Abstract
Designer nanoscaled materials have the potential to revolutionize diagnosis and treatment for glioma. This review summarizes current progress in nanoparticle-based therapies for glioma treatment including targeting, drug delivery, gene delivery, and direct tumor ablation. Preclinical and current human clinical trials are discussed. Although progress in the field has been significant over the past decade, many successful strategies demonstrated in the laboratory have yet to be implemented in human clinical trials. Looking forward, we provide examples of combined treatment strategies, which harness the potential for nanoparticles to interact with their biochemical environment, and simultaneously with externally applied photons or magnetic fields. We present our notion of the “ideal” nanoparticle for glioma, a concept that may soon be realized.
Keywords: Drug delivery, glioblastoma multiforme, nanotechnology, nanoparticle, nanomedicine, theranostic
INTRODUCTION
The ability to manipulate atoms, design supramolecular structures, and generate useful function at the nanoscale provides exciting opportunities for the treatment of human disease. Bionanotechnology is specifically devoted to materials possessing sub-100 nm dimensions, and the field possesses an interdisciplinary conceptual breadth that can bring practitioners of quantum physics and neurosurgery into the same discussion. The fabrication of useful architectures, made up of multiple base parts each with their own structural or functional role, is the overarching principle in most modern biomedical applications of nanotechnology.[22,89,158] Discrete molecular forces – including chemical bonding, electrostatics, steric interaction, and physical adsorption – are often harnessed in tandem to generate a 3-dimensional supramolecular layer cake, with an overall function that benefits from each of its chemical constituents.[92,100] Within this nanoscaled assembly, specific materials are included to provide desired properties such as eluding immune recognition, crossing of biological barriers, providing contrast in medical imaging, tumor targeting, releasing a drug, or delivering gene therapy.[19] In the field of neurosurgery, and specifically glioma therapeutics, there is great interest, and much skepticism, in the rapidly developing application of nanoscaled therapeutics.[18,181] Many see nanotechnology as a means to attack glioma at its source−the individual mutated genes, tumor stem cells, or individual cellular metastases that represent barriers to a cure. Materials designed with nanoscopic dimensions are able to signal, home, and induce damage in a coordinated fashion at the subcellular level, permitting an unparalleled degree of control over the targeted action of therapeutics.[135]
Glioma arises within the confines of a variably intact blood–brain barrier (BBB),[101] is surrounded by functional brain tissue that requires preservation, spreads diffusely beyond the gross tumor margin,[55,101] is prone to chemotherapy resistance by efflux and direct drug inactivation,[147] and can be rapidly lethal. The intersection of these obstacles in treatment makes glioma a challenging pathological entity, and at the same time a worthwhile target for investigation using a tailored molecular-scale approach.[12] Even with current optimal treatment, including surgery, chemotherapy, and radiation, high-grade glioblastoma (GBM, WHO grade IV) is associated with an average survival of 12–15 months.[98,160] Progression-free survival is <24 weeks after recurrence,[136,159] and the 5-year survival is <5%.[49,127] As a standard-of-care for GBM, radiation therapy is known to extend survival approximately 2-fold,[48,172] and the drug temozolomide added to radiation increases survival by an additional ~2.5 months.[125,159]
Given the modest treatment benefits of traditional therapy, the investigation of nanostructured drug formulations has intensified and has profited from the significant experience in treatment of non-glioma neoplastic disease.[70,96] The first approval of an antineoplastic nanotherapeutic by the US Food and Drug Administration (FDA) occurred in 1994 for the treatment of acute lymphocytic leukemia (ALL). The drug, Oncaspar, was a poly (ethylene glycol) (PEG)-coated L-asparaginase nanoparticle (NP) that demonstrated increased plasma half-life and decreased immunogenicity compared to native L-asparaginase.[130,142] To date, oncaspar remains in clinical use for ALL. A liposomal preparation of doxorubicin (Doxil) followed in 1995, having been found similarly to increase circulation half-life, and was approved for treatment of Kaposi sarcoma.[50,72] Liposomal formulations of vincristine, daunorubicin, and cytarabine have been approved for clinical use in systemic cancers,[69,144] with liposomal cytarabine (DepoCyt) uniquely indicated for intrathecal administration in lymphomatous meningitis, and currently in phase 1 and 2 trials for central nervous system (CNS) metastases from melanoma and breast cancer.[27,28]
As illustrated by the examples of Oncaspar and DepoCyt, biologically applied nanotechnology has utilized the concepts of polymeric[23] and liposomal NP systems. Drugs have been successfully loaded to the particle surface, and also within the core of both of these NPs.[94] Other biologically relevant nanoscaled structures include solid lipid NPs,[122,134] metal−polymer core-shell NPs,[89] carbon nanotubes,[195] quantum dots,[71] dendrimeric NPs,[7] and virus-based nanocarriers.[150] More complex structures with intriguing names such as nanodiamonds[84,182] and nanoworms[2,83] have exploited the influence of shape on function. In each of these systems, anchoring materials are present and provide a base structure, terminal surface groups allow conjugation of functional biomolecules, nontoxic polymers help to to avoid immunogenicity, degradable materials can provide pH- or enzyme-dependent release, and porous materials can help load or unload useful compounds.[129] The promise of bionanotechnology lies in the vast ability for modification; future technologies can be incorporated into existing multi-component constructs with relative ease. In our opinion, the application of ultrasmall customized therapeutics to glioma treatment will result in many further advances applied both in the OR and in the clinic. Our discussion here details current progress in nanotherapeutics specifically for glioma, and explores relevant advances in the field that may ultimately transform the current notion of poor prognosis.
TUNABLE NANOMATERIALS FOR GLIOMA IMAGING
The ideal nanoscaled imaging agent has the potential to cross the BBB and interact with the tumor microenvironment, providing detail about a specific cellular population of interest. The enhanced permeability and retention (EPR) phenomenon of NP accumulation within tumors was first reported in the 1980s, and nanomaterials were subsequently discovered to traverse the intact BBB in 1995.[96,97] Harnessing the EPR effect required an approximate size constraint of 30–100 nm. Within this regime, NPs would extravasate from poorly differentiated neoplastic vessels.[61,146] The slower diffusion rate of the particle combined with the limited intratumoral lymphatic drainage trapped the particle within the tumor mass instead of allowing it to re-enter the systemic circulation.[110,115,178] The EPR accumulation of NPs within glioma, as well as macrophage uptake of NPs, both facilitate imaging contrast that can persist beyond the time when NP has been eliminated from the bloodstream.[167]
Nanoscaled materials can be modified to provide visualization on conventional imaging modalities. Contrast may derive from a magnetic resonance (MR)- or computed tomography (CT)-visible metal,[9] MR-active nonmetal (e.g.,19F,13C),[57] PET-active radioisotope (e.g.,18F,13N,11C),[124,131] or chromophore/fluorophore-containing biomolecule.[158] Less well-known modalities also benefit from the use of nanomaterials, as in photo-acoustic imaging[174,188] where photon absorption (e.g., near-infrared (NIR) light absorption by gold NPs[103] or carbon nanotubes[40]) produces microscopic temperature fluctuations in the vicinity of the particle and resulting acoustic waves are detected as ultrasound.
For magnetic resonance imaging (MRI), image contrast is conveniently generated by the superparamagnetic property of certain NPs including those made of iron oxide.[157] Superparamagnetism denotes the presence of ultrasmall discrete magnetic domains, which fluctuate continuously at rest, but become poled within an external magnetic field and act as a coherent strong moment.[89,121] While iron oxide NPs historically have been used to generate intravoxel signal dephasing, and thus darken the appearance of T2- and T2*-weighted images, more recent modifications have given iron oxide NPs the ability to accelerate T1 relaxation, and thus provide “bright” contrast on T1-weighted scans. Such modifications include doping the metal core with gadolinium or manganese,[44,68] or manipulation of nonmetallic coating size.[150] Figure 1 shows work from our own group that highlights the appearance of iron oxide NPs in vivo. Gadolinium-enhanced imaging of an orthotopic implanted GBM6 tumor at high magnetic field [Figure 1a] is compared with a T2*-weighted image after PEG-chitosan NP administration, NPs highlight the cerebral microvasculature as well as tumor [Figure 1b]. These NPs can accumulate in high quantities within the tumor yielding contrast as shown in a 3-D reconstruction [Figure 1c]. A photograph of the implanted tumor is provided for comparison [Figure 1d].
Figure 1.
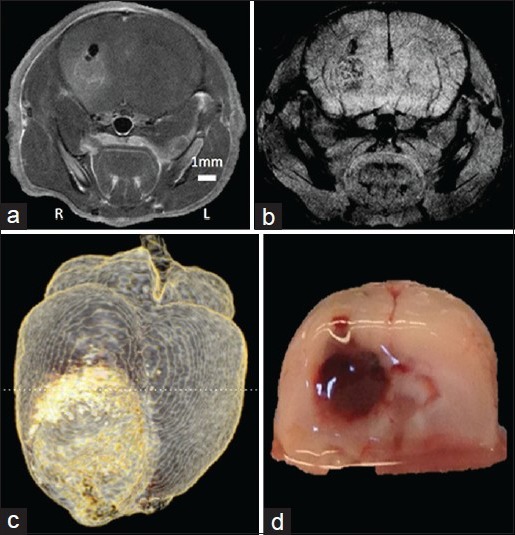
In vivo administration of iron oxide nanoparticles. (a) Gd-enhanced T1-weighted image and (b) iron oxide NP-enhanced T2*-weighted image of mouse glioblastoma tumor (GBM6). (c) 3D reconstruction of a T2-weighted image with inverted contrast after NP injection. (d) Coronal cross-section photograph of the brain for comparison, near the posterior extent of the tumor
Nanomaterials have tunable size, hydrophobicity, and surface charge [Table 1]. These properties can be adjusted to facilitate tumor homing and to avoid rapid elimination. NPs with sizes between 15 and 100 nm are ideal for ensuring long-circulation times in blood. Below a hydrodynamic size of 10–20 nm, particles will be rapidly filtered by the kidneys, and at sizes greater than ~150 nm, particles will be sequestered by the reticuloendothelial system (RES), with uptake into the spleen.[75,178] Although particle uptake by the liver will inevitably occur even within this ideal size range, half-life in circulation can remain long (2–40 h).[126] To optimize plasma half-life, Geng et al. constructed tube-like PEG-poly (caprolactone) micelles with small diameters in cylindrical cross-section (~20 nm), and very large cylindrical length (~18 μm).[54] The group found these structures would persist in the bloodstream for >5 days since they were able to fit lengthwise through tight microvascular spaces and were sufficiently long to mechanically hinder uptake into macrophages.[54]
Table 1.
Fundamental nanomaterial characteristics and their observed impact on tumor localization
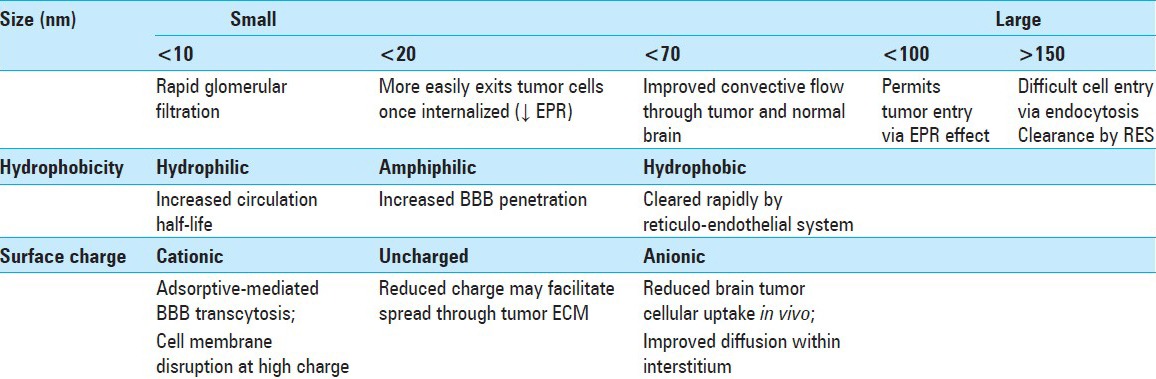
Hydrophobic drugs and surfaces are known to be targets for opsonization, and modifying a nanomaterial surface to be more hydrophilic (e.g., coating with PEG, chitosan, or albumin)[108,171] can increase circulation time. Amphiphilic molecules such as poloxamer 188 or polysorbate 80 have been used to provide the same circulation benefits by presenting a hydrophilic surface, while retaining internal hydrophobic regions that facilitate entry through the brain endothelium.[162,173] Surface charge can be altered by incorporating small molecules or polyelectrolytes. Positively charged surfaces promote BBB penetration by inducing physical adsorption to the endothelium.[107,116] In culture, cationic NPs are readily taken up into the cells at the periphery of tumor spheres, while anionic NPs demonstrate lower intracellular uptake.[90] Curiously, anionic particles show improved distribution throughout the space external to the cells. Zhou and colleagues formulated a poly (L-lysine) NP coated with acid-labile β-carboxylic amide groups, whose charge switches in the presence of acidic tumor microenvironment to exploit the apparent diffusive benefits of negative charge with the permeability benefits of positive charge.[149,184,198]
The potential to maintain a high plasma concentration and interact favorably with the blood−tumor interface make NPs highly useful for glioma imaging. Particles that can generate contrast on two,[171] or even three[92] unique imaging modalities have been constructed, with preferential accumulation seen within the tumor mass over normal brain. A current human clinical trial is in progress, testing the application of a magnetite NP coated with polyglucose sorbitol carboxymethylether (ferumoxytol) compared with standard gadolinium contrast for assessment of BBB permeability changes after combination chemotherapy in glioma.[29] The study seeks to use the NP as a means of calculating tumor blood volume,[26] and to show a benefit of the NP in distinguishing pseudo progression from tumor recurrence. There has yet to be a human clinical trial for imaging using a biochemically targeted NP, although such a trial will certainly have value given the high affinity of targeted particles for glioma and the relatively low toxicity of such materials.[171] Biochemically targeted materials will likely be necessary to provide uptake in areas with minimal EPR effect as vascular permeability may be regionally heterogeneous within the glioma mass.[47] In the following section, we discuss the role of molecular targeting as it pertains to NP drug delivery.
DRUG DELIVERY AND GLIOMA TARGETING
The use of targeted nanomaterials for drug delivery has intensified over the past decade.[128] Recent work in animal glioma models has used targeted NPs to delay tumor growth and improve survival.[62,63,183] A majority of research, to date, has utilized orthotopic tumor models in mice and rats, including implanted human (e.g., U87, T98G, GBM6) and mouse (e.g., C6) cell lines. Transgenic murine models of glioma have also been used,[66] and although these may not recapitulate human tumor biology in the same manner as implanted human cells, they are thought to better mimic the tumor microenvironment, tumor cell–stroma interactions, and invasive behavior.[43] Intracranial murine tumors commonly grow to lethal size by ~2 months, and intervention via NP therapeutics is usually performed ~1 month after implantation, once the tumor has reached intermediate size.[9] Reviewing the body of literature, it is common to see successful studies in rats and mice with a reported median survival increase of ~20 days,[21] with some studies demonstrating long-term remission in a percentage of the treated cohort.[155] Given the large number of successful animal studies over the past decade, and the low reported toxicity of these synthesized materials,[88] we expect to see clinical trials soon appear for targeted particle systems in glioma. Rigorous testing of short- and long-term particle safety will need to be accomplished,[67] and more detailed study of particle bio distribution will be necessary in large animal models.[117,152] Although a great number of laboratories are adept at small-batch NP synthesis, scaling up the synthetic volume and maintaining target molecule attachment under conditions of clinical-grade sterility require time and funding.[21]
Our optimism with regard to the arrival of clinical trials for glioma-targeted nanotherapeutics is galvanized by a number of existing trials for non-CNS pathology. For instance, drug-carrying liposomes targeted to the transferrin receptor are in phase II clinical trials for gastric and esophageal adenocarcinoma,[34] and PEGylated poly(lactic-co-glycolic acid) (PLGA) NPs targeted to glutamate carboxypeptidase II are in phase 2 trials for multiple solid tumors, including prostate cancer.[30] Toward the treatment of recurrent glioblastoma, trials for an intravenous nontargeted nanoliposomal formulation of the topoisomerase 1 inhibitor irinotecan (CPT-11) have been initiated, with a phase 1 trial currently underway for single-agent therapy.[31] This same agent has shown promise when injected intracranially, and a phase 1 trial is enrolling patients for convection-enhanced delivery (CED) of CPT-11 at escalating doses from 20 to 80 mg.[33]
To achieve maximal tumor uptake in vivo, a NP can be conjugated to a homing agent that seeks a target expressed both on tumor cells and on tumor-associated vascular endothelium. Table 2 presents an alphabetized list of the most common homing targets found to increase NP uptake in glioma. The table also includes a list of popular polymeric coatings, NP configurations, and (nongenetic) therapeutic agents used in current research.
Table 2.
Example homing targets, polymeric coatings, nanoparticle configurations, and therapeutic agents utilized in nanoparticle drug delivery for glioma
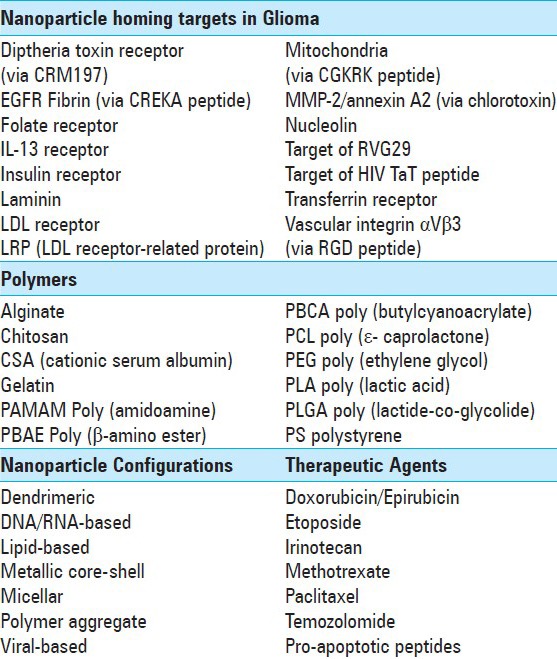
The targets shown in Table 2 include a number of receptors overexpressed on rapidly dividing cells. For instance, the transferrin receptor is normally expressed on brain endothelial cells, as well as hepatocyte, erythroid, and placental cells.[166] In the setting of a brain neoplasm, vascular expression of transferrin receptor is upregulated.[163] Zhang et al. delivered PEGylated immunoliposomes past the BBB to mice bearing U87 glioma xenografts via targeting antibodies that bound transferrin and insulin receptors.[194] Further research with insulin receptor targeting alone also resulted in increased NP accumulation.[164] Expression of the folic acid receptor at the BBB has been found to facilitate brain entry of drug-loaded targeted NPs,[79] and targeting of epidermal growth factor receptor (EGFR) overexpression on intracranial U87 tumors has similarly facilitated antineoplastic activity, with encapsulated chemotherapeutics delivered both intravenously[114] and via CED.[63] A related EGF peptide known as heparin-binding EGF-like growth factor (HB-EGF) has been discovered on the endothelial surface, and is known to bind diphtheria toxin.[51] The use of mutated nontoxic formulations of diphtheria toxin has correspondingly been shown to facilitate BBB crossing.[52] Conjugation of IL-13 to liposomes has increased drug transport to intracranial U251 implants as glioma cells overexpress the IL-13 receptor α2.[109] More esoteric receptor-targeting strategies have also shown promising results in glioma, such as the conjugation of rabies virus glycoprotein 29 (RVG29) peptides to dendrimeric NPs.[106] The increased brain uptake was attributed to binding of RVG29 to GABAB or nicotinic acetylcholine receptors.[106] A final popular target is the overexpressed low density lipoprotein (LDL) receptor, although LDL receptor-related proteins (LRP) also function well for targeting. While NP coating with agents including apoE or polysorbate 80 have induced binding to the LDL receptor,[95] attachment of β-amyloid precursor protein has resulted in binding to LRP.[93] LRP has been found both on glioma cells and BBB endothelium, with specific intracranial targeting and drug delivery having been demonstrated in a mouse U87 model.[41,183]
Similar to the case of LRP, other nonantibody proteins are overexpressed by glioma and appear in detectable quantities on the endothelium of tumor vessels. Examples include laminin 411,[42] nucleolin,[46] and fibrin.[25] The penta-peptide CREKA (cysteine−arginine−glutamic acid−lysine−alanine) was chosen with specific binding affinity for fibrin, and was found to yield particle deposition within GBM tumors 1 h after IV injection.[25] Other successful examples of short peptide binding include the tri-peptide RGD (arginine−glycine−aspartic acid) that binds to αVβ3 integrin on immature endothelial cells,[10,20] and the penta-peptide CGKRK (cysteine−glycine−lysine−arginine−lysine) that binds to heparan sulfate on tumor endothelium.[2]
The 36-amino acid peptide chlorotoxin (CTX) has been the subject of much focused research.[88,89,168,169] CTX is derived from the venom of the scorpion Leiurus quinquestriatus, and can facilitate NP entry across the BBB into tumor cells via binding of overexpressed matrix metalloproteinase-2 and annexin A2.[86] Figure 2 demonstrates accumulation of fluorescently labeled CTX-conjugated iron oxide NPs within a GFP expressing C6 glioma xenograft, by ex vivo fluorescence imaging, histology/iron staining, and detection of fluorescent label within individual cells.
Figure 2.
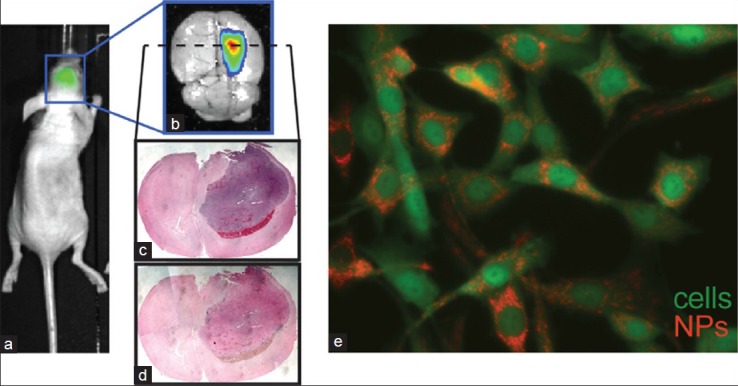
Targeting of iron oxide nanoparticles to orthotopic C6 glioma xenograft tumors in mice. (a) IVIS bioluminescent imaging of luciferase signal demonstrates tumor location. (b) Fluorescence imaging of red channel (710 nm) shows concentrated presence of cy5.5 fluorophore-labeled NPs within the glioma mass. (c) Hemotoxylin and eosin, and (d) prussian blue/nuclear fast red stained sections of the tumor show accumulation of iron oxide 24 h after injection. (e) Fluorescence microscopy of C6 cells loaded with CTX/cy5.5-bound NPs in vitro
While NPs are typically thought of as passive smart delivery vehicles, they can also be engineered to actively move throughout the tumor. Recent work has opened up the possibility to engineer NPs that migrate throughout the tumor with targeting agents that “walk” along antigen receptors.[132] Cells can also be used as delivery vehicles for NPs to provide active migration to and throughout the tumor. Microglia have been used for such a purpose as they are chemo attracted to brain tumors.[138,139] Neuronal stem cells (NSCs) have been used as delivery vehicles for glioma; NSCs have high specificity to brain tumor tissue and are able to actively move throughout the tumor.[1] The majority of this work has been performed using NSCs engineered to express oncolytic viruses or tumor suppressor proteins.[140] Importantly, NSCs can be loaded with NPs without affecting their normal cellular function and can be tracked using MRI.[14] We foresee NSCs being used as a Trojan horse to deliver multifunctional NPs to gliomas. The ability to track NSCs, as well as engineered T cells[13] and dendritic cells[6] for immunotherapy[137] when loaded with NPs will provide a more useful platform for optimizing these therapies. Imaging data could reveal if a therapeutic response is correlated with successful accumulation of cells in the tumor and with appropriate tumor eradication.
NANOPARTICLE GENE THERAPY
Nanotechnology provides tools to overcome current limitations in nonviral glioma gene delivery, and we believe that nanomaterial-facilitated gene therapy will eventually be incorporated into routine glioma management. Avenues of gene therapy for glioma include: (i) replacement of damaged genes with functional counterparts, (ii) knockdown of proteins required for glioma cell survival using small interfering RNAs (siRNAs) or short hairpin RNAs (shRNAs), (iii) delivery of genes that code for enzymes that convert inert prodrugs into cytotoxic compounds, and (iv) modulation of the stromal compartment by inhibiting angiogenesis or activation of the immune system. Gene therapy offers significant advantages over small-molecule drugs, as many targets are currently “undruggable” with existing therapeutics.[176] Furthermore, genetic therapies may facilitate key cellular transformations such as terminal differentiation of brain tumor stem cells.[162] The translation of gene therapies into the clinic has been hindered by the lack of a safe and effective gene delivery vehicle. Most clinical trials utilize viral vectors as they are effective at transferring genetic material into target cells. However, systemically administered viral gene transfection is limited by a rapid clearance rate due to recognition by the immune system and lack of tumor penetration caused by their large size (~100 nm).[99]
NPs have been proven capable of binding and protecting a nucleic acid payload. This was achieved initially by encapsulating nucleic acids into liposomes. The surface of the liposome can be modified with targeting and imaging agents with the nucleic acid protected in the liposome core. An early-adopted DNA gene therapy utilized liposomes to deliver a suicide gene, herpes simplex virus thymidine kinase, along with ganciclovir as the prodrug.[37] Cationic liposomes were also used to deliver the gene encoding interferon-β (IFN-β). Results in mice prompted a limited human trial using intratumoral injection of this formulation in five patients, with two glioma tumors demonstrating growth arrest for 10 weeks, and two others showing size reduction that persisted for ~16 months.[187] However, to date, this drug has not progressed past stage 1 clinical trials.
In addition to lipid particles, cationic polymer or core-shell NPs may also bind negatively charged nucleic acids through electrostatic interaction, and the condensation of these nucleic acids into the polymer layer may provide them with a means of protection. Nucleic acid protection can be challenging in complex fluids such as blood, where particle aggregation or nonspecific binding of serum proteins and cells can occur. To further protect nucleic acids, NPs can be stabilized through coating with PEG[133,193] or zwitterionic polymers[185] that create a hydration layer surrounding the NP. A particle designed in this fashion delivered tumor necrosis factor related apoptosis inducing ligand (TRAIL) by encapsulation within PEG/PLA NPs. TRAIL induces cell death in glioma cells that have the appropriate receptors overexpressed on their surface.[189]
More recently, significant focus has been placed on siRNA delivery to knockdown expression of genes required for glioma cell survival.[170] Cationic liposome-mediated delivery of c-Met siRNA decreased c-Met expression in orthotopic glioblastoma tumors in mice, and suppressed tumor growth.[76] Effective delivery of siRNA against EGFR was also demonstrated, both with intravenous liposomal delivery to a subcutaneous implanted U251 glioma model,[81] and by attachment to crosslinked dendrimeric iron oxide NPs followed by intracranial delivery to a transgenic glioma model.[3] Modified siRNAs have also been constructed that are protected from nuclease degradation and are readily taken up into cells.[24,85,177,180] These modified siRNAs provide the opportunity to focus NP engineering strategies away from siRNA protection and toward prolonging circulation time, increasing site-specific delivery, and promoting distribution throughout the tumor. By reducing the design constrains on NPs, we may thus simplify their construction and accelerate clinical translation.
On the horizon are exciting gene therapies whose application to glioma may be facilitated by nanoscaled delivery agents. For instance, spherical nucleic acid NP conjugates have been constructed from gold NPs coated in a densely packed, highly oriented layer of siRNA. These structures have been found to be well protected from nuclease degradation and provide highly efficient knockdown.[74,141] Clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology has quickly become a mainstream tool to regulate gene expression.[35,113,143] A Cas9 nuclease attached to an RNA guide makes a double-strand break at the RNA target sequence in the genome. Modified Cas9 can also induce DNA base methylation, chromatin modification, and even activation of gene expression. RNA activation (RNAa) also allows control of gene expression, and to date it has been less widely adopted.[73,82,102] Using RNAa, double-stranded ribonucleotide segments are delivered, which interact with the promoter region of a gene to induce expression, providing a means to reactivate tumor suppressor genes without the need to deliver the entire coding sequence.
Human clinical trials have shown some success with NP siRNA delivery, indicating the approach may soon be a viable option for glioma.[38] We are currently at a point where the library of known glioma targeting agents is progressively being applied to the transport of genetic material in small animal models. The targeting agents can facilitate trafficking not only to the glioma cell itself, but also to the nucleus or perinuclear region. NPs targeted with the peptide chlorotoxin, for instance, were found to promote localization in the perinuclear region with resulting high transfection efficiencies.[87,88] Genetic therapy in glioma is likely to yield optimum benefit as part of a combined treatment strategy. The use of gene therapy via NPs, in combination with chemotherapy drugs attached to the particle, could provide maximal benefit due to the co-localization of therapy. As we will discuss next, the third arm of a combined future treatment strategy can be tumor ablation using ingeniously applied chemistry and biophysics, to promote energy deposition at the site of the NP.
NANOMATERIAL TISSUE ABLATION–CREATIVE APPROACHES TO NANONEUROSURGERY
Successful NP drug or gene delivery must adhere to a delicate chemical and biological scheme including: (i) strong attachment of the therapeutic payload in high quantities, (ii) guarding of the beneficial agent from detachment in the bloodstream, (iii) carrying of the drug or gene into the tumor, and (iv) release of the payload once inside the cell. The chemistry of NP design often involves a limited number of competing reactive sites to attach drug and targeting agent, making the addition of both at appropriate quantity a challenge. In contrast, it is conceptually less complex to design a NP, which simply delivers itself to a glioma mass. Once internalized, NPs can be “activated” from outside the body in a number of ways−including photons and magnetic fields−causing them to release energy and ablate tissue with a level of precision that is determined by their targeting efficiency. The particles are otherwise biocompatible and nontoxic to cells, until the external trigger is initiated. If delivered appropriately, particles can accomplish selective tumor destruction, with no entry tract and minimal off-target tissue damage. Such a nanosurgical approach could prove of great value especially for the treatment of deeply situated glioma, in which location makes conventional surgery counterproductive.
The interaction between externally applied photons and an internalized material was the conceptual basis of photodynamic therapy (PDT) − a principle first described over 100 years ago.[120] Embedded photosensitizers were found to generate singlet oxygen (1O2) when excited with the appropriate wavelength, and would result in free radical damage at a distance of ~100 nm.[119] The visible light-activated (630 nm) compounds porfimer sodium and 5-aminolevulinic acid are well known examples of photodynamic agents. Porfimir sodium is in clinical use for nonsmall cell lung cancer and esophageal cancer,[80] and is also in clinical trials for glioma.[32] PDT has been under investigation for neurosurgical purposes for over two decades, and photoactivators have many novel applications through their incorporation into nanoscaled materials.[8] Santos et al. recently injected single-walled carbon nanotubes into murine temozolomide-resistant glioma flank tumors. These nanotubes absorb NIR light from an external laser source without the need for an additional photoactivator, resulting in radiative relaxation and hyperthermic tumor ablation.[145] A similar concept has been demonstrated for intracranial tumors using an external NIR source that interacts with hybrid silica-gold nanoshells.[39] Direct neurosurgical investigation of this technology is currently expanding, with lasers positioned either adjacent to an open resection cavity, or intracranially using long needles similar to those for laser-induced interstitial thermotherapy (LITT).[161,190] Particles can either be injected intravenously or directly into the brain using the principles of CED.[5,11]
NPs can also interact with photons outside the UV-visible infrared region of the electromagnetic spectrum, and can increase the tissue-ablating potential of very low frequencies. In the radiofrequency (RF) regime, shortwave (13.56 MHz) RF fields emitted at low power will induce negligible damage to tissues on their own.[53] When these RF waves encounter an electron-dense nanomaterial (e.g., metallic NP, carbon nanotube, quantum dot), the vibrational energy release from RF-induced electron movement can result in amplified resistive heating, which occurs on a length scale of ~100 μm around the particle.[58] The NPs effectively focus the energy from the broadly applied RF field onto specific sites of interest. Heating at these microscale dimensions can occur at a rate of 1–3°C/s, with local boiling temperatures attainable within minutes.[58,59] This is in contrast to standard RF ablation, which involves higher delivered power, and damages bulk tissue within a radius of 2–4 cm from a probe tip. While standard RF abliation has been involved in human clinical trials for glioma,[45,179] and although applications of targetted NP−RF ablation have been successfully demonstrated in animal models of nonglioma neoplasms,[16,60] further investigation using targetted RF nanotherapeutics for glioma is currently warranted.
Within the high-frequency spectral range, X- and gamma-rays are known to interact with metalic NPs, especially those composed of metals having a high atomic number.[36] When high-energy photons encounter an appropriate electron-dense heavy metal, they yield a localized deposition of energy due to inner-shell electron transitions in the metal atom, followed by relaxation and local emission of photons and electrons from the metal itself (Auger effect).[36,123] This electron cascade then triggers free radical formation in the surrounding solution, which can damage DNA, cell membranes, or cellular machinery. As perceived by the involved tumor cells receiving radiation damage, this spatially localized deposition of X- or gamma-rays energy acts similar to the high linear energy transfer (LET) behavior of particle radiation.[17] Although this effect also occurs with individual metal atoms, the NP delivery scheme provides a biocompatible polymer coating and prevents toxicity of the metal.[192] Success has been achieved with this approach using NPs composed of silver[105] and gadolinium,[36] although the most widely adopted material in this respect has been gold.[4,15,64] Hainfeld et al. demonstrated good response of intracranial Tu-2449 × enograft gliomas to 30 and 35 Gy doses of 100 kVp X-rays, administered in the presence of nontargeted gold NPs.[65] In this study, 5 of 9 mice remained alive one year after treatment, compared with 2 of 11 mice in the radiation-only group. Combined technologies have also been described, such as the self-lighting PDT technique.[19] In this method, ionizing radiation is applied externally to activate photon release from a scintillation-luminescent NP. Visible light produced by the particle then activates adjacent PDT-active photosensitizers, accomplishing the 1O2-dependent action of PDT, and circumventing the problem of visible light penetration through biological tissue.
Despite the body of research using photons to activate biologically internalized NPs, perhaps the greatest progress in external manipulation of NPs has been with magnetic fields.[151] Magnetic materials, including NPs of appropriate composition, undergo magnetic moment hysteresis and enhanced Brownian motion when subjected to a rapidly alternating field.[77,151,175] These effects contribute to thermal energy release and result in localized heating. The principles of magnetic hyperthermia have been under investigation for over 60 years,[56] and the first applications of this technology for treatment of glioma were initiated in the late 1980s by Stea et al., using ferromagnetic seeds.[153,154] A wide range of preclinical studies in animals have demonstrated significant survival benefit in glioma when using superparamagnetic iron oxide NPs followed by magnetic hyperthermia.[78,104,186] This success prompted human clinical trials based in Germany using an aminosilane-coated iron oxide NP, with magnetic thermotherapy added to conventional treatment. The first results were published in 2007,[77,111] and the most recent were published in 2011.[112,165] The combined therapy yielded an average survival of 13.4 months after glioma recurrence, in a cohort of 59 patients. Comparison against a conventional treatment cohort with comparable tumor size and demographics showed a survival of 6.2 months after recurrence. The study selected patients with supratentorial GBM, up to 3 foci, a maximum tumor size of 7 cm, and a Karnofsky score >60 at the time of enrollment. NPs were delivered to the tumor through direct intracranial injection (concentration 112 mg/ml) of ~4.5 ml of solution, and were exposed to a magnetic field alternating at 100 kHz. Transient intratumoral temperatures of approximately 51°C were reached. Figure 3 displays representative CT-based maps of NP after instillation for this therapy. After postmortem study of relevant brain tissue, the authors concluded that magnetic heating contributed to additional coagulative necrosis in the areas containing the NP.[165]
Figure 3.
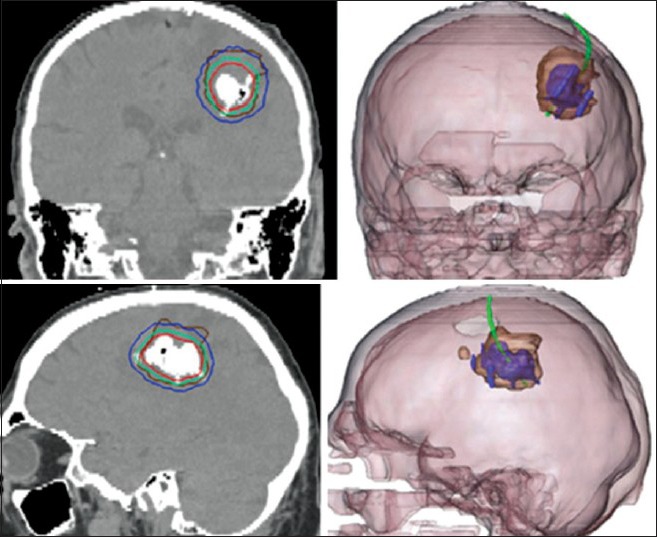
Human intratumoral injection of iron oxide NP, for clinical study of magnetic hyperthermia. (a) Coronal CT image displays hyperdense NP mass, with surrounding isothermic lines of simulated treatment temperatures (red=50°C, blue=40°C). (b) Fused CT-MRI images showing enhancing glioma margin (brown), with respect to the iron oxide infusion (purple). Adapted with permission from Maier-Hauff et al.[112]
TOWARD A CURE–NANOTECHNOLOGY IN THE OPERATING ROOM AND IN THE CLINIC
The methods of NP tissue ablation and of NP drug−gene delivery, when viewed together, provide a glimpse of the great potential that nanotechnology has in the field of glioma. To date, the literature on these approaches has remained discrete, and the presence of an integrated literature exploring the potential of combined targeting, molecular therapeutics, and photon/magnetic ablation remain in the formative stages. Rather than simply allowing treatment through a single modality, nanotechnology can act as a platform for multi-modal glioma treatment, employing many useful approaches simultaneously. We envision a treatment scheme that incorporates a number of therapeutic strategies via a common nanoscaled agent for targeted delivery.
Over the past decade, there has been much speculation with regard to the “theranostic” potential of NP materials. Utilizing a single vehicle to assist in both diagnosis and treatment brings the worlds of clinic and the operating room closer together. It also brings exciting principles of physics and spectroscopy closer to the direct management of glioma. From a purely conjectural standpoint, we reflect on a hypothetical particle that is injected intraoperatively after resection, with magnets closely positioned around the resection cavity to draw iron oxide NP quickly to the margins of the tumor bed.[191,196] Such a NP would carry one or more molecular targeting agents to promote internalization past the BBB and to the tumor cell nucleus. After surgery, based on the estimated degree of successful NP delivery−as gauged by the superparamagnetic signature on MRI−radiation dose for NP-enhanced ionizing-beam therapy could be chosen. Such therapy would act synergistically with the otherwise prohibitively hydrophobic chemotherapeutic drug that was simultaneously bound to the particle.
A second, and separate, hypothetical scenario involves the treatment of gliomas that are poor candidates for resection due to deep intracerebral location. In this setting of highly sensitive surrounding anatomy, CED of the NP formulation (potentially performed at the same time as stereotactic biopsy) would be followed by imaging to confirm the absence of off-target particle diffusion (e.g., to the brainstem or near large cerebral vasculature). Subsequently, magnetic convective heating would be applied, and would act along with the combined action of oral chemotherapy and NP-bound gene therapy.
Such hypothetical combined therapeutic strategies may soon present viable options for clinical testing.[197] One concept explored by Karabeber et al. used NPs coated with a Raman-active 4,4´-bipyridine dye loaded in between a gold core and a silica outer shell.[83,92] The group performed a sequential resection of tumor in an infiltrative glioma model, while applying a handheld surface-enhanced Raman scattering (SERS) probe to determine if neoplastic cells remained at the margins [Figure 4]. The study found that resection of all Raman-active microscopic tumor foci resulted in the absence of local tumor cells on follow-up immunohistochemistry. The NPs, in this example provided intraoperative feedback with cellular precision. Another example from Veiseh et al. described a dual MR- and NIR-active NP, targeted with chlorotoxin to pass the BBB.[171] This same particle has been recently conjugated with O6-benzylguanine and injected directly into GBM6 gliomas, with excellent visualization on MRI, minimal off-target toxicity, and demonstration of survival benefit.[156]
Figure 4.
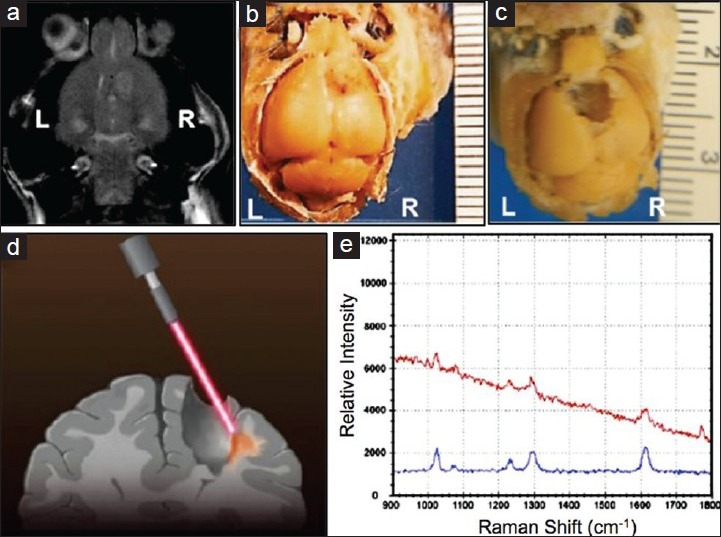
Intraoperative spectroscopy. A handheld SERS probe assisted in optimizing surgical resection of infiltrative glioma from the brain of a mouse, after Raman-active nanoparticles were delivered intravenously. Tumor site before (a and b) and after (c) resection are displayed, as well as a schematic of the handheld probe in use (d), and an example spectrum of the particle detected in cells at the tumor margin (e). Adapted with permission from Karabeber et al.[83]
A schematic of our idealized future nanotherapeutic incorporating a number of the features described above is displayed in Figure 5. The field of nanotechnology provides an array of options for the improved diagnosis and treatment of glioma. The principles of smart molecular design allow us to choose simultaneous treatment strategies that work synergistically to eradicate tumor cells both within the enhancing margin and beyond. The existing body of work and current clinical trials suggest that such combined therapeutic strategies will likely be ready for clinical testing within the next 5–10 years. Rather than a substitute for surgical therapy, nanoscaled treatment modalities provide an adjunct to modern surgical strategies−improving the extent of resection, working noninvasively to eradicate tumor cells remaining after surgery, and targeting the biomolecular mechanisms that make glioma a challenging neoplasm.
Figure 5.
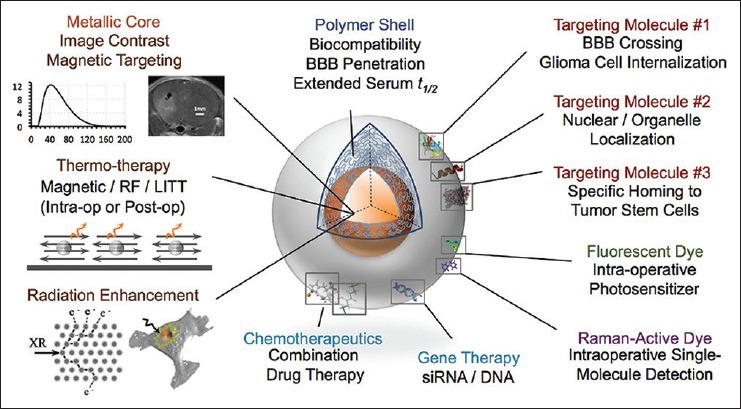
Conceptualized ideal nanoparticle. A metal core is used for image contrast and radiation/magnetic therapy, while the polymeric shell provides biocompatibility and functional sites for attachment of homing molecules, nucleic acids, chemotherapeutics, and optically active moieties
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2015/6/2/45/151334
Contributor Information
Peter A. Chiarelli, Email: pac47@uw.edu.
Forrest M. Kievit, Email: fmkemt@uw.edu.
Miqin Zhang, Email: mzhang@u.washington.edu.
Richard G. Ellenbogen, Email: rge@uw.edu.
REFERENCES
- 1.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–51. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agemy L, Friedmann-Morvinski D, Kotamraju VR, Roth L, Sugahara KN, Girard OM, et al. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc Natl Acad Sci U S A. 2011;108:17450–5. doi: 10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal A, Min DH, Singh N, Zhu H, Birjiniuk A, von Maltzahn G, et al. Functional delivery of siRNA in mice using dendriworms. ACS Nano. 2009;3:2495–504. doi: 10.1021/nn900201e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Zaki A, Joh D, Cheng Z, De Barros AL, Kao G, Dorsey J, et al. Gold-loaded polymeric micelles for computed tomography-guided radiation therapy treatment and radiosensitization. ACS Nano. 2014;8:104–12. doi: 10.1021/nn405701q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials. 2009;30:2302–18. doi: 10.1016/j.biomaterials.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:E257–67. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]
- 7.Bai CZ, Choi S, Nam K, An S, Park JS. Arginine modified PAMAM dendrimer for interferon beta gene delivery to malignant glioma. Int J Pharm. 2013;445:79–87. doi: 10.1016/j.ijpharm.2013.01.057. [DOI] [PubMed] [Google Scholar]
- 8.Bechet D, Couleaud P, Frochot C, Viriot ML, Guillemin F, Barberi-Heyob M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008;26:612–21. doi: 10.1016/j.tibtech.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Bhojani MS, Van Dort M, Rehemtulla A, Ross BD. Targeted imaging and therapy of brain cancer using theranostic nanoparticles. Mol Pharm. 2010;7:1921–9. doi: 10.1021/mp100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibby DC, Talmadge JE, Dalal MK, Kurz SG, Chytil KM, Barry SE, et al. Pharmacokinetics and biodistribution of RGD-targeted doxorubicin-loaded nanoparticles in tumor-bearing mice. Int J Pharm. 2005;293:281–90. doi: 10.1016/j.ijpharm.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–80. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandsma D, van den Bent MJ. Molecular targeted therapies and chemotherapy in malignant gliomas. Curr Opin Oncol. 2007;19:598–605. doi: 10.1097/CCO.0b013e3282f0313b. [DOI] [PubMed] [Google Scholar]
- 13.Brown CE, Starr R, Martinez C, Aguilar B, D’Apuzzo M, Todorov I, et al. Recognition and Killing of Brain Tumor Stem-Like Initiating Cells by CD8(+) Cytolytic T Cells. Cancer Res. 2009;69:8886–93. doi: 10.1158/0008-5472.CAN-09-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141–7. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 15.Burger N, Biswas A, Barzan D, Kirchner A, Hosser H, Hausmann M, et al. A method for the efficient cellular uptake and retention of small modified gold nanoparticles for the radiosensitization of cells. Nanomedicine. 2014;10:1365–73. doi: 10.1016/j.nano.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Cardinal J, Klune JR, Chory E, Jeyabalan G, Kanzius JS, Nalesnik M, et al. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery. 2008;144:125–32. doi: 10.1016/j.surg.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter JD, Cheng NN, Qu Y, Suarez GD, Guo T. Nanoscale energy deposition by X-ray absorbing nanostructures. J Phys Chem B. 2007;111:11622–5. doi: 10.1021/jp075253u. [DOI] [PubMed] [Google Scholar]
- 18.Caruso G, Caffo M, Alafaci C, Raudino G, Cafarella D, Lucerna S, et al. Could nanoparticle systems have a role in the treatment of cerebral gliomas? Nanomedicine. 2011;7:744–52. doi: 10.1016/j.nano.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Chen W, Zhang J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J Nanosci Nanotechnol. 2006;6:1159–66. doi: 10.1166/jnn.2006.327. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Plasencia C, Hou Y, Neamati N. Synthesis and biological evaluation of dimeric RGD peptide-paclitaxel conjugate as a model for integrin-targeted drug delivery. J Med Chem. 2005;48:1098–106. doi: 10.1021/jm049165z. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Morshed RA, Auffinger B, Tobias AL, Lesniak MS. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv Drug Deliv Rev. 2014;66:42–57. doi: 10.1016/j.addr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chertok B, Webber MJ, Succi MD, Langer R. Drug delivery interfaces in the 21 st century: From science fiction ideas to viable technologies. Mol Pharm. 2013;10:3531–43. doi: 10.1021/mp4003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien AJ, Illi JA, Ko AH, Korn WM, Fong L, Chen LM, et al. A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res. 2009;15:5569–75. doi: 10.1158/1078-0432.CCR-09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu YL, Rana TM. siRNA function in RNAi: A chemical modification analysis. RNA. 2003;9:1034–48. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung EJ, Cheng Y, Morshed R, Nord K, Han Y, Wegscheid ML, et al. Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma. Biomaterials. 2014;35:1249–56. doi: 10.1016/j.biomaterials.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov. Assessing Dynamic Magnetic Resonance (MR) Imaging in Patients With Recurrent High Grade Glioma Receiving Chemotherapy. [Last accessed on 2014 Aug 01]. Available from: http://clinicaltrials.gov/ct2/show/NCT00769093 .
- 27.ClinicalTrials.gov. A Clinical Trial to Assess the Safety and Efficacy of the Treatment of Patients With Metastasis From Malignant Melanoma-Treatment Consists of the Substances Lomustine (Capsules) and Cytarabine (Injected Into an Area Near the Spinal Cord), Accompanied by Radiotherapy of the Brain. [Last accessed on 2014 Aug 01]. Available from: http://clinicaltrials.gov/show/NCT01563614 .
- 28.ClinicalTrials.gov. Intrathecal Chemotherapy With Liposomal Cytarabine (DepoCyte®) in Leptomeningeal Metastases of Breast Cancer Versus no Intrathecal Treatment. [Last accessed on 2014 Aug 01]. Available from: http://clinicaltrials.gov/ct2/show/NCT01645839 .
- 29.ClinicalTrials.gov. Magnetic Resonance (MR) Imaging Study Using Ferumoxytol to Assess Early Tumor Response in Patients With Glioblastoma Multiforme. [Last accessed on 2014 Aug 01]. Available from: http://clinicaltrials.gov/ct2/show/NCT00660543 .
- 30.ClinicalTrials.gov. A Phase 2 Study to Determine the Safety and Efficacy of BIND-014 (Docetaxel Nanoparticles for Injectable Suspension), Administered to Patients With Metastatic Castration-Resistant Prostate Cancer. [Last accessed on 2014 Aug 01]. Available from: http://clinicaltrials.gov/ct2/show/NCT01812746 .
- 31.ClinicalTrials.gov. A Phase I Trial of Nanoliposomal CPT-11 (NL CPT-11) in Patients With Recurrent High. Grade Gliomas. [Last accessed on 2014 Aug 01]. Available from: http://clinicaltrials.gov/ct2/show/NCT00734682 .
- 32.ClinicalTrials.gov. Photodynamic Therapy (PDT) for Brain Tumors. [Last accessed on 2014 Aug 01]. Available from http://clinicaltrials.gov/ct2/show/NCT01682746 .
- 33.ClinicalTrials.gov. Study of Convection-Enhanced, Image-Assisted Delivery of Liposomal-Irinotecan. Recurrent High Grade Glioma. [Last accessed on 2014 Aug 01]. Available from: http://clinicaltrials.gov/ct2/show/NCT02022644 .
- 34.ClinicalTrials.gov. Study of MBP-426 in Patients With Second Line Gastric, Gastroesophageal, or Esophageal Adenocarcinoma. [Last accessed on 2014 Augt 01]. Available from: http://clinicaltrials.gov/ct2/show/NCT00964080 .
- 35.Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339:819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coulter JA, Hyland WB, Nicol J, Currell FJ. Radiosensitising nanoparticles as novel cancer therapeutics--pipe dream or realistic prospect? Clin Oncol. 2013;25:593–603. doi: 10.1016/j.clon.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 37.David S, Montier T, Carmoy N, Resnier P, Clavreul A, Mevel M, et al. Treatment efficacy of DNA lipid nanocapsules and DNA multimodular systems after systemic administration in a human glioma model. J Gene Med. 2012;14:769–75. doi: 10.1002/jgm.2683. [DOI] [PubMed] [Google Scholar]
- 38.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day ES, Thompson PA, Zhang L, Lewinski NA, Ahmed N, Drezek RA, et al. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol. 2011;104:55–63. doi: 10.1007/s11060-010-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De la Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3:557–62. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106:1534–44. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 42.Ding H, Inoue S, Ljubimov AV, Patil R, Portilla-Arias J, Hu J, et al. Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release [corrected] Proc Natl Acad Sci U S A. 2010;107:18143–8. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dougherty JD, Fomchenko EI, Akuffo AA, Schmidt E, Helmy KY, Bazzoli E, et al. Candidate pathways for promoting differentiation or quiescence of oligodendrocyte progenitor-like cells in glioma. Cancer Res. 2012;72:4856–68. doi: 10.1158/0008-5472.CAN-11-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faucher L, Guay-Begin AA, Lagueux J, Cote MF, Petitclerc E, Fortin MA. Ultra-small gadolinium oxide nanoparticles to image brain cancer cells in vivo with MRI. Contrast media and molecular imaging. 2011;6:209–18. doi: 10.1002/cmmi.420. [DOI] [PubMed] [Google Scholar]
- 45.Fiorentini G, Giovanis P, Rossi S, Dentico P, Paola R, Turrisi G, et al. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. In vivo. 2006;20:721–4. [PubMed] [Google Scholar]
- 46.Fogal V, Sugahara KN, Ruoslahti E, Christian S. Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis. 2009;12:91–100. doi: 10.1007/s10456-009-9137-5. [DOI] [PubMed] [Google Scholar]
- 47.Fukumura D, Jain RK. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J Cell Biochem. 2007;101:937–49. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 48.Fulton DS, Urtasun RC, Scott-Brown I, Johnson ES, Mielke B, Curry B, et al. Increasing radiation dose intensity using hyperfractionation in patients with malignant glioma. Final report of a prospective phase I-II dose response study. J Neurooncol. 1992;14:63–72. doi: 10.1007/BF00170946. [DOI] [PubMed] [Google Scholar]
- 49.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 50.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin Pharmacokinet. 2003;42:419–36. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 51.Gaillard PJ, de Boer AG. A novel opportunity for targeted drug delivery to the brain. J Control Release. 2006;116:e60–2. doi: 10.1016/j.jconrel.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 52.Gaillard PJ, Visser CC, de Boer AG. Targeted delivery across the blood-brain barrier. Expert Opin Drug Deliv. 2005;2:299–309. doi: 10.1517/17425247.2.2.299. [DOI] [PubMed] [Google Scholar]
- 53.Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–65. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 54.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–55. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Germano IM, Binello E. Stem cells and gliomas: Past, present, and future. J Neurooncol. 2014;119:547–55. doi: 10.1007/s11060-014-1498-y. [DOI] [PubMed] [Google Scholar]
- 56.Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg. 1957;146:596–606. doi: 10.1097/00000658-195710000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giraudeau C, Geffroy F, Meriaux S, Boumezbeur F, Robert P, Port M, et al. 19F molecular MR imaging for detection of brain tumor angiogenesis: In vivo validation using targeted PFOB nanoparticles. Angiogenesis. 2013;16:171–9. doi: 10.1007/s10456-012-9310-0. [DOI] [PubMed] [Google Scholar]
- 58.Glazer ES, Curley SA. Non-invasive radiofrequency ablation of malignancies mediated by quantum dots, gold nanoparticles and carbon nanotubes. Ther Deliv. 2011;2:1325–30. doi: 10.4155/tde.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glazer ES, Curley SA. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer. 2010;116:3285–93. doi: 10.1002/cncr.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glazer ES, Massey KL, Zhu C, Curley SA. Pancreatic carcinoma cells are susceptible to noninvasive radio frequency fields after treatment with targeted gold nanoparticles. Surgery. 2010;148:319–24. doi: 10.1016/j.surg.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Groothuis DR. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro Oncol. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–20. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Hadjipanayis CG, Machaidze R, Kaluzova M, Wang L, Schuette AJ, Chen H, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–12. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–15. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 65.Hainfeld JF, Smilowitz HM, O’Connor MJ, Dilmanian FA, Slatkin DN. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine. 2013;8:1601–9. doi: 10.2217/nnm.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling Adult Gliomas Using RCAS/t-va Technology. Transl Oncol. 2009;2:89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harris G, Palosaari T, Magdolenova Z, Mennecozzi M, Gineste JM, Saavedra L, et al. Iron oxide nanoparticle toxicity testing using high throughput analysis and high content imaging. Nanotoxicology. 2013 doi: 10.3109/17435390.2013.816797. [Ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Huang J, Xie J, Chen K, Bu L, Lee S, Cheng Z, et al. HSA coated MnO nanoparticles with prominent MRI contrast for tumor imaging. Chem Commun. 2010;46:6684–6. doi: 10.1039/c0cc01041c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid nanocapsules: A new platform for nanomedicine. Int J Pharm. 2009;379:201–9. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 70.Jabir NR, Tabrez S, Ashraf GM, Shakil S, Damanhouri GA, Kamal MA. Nanotechnology-based approaches in anticancer research. Int J Nanomed. 2012;7:4391–408. doi: 10.2147/IJN.S33838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackson H, Muhammad O, Daneshvar H, Nelms J, Popescu A, Vogelbaum MA, et al. Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery. 2007;60:524–9. doi: 10.1227/01.NEU.0000255334.95532.DD. [DOI] [PubMed] [Google Scholar]
- 72.James JS. DOXIL approved for KS. AIDS treatment news. 1995;(no 236):6. [PubMed] [Google Scholar]
- 73.Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Chem Biol. 2007;3:166–73. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 74.Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM, et al. Spherical Nucleic Acid Nanoparticle Conjugates as an RNAi-Based Therapy for Glioblastoma. Sci Transl Med. 2013;5:209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–50. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 76.Jin J, Bae KH, Yang H, Lee SJ, Kim H, Kim Y, et al. In Vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem. 2011;22:2568–72. doi: 10.1021/bc200406n. [DOI] [PubMed] [Google Scholar]
- 77.Jordan A, Maier-Hauff K. Magnetic nanoparticles for intracranial thermotherapy. J Nanosci and Nanotechnol. 2007;7:4604–6. [PubMed] [Google Scholar]
- 78.Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol. 2006;78:7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 79.Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood-brain barrier: Chemical modifications of drugs or drug-nanoparticles? Drug Discov Today. 2008;13:1099–106. doi: 10.1016/j.drudis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Juzenas P, Chen W, Sun YP, Coelho MA, Generalov R, Generalova N, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008;60:1600–14. doi: 10.1016/j.addr.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang CS, Zhang ZY, Jia ZF, Wang GX, Qiu MZ, Zhou HX, et al. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006;13:530–8. doi: 10.1038/sj.cgt.7700932. [DOI] [PubMed] [Google Scholar]
- 82.Kang MR, Yang G, Place RF, Charisse K, Epstein-Barash H, Manoharan M, et al. Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth. Cancer Res. 2012;72:5069–79. doi: 10.1158/0008-5472.CAN-12-1871. [DOI] [PubMed] [Google Scholar]
- 83.Karabeber H, Huang R, Iacono P, Samii JM, Pitter K, Holland EC, et al. Guiding brain tumor resection using surface-enhanced raman scattering nanoparticles and a hand-held raman scanner. ACS Nano. 2014 doi: 10.1021/nn503948b. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaur R, Badea I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int J Nanomed. 2013;8:203–20. doi: 10.2147/IJN.S37348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kenski DM, Butora G, Willingham AT, Cooper AJ, Fu W, Qi N, et al. siRNA-optimized Modifications for Enhanced In Vivo Activity. Mol Ther Nucleic Acids. 2012;1:e5. doi: 10.1038/mtna.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kesavan K, Ratliff J, Johnson EW, Dahlberg W, Asara JM, Misra P, et al. Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. The J Biol Chem. 2010;285:4366–74. doi: 10.1074/jbc.M109.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, et al. PEI-PEG-Chitosan copolymer coated iron oxide nanoparticles for safe gene delivery: Synthesis, complexation, and transfection. Adv Funct Mater. 2009;19:2244–51. doi: 10.1002/adfm.200801844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, et al. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano. 2010;4:4587–94. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kievit FM, Zhang M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res. 2011;44:853–62. doi: 10.1021/ar2000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat Nanotechnol. 2010;5:465–72. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim TH, Lee S, Chen X. Nanotheranostics for personalized medicine. Expert Rev Mol Diagn. 2013;13:257–69. doi: 10.1586/erm.13.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med. 2012;18:829–34. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–40. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 94.Kraft JC, Freeling JP, Wang Z, Ho RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103:29–52. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kreuter J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB) J Microencapsul. 2013;30:49–54. doi: 10.3109/02652048.2012.692491. [DOI] [PubMed] [Google Scholar]
- 96.Kreuter J. Nanoparticles--a historical perspective. Int J Pharm. 2007;331:1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 97.Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles) Brain Res. 1995;674:171–4. doi: 10.1016/0006-8993(95)00023-j. [DOI] [PubMed] [Google Scholar]
- 98.Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 2007;130:2596–606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- 99.Kwiatkowska A, Nandhu MS, Behera P, Chiocca EA, Viapiano MS. Strategies in gene therapy for glioblastoma. Cancers. 2013;5:1271–305. doi: 10.3390/cancers5041271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Langer R. Biomaterials and biotechnology: From the discovery of the first angiogenesis inhibitors to the development of controlled drug delivery systems and the foundation of tissue engineering. J Biomed Mater Res Part A. 2013;101:2449–55. doi: 10.1002/jbm.a.34811. [DOI] [PubMed] [Google Scholar]
- 101.Leten C, Struys T, Dresselaers T, Himmelreich U. In vivo and ex vivo assessment of the blood brain barrier integrity in different glioblastoma animal models. J Neurooncol. 2014;119:297–306. doi: 10.1007/s11060-014-1514-2. [DOI] [PubMed] [Google Scholar]
- 102.Li LC, Okino ST, Zhao H, Pookot D, Place RF, Urakami S, et al. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006;103:17337–42. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li ML, Wang JC, Schwartz JA, Gill-Sharp KL, Stoica G, Wang LV. In-vivo photoacoustic microscopy of nanoshell extravasation from solid tumor vasculature. J Biomed Opt. 2009;14:010507. doi: 10.1117/1.3081556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L, Ni F, Zhang J, Wang C, Lu X, Guo Z, et al. Thermal analysis in the rat glioma model during directly multipoint injection hyperthermia incorporating magnetic nanoparticles. J Nanosci Nanotechnol. 2011;11:10333–8. doi: 10.1166/jnn.2011.5010. [DOI] [PubMed] [Google Scholar]
- 105.Liu P, Huang Z, Chen Z, Xu R, Wu H, Zang F, et al. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale. 2013;5:11829–36. doi: 10.1039/c3nr01351k. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30:4195–202. doi: 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 107.Lu W. Adsorptive-mediated brain delivery systems. Curr Pharm Biotechnol. 2012;13:2340–8. doi: 10.2174/138920112803341851. [DOI] [PubMed] [Google Scholar]
- 108.Lu W, Tan YZ, Hu KL, Jiang XG. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int J Pharm. 2005;295:247–60. doi: 10.1016/j.ijpharm.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 109.Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther. 2006;5:3162–9. doi: 10.1158/1535-7163.MCT-06-0480. [DOI] [PubMed] [Google Scholar]
- 110.Maeda HG, Fang J. EPR effect and polymeric drugs: A paradigm shift for cancer chemotherapy in the 21st century. Adv Polym Sci. 2006;193:103–21. [Google Scholar]
- 111.Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81:53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 112.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317–24. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mali P, Yang LH, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-Guided Human Genome Engineering via Cas9. Science. 2013;339:823–6. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65:11631–8. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 115.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 116.Mattix B, Moore T, Uvarov O, Pollard S, O’Donnell L, Park K, et al. Effects of polymeric nanoparticle surface properties on interaction with brain tumor environment. Nano Life. 2013;3:1343003. doi: 10.1142/S1793984413430034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Maurizi L, Sakulkhu U, Gramoun A, Vallee JP, Hofmann H. A fast and reproducible method to quantify magnetic nanoparticle biodistribution. Analyst. 2014;139:1184–91. doi: 10.1039/c3an02153j. [DOI] [PubMed] [Google Scholar]
- 118.Meyers JD, Doane T, Burda C, Basilion JP. Nanoparticles for imaging and treating brain cancer. Nanomedicine. 2013;8:123–43. doi: 10.2217/nnm.12.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moan J, Juzenas P. Singlet oxygen in photosensitization. J Environ Pathol Toxicol Oncol. 2006;25:29–50. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 120.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–600. [PubMed] [Google Scholar]
- 121.Mok H, Zhang M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin Drug Deliv. 2013;10:73–87. doi: 10.1517/17425247.2013.747507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nguyen DN, Mahon KP, Chikh G, Kim P, Chung H, Vicari AP, et al. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc Natl Acad Sci U S A. 2012;109:E797–803. doi: 10.1073/pnas.1121423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Donoghue JA, Wheldon TE. Targeted radiotherapy using Auger electron emitters. Phys Med Biol. 1996;41:1973–92. doi: 10.1088/0031-9155/41/10/009. [DOI] [PubMed] [Google Scholar]
- 124.Oku N, Yamashita M, Katayama Y, Urakami T, Hatanaka K, Shimizu K, et al. PET imaging of brain cancer with positron emitter-labeled liposomes. Int J Pharm. 2011;403:170–7. doi: 10.1016/j.ijpharm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 125.Olson JJ, Nayak L, Ormond DR, Wen PY, Kalkanis SN, Committee AC. The role of cytotoxic chemotherapy in the management of progressive glioblastoma: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118:501–55. doi: 10.1007/s11060-013-1338-5. [DOI] [PubMed] [Google Scholar]
- 126.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 127.Palanichamy K, Erkkinen M, Chakravarti A. Predictive and prognostic markers in human glioblastomas. Curr Treat Options Oncol. 2006;7:490–504. doi: 10.1007/s11864-006-0024-7. [DOI] [PubMed] [Google Scholar]
- 128.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 129.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9:1909–15. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 130.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9:615–27. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 131.Plotkin M, Gneveckow U, Meier-Hauff K, Amthauer H, Feussner A, Denecke T, et al. 18F-FET PET for planning of thermotherapy using magnetic nanoparticles in recurrent glioblastoma. Int J Hyperthermia. 2006;22:319–25. doi: 10.1080/02656730600734128. [DOI] [PubMed] [Google Scholar]
- 132.Preiner J, Kodera N, Tang J, Ebner A, Brameshuber M, Blaas D, et al. IgGs are made for walking on bacterial and viral surfaces. Nat Commun. 2014;5:4394. doi: 10.1038/ncomms5394. [DOI] [PubMed] [Google Scholar]
- 133.Prime KL, Whitesides GM. Self-assembled organic monolayers-Model systems for studying adsorption of proteins at surfaces. Science. 1991;252:1164–7. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 134.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–80. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rahman M, Hoh B, Kohler N, Dunbar EM, Murad GJ. The future of glioma treatment: Stem cells, nanotechnology and personalized medicine. Future Oncol. 2012;8:1149–56. doi: 10.2217/fon.12.111. [DOI] [PubMed] [Google Scholar]
- 136.Reardon DA, Desjardins A, Peters KB, Vredenburgh JJ, Gururangan S, Sampson JH, et al. Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer. 2011;117:5351–8. doi: 10.1002/cncr.26188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reardon DA, Freeman G, Wu C, Chiocca EA, Wucherpfennig KW, Wen PY, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2014;16:1441–58. doi: 10.1093/neuonc/nou212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ribot E, Bouzier-Sore AK, Bouchaud V, Miraux S, Delville MH, Franconi JM, et al. Microglia used as vehicles for both inducible thymidine kinase gene therapy and MRI contrast agents for glioma therapy. Cancer Gene Ther. 2007;14:724–37. doi: 10.1038/sj.cgt.7701060. [DOI] [PubMed] [Google Scholar]
- 139.Ribot EJ, Miraux S, Konsman JP, Bouchaud V, Pourtau L, Delville MH, et al. In vivo MR tracking of therapeutic microglia to a human glioma model. Nmr Biomed. 2011;24:1361–8. doi: 10.1002/nbm.1699. [DOI] [PubMed] [Google Scholar]
- 140.Roger M, Clavreul A, Venier-Julienne MC, Passirani C, Montero-Menei C, Menei P. The potential of combinations of drug-loaded nanoparticle systems and adult stem cells for glioma therapy. Biomaterials. 2011;32:2106–16. doi: 10.1016/j.biomaterials.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 141.Rouge JL, Hao L, Wu XA, Briley WE, Mirkin CA. Spherical nucleic acids as a divergent platform for synthesizing RNA–nanoparticle conjugates through enzymatic ligation. ACS Nano. 2014;8:8837–43. doi: 10.1021/nn503601s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rytting M. Peg-asparaginase for acute lymphoblastic leukemia. Expert Opin Biol Ther. 2010;10:833–9. doi: 10.1517/14712591003769808. [DOI] [PubMed] [Google Scholar]
- 143.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–55. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: Focus on cancer. Int J Nanomed. 2014;9:467–83. doi: 10.2147/IJN.S36654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Santos T, Fang X, Chen MT, Wang W, Ferreira R, Jhaveri N, et al. Sequential administration of carbon nanotubes and near-infrared radiation for the treatment of gliomas. Front Oncol. 2014;4:180. doi: 10.3389/fonc.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sarin H, Kanevsky AS, Wu H, Sousa AA, Wilson CM, Aronova MA, et al. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51. doi: 10.1186/1479-5876-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14:2900–8. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Senpan A, Caruthers SD, Rhee I, Mauro NA, Pan D, Hu G, et al. Conquering the dark side: Colloidal iron oxide nanoparticles. ACS Nano. 2009;3:3917–26. doi: 10.1021/nn900819y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen Y, Zhou Z, Sui M, Tang J, Xu P, Van Kirk EA, et al. Charge-reversal polyamidoamine dendrimer for cascade nuclear drug delivery. Nanomedicine. 2010;5:1205–17. doi: 10.2217/nnm.10.86. [DOI] [PubMed] [Google Scholar]
- 150.Shriver LP, Koudelka KJ, Manchester M. Viral nanoparticles associate with regions of inflammation and blood brain barrier disruption during CNS infection. J Neuroimmunol. 2009;211:66–72. doi: 10.1016/j.jneuroim.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Silva AC, Oliveira TR, Mamani JB, Malheiros SM, Malavolta L, Pavon LF, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomed. 2011;6:591–603. doi: 10.2147/IJN.S14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Simon LC, Sabliov CM. The effect of nanoparticle properties, detection method, delivery route and animal model on poly (lactic-co-glycolic) acid nanoparticles biodistribution in mice and rats. Drug Metab Rev. 2014;46:128–41. doi: 10.3109/03602532.2013.864664. [DOI] [PubMed] [Google Scholar]
- 153.Stea B, Cetas TC, Cassady JR, Guthkelch AN, Iacono R, Lulu B, et al. Interstitial thermoradiotherapy of brain tumors: Preliminary results of a phase I clinical trial. Int J Radiat Oncol Biol Phys. 1990;19:1463–71. doi: 10.1016/0360-3016(90)90359-r. [DOI] [PubMed] [Google Scholar]
- 154.Stea B, Rossman K, Kittelson J, Shetter A, Hamilton A, Cassady JR. Interstitial irradiation versus interstitial thermoradiotherapy for supratentorial malignant gliomas: A comparative survival analysis. Int J Radiat Oncol Biol Phys. 1994;30:591–600. doi: 10.1016/0360-3016(92)90945-e. [DOI] [PubMed] [Google Scholar]
- 155.Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS, et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109:759–67. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 156.Stephen ZR, Kievit FM, Veiseh O, Chiarelli PA, Fang C, Wang K, et al. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O-benzylguanine to brain tumors. ACS Nano. 2014;8:10383–95. doi: 10.1021/nn503735w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stephen ZR, Kievit FM, Zhang M. Magnetite nanoparticles for Medical MR Imaging. Mater Today. 2011;14:330–8. doi: 10.1016/S1369-7021(11)70163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stojanov K, Zuhorn IS, Dierckx RA, de Vries EF. Imaging of cells and nanoparticles: Implications for drug delivery to the brain. Pharm Res. 2012;29:3213–34. doi: 10.1007/s11095-012-0826-1. [DOI] [PubMed] [Google Scholar]
- 159.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 160.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 161.Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): A novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013;113:495–503. doi: 10.1007/s11060-013-1142-2. [DOI] [PubMed] [Google Scholar]
- 162.Tzeng SY, Green JJ. Therapeutic nanomedicine for brain cancer. Ther Deliv. 2013;4:687–704. doi: 10.4155/tde.13.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB) Eur J Pharm Biopharm. 2009;71:251–6. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 164.Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood-brain barrier (BBB) J Drug Target. 2011;19:125–32. doi: 10.3109/10611861003734001. [DOI] [PubMed] [Google Scholar]
- 165.van Landeghem FK, Maier-Hauff K, Jordan A, Hoffmann KT, Gneveckow U, Scholz R, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–7. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 166.van Rooy I, Mastrobattista E, Storm G, Hennink WE, Schiffelers RM. Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain. J Control Release. 2011;150:30–6. doi: 10.1016/j.jconrel.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 167.Varallyay P, Nesbit G, Muldoon LL, Nixon RR, Delashaw J, Cohen JI, et al. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am J Neuroradiol. 2002;23:510–9. [PMC free article] [PubMed] [Google Scholar]
- 168.Veiseh O, Gunn JW, Kievit FM, Sun C, Fang C, Lee JS, et al. Inhibition of tumor-cell invasion with chlorotoxin-bound superparamagnetic nanoparticles. Small. 2009;5:256–64. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Veiseh O, Kievit FM, Fang C, Mu N, Jana S, Leung MC, et al. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials. 2010;31:8032–42. doi: 10.1016/j.biomaterials.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Veiseh O, Kievit FM, Mok H, Ayesh J, Clark C, Fang C, et al. Cell transcytosing poly-arginine coated magnetic nanovector for safe and effective siRNA delivery. Biomaterials. 2011;32:5717–25. doi: 10.1016/j.biomaterials.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Veiseh O, Sun C, Fang C, Bhattarai N, Gunn J, Kievit F, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009;69:6200–7. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 173.Wang CX, Huang LS, Hou LB, Jiang L, Yan ZT, Wang YL, et al. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009;1261:91–9. doi: 10.1016/j.brainres.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 174.Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat Biotechnol. 2003;21:803–6. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 175.Wankhede M, Bouras A, Kaluzova M, Hadjipanayis CG. Magnetic nanoparticles: An emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol. 2012;5:173–86. doi: 10.1586/ecp.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Weinstein S, Peer D. RNAi nanomedicines: Challenges and opportunities within the immune system. Nanotechnology. 2010;21:232001. doi: 10.1088/0957-4484/21/23/232001. [DOI] [PubMed] [Google Scholar]
- 177.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–22. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 179.Wismeth C, Dudel C, Pascher C, Ramm P, Pietsch T, Hirschmann B, et al. Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas: Phase I clinical results. J Neurooncol. 2010;98:395–405. doi: 10.1007/s11060-009-0093-0. [DOI] [PubMed] [Google Scholar]
- 180.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 181.Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64:686–700. doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 182.Xi G, Robinson E, Mania-Farnell B, Vanin EF, Shim KW, Takao T, et al. Convection-enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatment. Nanomedicine. 2014;10:381–91. doi: 10.1016/j.nano.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 183.Xin H, Sha X, Jiang X, Zhang W, Chen L, Fang X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33:8167–76. doi: 10.1016/j.biomaterials.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 184.Xu P, Van Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew Chem Int Ed Engl. 2007;46:4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- 185.Yang W, Liu SJ, Bai T, Keefe AJ, Zhang L, Ella-Menye JR, et al. Poly (carboxybetaine) nanomaterials enable long circulation and prevent polymer-specific antibody production. Nano Today. 2014;9:10–6. [Google Scholar]
- 186.Yi GQ, Gu B, Chen LK. The safety and efficacy of magnetic nano-iron hyperthermia therapy on rat brain glioma. Tumour Biol. 2014;35:2445–9. doi: 10.1007/s13277-013-1324-8. [DOI] [PubMed] [Google Scholar]
- 187.Yoshida J, Mizuno M, Fujii M, Kajita Y, Nakahara N, Hatano M, et al. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum Gene Ther. 2004;15:77–86. doi: 10.1089/10430340460732472. [DOI] [PubMed] [Google Scholar]
- 188.Yuan Z, Jiang H. Photoacoustic tomography for imaging nanoparticles. Methods Mol Biol. 2010;624:309–24. doi: 10.1007/978-1-60761-609-2_21. [DOI] [PubMed] [Google Scholar]
- 189.Zhan C, Wei X, Qian J, Feng L, Zhu J, Lu W. Co-delivery of TRAIL gene enhances the anti-glioblastoma effect of paclitaxel in vitro and in vivo. J Control Release. 2012;160:630–6. doi: 10.1016/j.jconrel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 190.Zhang J, Jin C, He ZZ, Liu J. Numerical simulations on conformable laser-induced interstitial thermotherapy through combined use of multi-beam heating and biodegradable nanoparticles. Lasers Med Sci. 2014;29:1505–16. doi: 10.1007/s10103-014-1558-8. [DOI] [PubMed] [Google Scholar]
- 191.Zhang J, Shin MC, Yang VC. Magnetic targeting of novel heparinized iron oxide nanoparticles evaluated in a 9L-glioma mouse model. Pharm Res. 2014;31:579–92. doi: 10.1007/s11095-013-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang XD, Wu D, Shen X, Chen J, Sun YM, Liu PX, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials. 2012;33:6408–19. doi: 10.1016/j.biomaterials.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 193.Zhang Y, Zhang YF, Bryant J, Charles A, Boado RJ, Pardridge WM. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res. 2004;10:3667–77. doi: 10.1158/1078-0432.CCR-03-0740. [DOI] [PubMed] [Google Scholar]
- 194.Zhang Y, Zhu C, Pardridge WM. Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol Ther. 2002;6:67–72. doi: 10.1006/mthe.2002.0633. [DOI] [PubMed] [Google Scholar]
- 195.Zhao D, Alizadeh D, Zhang L, Liu W, Farrukh O, Manuel E, et al. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin Cancer Res. 2011;17:771–82. doi: 10.1158/1078-0432.CCR-10-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou J, Zhang J, Gao W. Enhanced and selective delivery of enzyme therapy to 9L-glioma tumor via magnetic targeting of PEG-modified, beta-glucosidase-conjugated iron oxide nanoparticles. Int J Nanomed. 2014;9:2905–17. doi: 10.2147/IJN.S59556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou Z, Sun Y, Shen J, Wei J, Yu C, Kong B, et al. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials. 2014;35:7470–8. doi: 10.1016/j.biomaterials.2014.04.063. [DOI] [PubMed] [Google Scholar]
- 198.Zhou ZS, Tang J, Fan M, Van Kirk EA, Murdoch WJ, Radosz M. Charge-reversal drug conjugate for targeted cancer cell nuclear drug delivery. Adv Funct Mater. 2009;19:3580–9. [Google Scholar]


