapies for treating and/or preventing androgenic alopecia. Skin Therapy Lett. 2012;17:1–4. [PubMed] [Google Scholar]
- 2.Leavitt M. Understanding and management of female pattern alopecia. Facial Plast Surg. 2008;24:414–27. doi: 10.1055/s-0028-1102905. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso M, Richter Appelt H, Tosti A, Viera MS, García M. The psychosocial impact of hair loss among men: A multinational European study. Curr Med Res Opin. 2005;21:1829–36. doi: 10.1185/030079905X61820. [DOI] [PubMed] [Google Scholar]
- 4.Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: Comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29:568–75. doi: 10.1016/0190-9622(93)70223-g. [DOI] [PubMed] [Google Scholar]
- 5.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. European Dermatology Forum (EDF).Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9(Suppl 6):S1–57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 6.Sclafani AP. Application of platelet-rich fibrin matrix in facial plastic surgery. Facial Plast Surg. 2009;25:270–6. doi: 10.1055/s-0029-1242033. [DOI] [PubMed] [Google Scholar]
- 7.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast Reconstr Surg. 2004;114:1502–8. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 8.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-González DJ, Méndez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: Key for regeneration. Int J Pept. 2012;2012:532519. doi: 10.1155/2012/532519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su HY, Hickford JG, The PH, Hill AM, Frampton CM, Bickerstaffe R. Increased vibrissa growth in transgenic mice expressing insulin-like growth factor 1. J Invest Dermatol. 1999;112:245–8. doi: 10.1046/j.1523-1747.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- 11.Tavakkol A, Elder JT, Griffiths CE, Cooper KD, Talwar H, Fisher GJ, et al. Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J Invest Dermatol. 1992;99:343–9. doi: 10.1111/1523-1747.ep12616668. [DOI] [PubMed] [Google Scholar]
- 12.Arshdeep, Kumaran MS. Platelet-rich plasma in dermatology: Boon or a bane? Indian J Dermatol Venereol Leprol. 2014;80:5–14. doi: 10.4103/0378-6323.125467. [DOI] [PubMed] [Google Scholar]
- 13.Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458–67. doi: 10.1097/01.prs.0000239560.29172.33. [DOI] [PubMed] [Google Scholar]
- 14.Lee EH, Kang JS, Kang DS, Han CS, Oh SH, Cho SB. Facilitated scalp measuring using phototrichogram with a headband and tapeline. Dermatol Surg. 2011;37:1150–2. doi: 10.1111/j.1524-4725.2011.02020.x. [DOI] [PubMed] [Google Scholar]
- 15.Jandali S, Low DW. From surgery to pharmacology to gene therapy: The past, present, and future of hair restoration. Ann Plast Surg. 2010;65:437–42. doi: 10.1097/SAP.0b013e3181d59f60. [DOI] [PubMed] [Google Scholar]
- 16.Semalty M, Semalty A, Joshi GP, Rawat MS. Hair growth and rejuvenation: An overview. J Dermatolog Treat. 2011;22:123–32. doi: 10.3109/09546630903578574. [DOI] [PubMed] [Google Scholar]
- 17.Han JH, Kwon OS, Chung JH, Cho KH, Eun HC, Kim KH. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J Dermatol Sci. 2004;34:91–8. doi: 10.1016/j.jdermsci.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Tosti A, Piraccini BM. Finasteride and the hair cycle. J Am Acad Dermatol. 2000;42:848–9. doi: 10.1067/mjd.2000.103272. [DOI] [PubMed] [Google Scholar]
- 19.Sawaya ME, Blume-Peytavi U, Mullins DL, Nusbaum BP, Whiting D, Nicholson DW, et al. Effects of finasteride on apoptosis and regulation of the human hair cycle. J Cutan Med Surg. 2002;6:1–9. doi: 10.1007/s10227-001-0024-y. [DOI] [PubMed] [Google Scholar]
- 20.de Rivero Vaccari JP, Sawaya ME, Brand F, 3rd, Nusbaum BP, Bauman AJ, Bramlett HM, et al. Caspase-1 level is higher in the scalp in androgenetic alopecia. Dermatol Surg. 2012;38:1033–9. doi: 10.1111/j.1524-4725.2012.02378.x. [DOI] [PubMed] [Google Scholar]
- 21.Li ZJ, Choi HI, Choi DK, Sohn KC, Im M, Seo YJ, et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–6. doi: 10.1111/j.1524-4725.2012.02394.x. [DOI] [PubMed] [Google Scholar]


