Abstract
OBJECTIVE
This study analyzed the lifetime health care expenditures and life years lost associated with diabetes in the U.S.
RESEARCH DESIGN AND METHODS
Data from the National Health Interview Survey (NHIS), the Medical Expenditure Panel Survey from 1997 to 2000, and the NHIS Linked Mortality Public-use Files with a mortality follow-up to 2006 were used to estimate age-, race-, sex-, and BMI-specific risk of diabetes, mortality, and annual health care expenditures for both patients with diabetes and those without diabetes. A Markov model populated by the risk and cost estimates was used to compute life years and total lifetime health care expenditures by age, race, sex, and BMI classifications for patients with diabetes and without diabetes.
RESULTS
Predicted life expectancy for patients with diabetes and without diabetes demonstrated an inverted U shape across most BMI classifications, with highest life expectancy being for the overweight. Lifetime health care expenditures were higher for whites than blacks and for females than males. Using U.S. adults aged 50 years as an example, we found that diabetic white females with a BMI >40 kg/m2 had 17.9 remaining life years and lifetime health expenditures of $185,609, whereas diabetic white females with normal weight had 22.2 remaining life years and lifetime health expenditures of $183,704.
CONCLUSIONS
Our results show that diabetes is associated with large decreases in life expectancy and large increases in lifetime health care expenditures. In addition to decreasing life expectancy by 3.3 to 18.7 years, diabetes increased lifetime health care expenditures by $8,946 to $159,380 depending on age-race-sex-BMI classification groups.
Introduction
The prevalence of diabetes has imposed a substantial health and economic burden to patients and society. It is a public health concern that the prevalence of diabetes remains high in high-income countries and has been increasing in low- and middle-income countries. In 2011, it was estimated that there were a total of 366 million people living with diabetes in the world, and this is expected to increase to 552 million by 2030 (1).
In the U.S., the prevalence of diabetes among adults aged 18 years or older has risen 11-fold in the last five decades (from 0.63% in 1958 to 7.0% in 2010) (2) and has increased by 150% in the last two decades (from 3.6% in 1991 to 9.0% in 2011) (3). In 2011, it was reported that 20.9 million Americans were diagnosed with diabetes (3). Data show a doubling of the incidence and prevalence of diabetes during 1990–2008 and a leveling off during 2008–2012 (4). In 2010, diabetes was estimated to be the seventh leading cause of death in the U.S. (5). In terms of medical expenditures, diabetes was a leading economic burden among heart disease, cancer, asthma, and hypertension in 2010 (6). The estimated economic cost of diabetes in 2012 was $245 billion, a 41% increase from a 2007 estimate of $174 billion (7).
The dramatic increase in the prevalence of diabetes is closely related to the increase in the number of overweight and obese populations (8). Studies have shown that overweight and obesity are key risk factors for the development of diabetes (8,9). The health and economic consequences of obesity (10,11) and diabetes (7,12–14) have been extensively studied separately, but little is known about the lifetime risk of obesity and diabetes in terms of life years lost and lifetime economic costs.
The aim of our study is to examine the burden of diabetes on the expected remaining life years and the associated expected health care expenditures for individuals by age, race, sex, and BMI. Previous studies such as by Narayan et al. (12) and Morgan et al. (14) have examined life years lost associated with diabetes by age, race, and sex. However, they did not take into account the effect of BMI, which impacts life expectancy through other chronic conditions. Because diabetes is closely related to degree of obesity, it is important to incorporate the impact of BMI in the analysis, in addition to age, race, and sex differences. Finkelstein et al. (15) computed life years lost associated with overweight and obesity but was not focused on life years lost associated with diabetes. In terms of cost estimation, various studies have provided estimates of economic costs of diabetes in the U.S. Zhuo et al. (16) attempted to estimate the economic burden of diabetes; however, their study only examined the direct medical costs of treating type 2 diabetes and diabetes complications. Using 2001–2011 claims data, Østbye et al. (17) examined the relationship between BMI and costs by major diagnostic categories, which included kidney and urinary tract diseases, controlling for age, sex, and race/ethnicity. However, none of the existing studies examined the total lifetime health care expenditures associated with diabetes by age-race-sex-BMI classification groups. Birnbaum et al. (18) estimated the incremental lifetime medical cost of treating a woman with diabetes, but they focused on women only and did not stratify their analysis by age, race, and BMI. A recent study by Zhuo et al. (19) estimated the excess lifetime medical spending attributed to diabetes, but their analysis was stratified by sex and age at diagnosis only. Daviglus et al. (20) and Lakdawalla et al. (21) studied the consequences of obesity on total health care cost and expenditures; however, the former was not focused on diabetes-related expenditures, and the latter only focused on diabetes-related average medical charges in people above the age of 65 years. The American Diabetes Association provided estimates of total lifetime health care expenditures associated with diabetes, but their study focused on the variations of lifetime health care expenditures of diabetes by age-group only (7). Our study is the first to investigate life years lost and lifetime health care expenditures associated with diabetes by age, race, sex, and BMI.
Research Design and Methods
Data
Our data were extracted from 1) the Sample Adult Data Files from the National Health Interview Survey (NHIS), a national probability sample of U.S., civilian, noninstitutionalized adults aged 18 years and older (22); 2) the NHIS Linked Mortality Public-use Files, which provide mortality follow-up data for the NHIS sample up to 31 December 2006 (23); and 3) the Household Component of the Medical Expenditure Panel Survey (MEPS-HC), which is a survey conducted by the Agency for Healthcare Research and Quality that provides detailed individual medical expenditure data (24). The NHIS is an ongoing, continuous, nationwide, cross-sectional survey of the U.S. population conducted by the National Center for Health Statistics and the Bureau of Census. A multistage probability sampling strategy is used each year to select households and individuals for the sample in the NHIS (22).
Personal identification numbers of the sample were used to link the individuals between the NHIS and the NHIS Linked Mortality Public-use Files (23). The individuals were further linked to the MEPS-HC. The set of households selected for each panel of the MEPS-HC is a subsample of households participating in the previous year’s NHIS; it provides nationally representative estimates of health care use, expenditures, sources of payment, and health insurance coverage (24).
The sample in our study was retrieved from NHIS years 1997–2000. We combined 4 years of data to increase our sample size to allow for stratified analyses (25). The NHIS years 1997–2000 were chosen to allow a longer mortality follow-up period to estimate the probability of death for patients with diabetes and those without diabetes. The exclusion criteria were as follows: 1) individuals with any missing data on the target variables; 2) individuals who have ever been diagnosed with cancer, because their BMI levels are less stable due to cancer treatments and appetite loss (11); 3) women pregnant at the time of survey, because BMI levels are unstable during pregnancy (11,26); and 4) underweight individuals, because this group may include heavy smokers, those with severe chronic diseases, and people with malignancies (26,27).
Risk Estimation
The probability of developing diabetes and the probability of death for individuals with and without diabetes were estimated by fitting an exponential survival function, controlling for sex, race (white, black, and other races), diabetes status, age at survey (age ≤19, 20≤ age ≤29, 30≤ age ≤39, 40≤ age ≤49, 50≤ age ≤59, 60≤ age ≤69, and age ≥70 years), and BMI. BMI was categorized based on the standards established by the World Health Organization: normal weight (18.5≤ BMI <25 kg/m2); overweight (25≤ BMI <30 kg/m2); and class I (30≤ BMI <35 kg/m2), class II (35≤ BMI <40 kg/m2), and class III (BMI ≥40 kg/m2) obese (28). In addition to the aforementioned covariates, the probabilities of death for individuals with and without diabetes were estimated by controlling for insurance status (with or without any form of health insurance), duration, and squared duration of diabetes.
Cost Estimation
Because a significant fraction of individuals (13.0%) had zero medical expenditures, the age-, race-, sex-, and BMI-specific annual health care expenditures for both patients with diabetes and those without diabetes were estimated using a two-part model (29,30). We estimated a logistic model in the first part and a generalized linear model with log-link and gamma-variance function for the second part, controlling for sex, race, BMI, age, diabetes status, insurance status, duration of diabetes, and squared duration of diabetes.
Markov Model
A three-state (no diabetes, diabetes, and death) Markov model was built. Transitions between states took place at a discrete interval of 1 year. The predicted values of age-, race-, sex-, and BMI-specific probabilities of developing diabetes, the probabilities of death, and the estimated annual health care expenditures with and without diabetes were used to populate the Markov model to compute life years lost and medical expenditure differentials between patients with diabetes and without diabetes. The probability of recovering from diabetes is small (31); therefore, it was assumed to be zero in this study.
The model was bootstrapped 1,000 times and the means and SEs of the following were computed for each age-race-sex-BMI classification group: 1) expected remaining life years, 2) expected total health care expenditures incurred during the remaining life years, 3) life years lost associated with diabetes, and 4) cost differentials between patients with diabetes and without diabetes. Life years lost associated with diabetes was calculated by subtracting the remaining life years of the individuals without diabetes with those of the patients with diabetes for each age-race-sex-BMI classification group. Cost differentials were obtained by taking the difference in lifetime medical costs between the patients with diabetes and those without diabetes. ANOVA tests have been conducted to compare the means of the predicted life years lost and cost differentials across age-race-sex-BMI classification groups (see details in Supplementary Table 20).
All estimations were adjusted for the complex sampling design in the NHIS (22). All dollar values are presented in U.S. dollars and at 2010 price levels, based on the Consumer Price Index for All Urban Consumers. Future costs were discounted to present values using an annual rate of 3%. STATA (StataCorp 2012, College Station, TX) was used to estimate risk probabilities and annual health care expenditures, and MATLAB (MATLAB Release 2012b; MathWorks, Natick, MA) was used to perform the Markov cohort analysis.
Results
Descriptive Statistics
Table 1 presents the summary statistics of our sample and the estimated population for both NHIS and MEPS data from 1997 to 2000. The NHIS sample contained 110,844 individuals, representing a population of 168,741,800 U.S. adults. Within the estimated population, 50.8% were male, 81.3% were white, 11.6% were black, and 7.1% were other races (including 2.3% Chinese, 1.4% Filipino, 0.7% Asian Indian, and 0.6% Indian American). The average BMI of the population estimates was 26.7 kg/m2; 42.5% were normal weight; 36.4% were overweight; and 14.4, 4.5, and 2.3% of the sample belonged to class I, II, and III obese, respectively. About 6.0% of the sample reported ever being diagnosed with diabetes. About 85% of the sample had health insurance coverage of any form. The mean age at diagnosis of diabetes was 46.8 years. The mean duration of diabetes was 9.25 years. The mean age at death was 70.6 years.
Table 1.
Descriptive statistics for U.S. adults in the sample and the population for NHIS and MEPS data, 1997–2000
| NHIS and mortality data | MEPS data | |||||||
|---|---|---|---|---|---|---|---|---|
| n* (%) | N† (%) | n* (%) | N† (%) | |||||
| Total | 110,844 | 168,741,800 | 29,369 | 80,361,966 | ||||
| Sex | ||||||||
| Male | 50,887 (45.9) | 85,684,842 (50.8) | 13,432 (45.7) | 38,358,002 (47.7) | ||||
| Female | 59,957 (54.1) | 83,056,958 (49.2) | 15,937 (54.3) | 42,003,964 (52.3) | ||||
| Race | ||||||||
| White | 86,110 (77.7) | 137,141,051 (81.3) | 16,426 (55.9) | 47,951,365 (59.7) | ||||
| Black | 15,933 (14.4) | 19,617,639 (11.6) | 3,154 (10.7) | 7,466,723 (9.3) | ||||
| Others | 8,801 (7.9) | 11,983,110 (7.1) | 9,789 (33.3) | 24,943,878 (31.0) | ||||
| BMI classification | ||||||||
| Normal weight | 46,585 (42.0) | 71,673,006 (42.5) | 11,778 (40.1) | 34,246,694 (42.6) | ||||
| Overweight | 40,087 (36.2) | 61,341,287 (36.4) | 10,767 (36.7) | 28,995,809 (36.1) | ||||
| Class I obese | 16,202 (14.6) | 24,239,950 (14.4) | 4,524 (15.4) | 11,517,485 (14.3) | ||||
| Class II obese | 5,145 (4.6) | 7,583,885 (4.5) | 1,469 (5.0) | 3,584,154 (4.5) | ||||
| Class III obese | 2,825 (2.6) | 3,903,572 (2.3) | 831 (2.8) | 2,017,824 (2.5) | ||||
| Ever diagnosed with diabetes | 7,368 (6.7) | 10,087,533 (6.0) | 2,078 (7.1) | 4,902,475 (6.1) | ||||
| Insured‡ | 92,340 (83.5) | 142,933,464 (84.9) | 25,404 (86.5) | 71,101,764 (88.5) | ||||
| NHIS | n | N | Min | Max | Mean | SD | ||
| BMI (kg/m2) | 110,844 | 168,741,800 | 18.5 | 85.8 | 26.7 | 5.29 | ||
| Age at diabetes diagnosis§ | 6,254 | 8,547,464 | 1 | 84 | 46.8 | 17.2 | ||
| Duration of diabetes‖ | 6,254 | 8,547,464 | 0 | 83 | 9.25 | 11.8 | ||
| Age at death | 6,071 | 7,903,792 | 20 | 93 | 70.6 | 16.2 | ||
| MEPS | n* (%) | N† (%) | ||||||
| Total annual health care expenditures at 2010 price levels | ||||||||
| $0 | 4,209 (14.3) | 10,172,135 (13.1) | ||||||
| $1-$499 | 7,572 (25.8) | 20,313,181 (26.0) | ||||||
| $500-$999 | 4,007 (13.6) | 11,009,942 (14.1) | ||||||
| $1,000–$1,499 | 2,622 (8.9) | 7,430,583 (9.4) | ||||||
| $1,500–$1,999 | 1,815 (6.2) | 4,954,863 (6.3) | ||||||
| $2,000–$2,499 | 1,342 (4.6) | 3,620,516 (4.7) | ||||||
| $2,500–$2,999 | 1,041 (3.5) | 2,917,876 (3.7) | ||||||
| $3,000–$3,499 | 821 (2.8) | 2,206,638 (2.8) | ||||||
| $3,500–$3,999 | 677 (2.3) | 1,783,732 (2.3) | ||||||
| $4,000–$4,499 | 560 (1.9) | 1,438,713 (1.9) | ||||||
| $4,500–$4,999 | 485 (1.7) | 1,365,389 (1.7) | ||||||
| >$5,000 | 4,218 (14.4) | 10,984,086 (14.1) | ||||||
| n | N | Min | Max | Mean | SD | |||
| Total health care expenditures at 2010 price levels¶ | 29,369 | 80,361,966 | 0 | $349,748 | $3,770.3 | $10,204.7 | ||
n, sample size.
N, estimated population size.
Individuals with any form of health insurance coverage.
Age first diagnosed with diabetes.
Duration of diabetes, defined as the difference between age at interview and age first diagnosed with diabetes.
Based on the Consumer Price Index for All Urban Consumers.
Table 1 also shows the summary statistics for the MEPS sample. It contained 29,369 individuals, representing 80,361,966 U.S. adults. Within the estimated population, 47.7% were male, 59.7% were white, 9.3% were black, and 31.0% were other races. The distribution of the sample across BMI classifications in the MEPS was comparable with that in the NHIS, where 42.6% were normal weight; 36.1% were overweight; and 14.3, 4.5, and 2.5% of the sample belonged to class I, II, and III obese, respectively. About 89% were insured in the sample. The mean of total health care expenditures was $3,770 with an SD of $10,205. Whereas 13.0% of the sample had $0 annual health care expenditures, the majority (26.0%) spent $1–$499 on health care annually.
Predicted Life Years Lost Associated With Diabetes
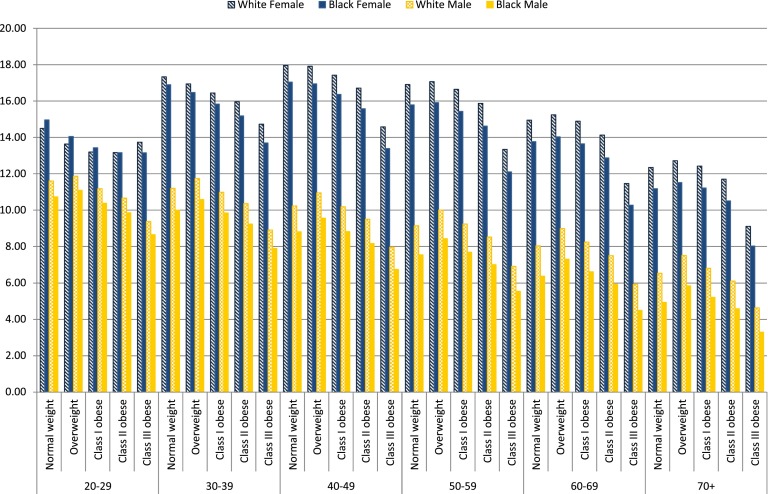
Tables 2 and 3 show the bootstrapped means and SEs of life years lost and the health care expenditure differentials associated with diabetes across age-race-sex-BMI classification groups for insured U.S. adults. Figure 1 demonstrates the patterns of life years lost associated with diabetes. Our results showed that whites lost more life years than blacks. Females lost more life years than males. White females, on average (computed as the arithmetic mean across age-race-sex-BMI classification groups), lost 14.77 life years compared with 13.90 life years for black females. White males lost 9.03 life years compared with 7.71 life years for black males. The life years lost associated with diabetes was higher for females than males. The average difference in life years lost was 5.74 years between white females and white males, 6.19 years between black females and black males, and 5.54 years between females and males of other races.
Table 2.
Bootstrapped means and SEs of life years lost associated with diabetes for insured U.S. adults, 1997–2000
| White female |
Black female |
Other female |
White male |
Black male |
Other male |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age‡ | BMI class | LYL | SE | LYL | SE | LYL | SE | LYL | SE | LYL | SE | LYL | SE |
| 20–29 | Normal weight | 14.50 | 2.69 | 14.96 | 2.88 | 13.16 | 3.96 | 11.60 | 2.82 | 10.73 | 3.11 | 11.86 | 4.07 |
| Overweight | 13.64 | 2.65 | 14.06 | 2.67 | 12.04 | 3.83 | 11.87 | 2.70 | 11.09 | 3.03 | 11.79 | 4.09 | |
| Class I obese | 13.20 | 2.70 | 13.43 | 2.70 | 11.50 | 3.86 | 11.18 | 3.02 | 10.38 | 3.22 | 11.00 | 4.22 | |
| Class II obese | 13.16 | 3.05 | 13.16 | 2.83 | 11.52 | 3.88 | 10.66 | 3.62 | 9.86 | 3.76 | 10.51 | 4.33 | |
| Class III obese | 13.74 | 2.79 | 13.15 | 2.56 | 12.70 | 3.77 | 9.38 | 4.15 | 8.65 | 4.06 | 9.05 | 4.38 | |
| 30–39 | Normal weight | 17.33 | 1.98 | 16.90 | 2.19 | 17.21 | 3.28 | 11.20 | 2.50 | 10.00 | 2.74 | 11.81 | 3.81 |
| Overweight | 16.94 | 1.98 | 16.48 | 2.01 | 16.53 | 3.25 | 11.74 | 2.43 | 10.58 | 2.70 | 12.14 | 3.92 | |
| Class I obese | 16.44 | 2.05 | 15.83 | 2.07 | 15.94 | 3.27 | 10.98 | 2.75 | 9.84 | 2.89 | 11.24 | 4.05 | |
| Class II obese | 15.96 | 2.38 | 15.18 | 2.22 | 15.54 | 3.23 | 10.36 | 3.35 | 9.24 | 3.42 | 10.60 | 4.16 | |
| Class III obese | 14.73 | 2.25 | 13.70 | 2.10 | 14.74 | 3.10 | 8.90 | 3.85 | 7.89 | 3.68 | 8.96 | 4.22 | |
| 40–49 | Normal weight | 17.96 | 1.57 | 17.04 | 1.84 | 18.74 | 2.74 | 10.23 | 2.27 | 8.81 | 2.45 | 11.06 | 3.59 |
| Overweight | 17.91 | 1.56 | 16.95 | 1.67 | 18.51 | 2.69 | 10.95 | 2.23 | 9.56 | 2.46 | 11.62 | 3.73 | |
| Class I obese | 17.43 | 1.67 | 16.37 | 1.76 | 17.92 | 2.75 | 10.19 | 2.55 | 8.82 | 2.65 | 10.72 | 3.89 | |
| Class II obese | 16.71 | 2.03 | 15.58 | 1.94 | 17.19 | 2.77 | 9.50 | 3.15 | 8.17 | 3.15 | 10.03 | 4.01 | |
| Class III obese | 14.58 | 2.00 | 13.39 | 1.90 | 15.20 | 2.82 | 7.96 | 3.64 | 6.75 | 3.39 | 8.34 | 4.11 | |
| 50–59 | Normal weight | 16.91 | 1.30 | 15.79 | 1.59 | 18.10 | 2.43 | 9.14 | 2.02 | 7.55 | 2.13 | 10.14 | 3.35 |
| Overweight | 17.07 | 1.30 | 15.91 | 1.43 | 18.13 | 2.40 | 10.00 | 2.00 | 8.43 | 2.18 | 10.88 | 3.52 | |
| Class I obese | 16.65 | 1.43 | 15.43 | 1.55 | 17.61 | 2.49 | 9.24 | 2.32 | 7.70 | 2.35 | 10.01 | 3.70 | |
| Class II obese | 15.87 | 1.81 | 14.62 | 1.74 | 16.81 | 2.54 | 8.52 | 2.91 | 7.01 | 2.81 | 9.28 | 3.83 | |
| Class III obese | 13.34 | 1.79 | 12.10 | 1.70 | 14.31 | 2.63 | 6.92 | 3.31 | 5.55 | 2.93 | 7.56 | 3.89 | |
| 60–69 | Normal weight | 14.95 | 1.06 | 13.77 | 1.35 | 16.33 | 2.19 | 8.03 | 1.77 | 6.38 | 1.83 | 9.14 | 3.09 |
| Overweight | 15.25 | 1.08 | 14.03 | 1.23 | 16.54 | 2.19 | 8.98 | 1.79 | 7.31 | 1.92 | 10.02 | 3.30 | |
| Class I obese | 14.89 | 1.22 | 13.65 | 1.36 | 16.11 | 2.29 | 8.24 | 2.10 | 6.62 | 2.09 | 9.19 | 3.49 | |
| Class II obese | 14.13 | 1.62 | 12.88 | 1.57 | 15.31 | 2.36 | 7.51 | 2.67 | 5.93 | 2.52 | 8.45 | 3.63 | |
| Class III obese | 11.46 | 1.60 | 10.28 | 1.52 | 12.63 | 2.44 | 5.93 | 3.00 | 4.50 | 2.55 | 6.76 | 3.69 | |
| 70+ | Normal weight | 12.35 | 0.81 | 11.18 | 1.09 | 13.79 | 1.90 | 6.54 | 1.42 | 4.94 | 1.44 | 7.69 | 2.66 |
| Overweight | 12.72 | 0.83 | 11.52 | 0.98 | 14.11 | 1.91 | 7.52 | 1.47 | 5.85 | 1.55 | 8.64 | 2.90 | |
| Class I obese | 12.43 | 1.00 | 11.22 | 1.13 | 13.77 | 2.03 | 6.82 | 1.74 | 5.22 | 1.69 | 7.86 | 3.07 | |
| Class II obese | 11.70 | 1.37 | 10.51 | 1.33 | 13.01 | 2.10 | 6.11 | 2.27 | 4.59 | 2.07 | 7.13 | 3.20 | |
| Class III obese | 9.11 | 1.33 | 8.03 | 1.26 | 10.32 | 2.13 | 4.64 | 2.49 | 3.30 | 2.03 | 5.53 | 3.19 | |
LYL, life years lost associated with diabetes.
Age refers to the age of patients entering into the Markov model.
Table 3.
Bootstrapped means and SEs of cost differentials (in 2010 U.S. dollars) associated with diabetes for insured U.S. adults, 1997–2000
| White female |
Black female |
Other female |
White male |
Black male |
Other male |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age‡ | BMI class | $* | SE | $ | SE | $ | SE | $ | SE | $ | SE | $ | SE |
| 20–29 | Normal weight | 117,651 | 25,686 | 105,397 | 25,346 | 115,781 | 24,367 | 75,281 | 26,778 | 66,207 | 26,566 | 89,550 | 32,210 |
| Overweight | 117,794 | 25,331 | 104,695 | 25,477 | 114,435 | 24,384 | 76,416 | 25,982 | 68,167 | 25,503 | 89,565 | 30,893 | |
| Class I obese | 137,074 | 31,197 | 120,465 | 29,309 | 131,781 | 29,191 | 81,932 | 30,396 | 72,845 | 30,325 | 94,931 | 35,540 | |
| Class II obese | 145,620 | 38,027 | 126,617 | 36,259 | 139,237 | 35,176 | 80,638 | 30,412 | 70,827 | 28,848 | 93,318 | 34,956 | |
| Class III obese | 113,592 | 32,693 | 97,094 | 29,757 | 109,519 | 31,076 | 127,148 | 73,809 | 109,679 | 64,082 | 145,992 | 82,330 | |
| 30–39 | Normal weight | 111,324 | 24,354 | 98,320 | 24,527 | 112,399 | 24,125 | 80,252 | 29,076 | 69,400 | 28,761 | 97,445 | 35,831 |
| Overweight | 112,910 | 24,372 | 99,080 | 25,038 | 112,727 | 24,539 | 82,742 | 28,356 | 72,440 | 27,731 | 98,958 | 34,564 | |
| Class I obese | 131,787 | 30,286 | 114,576 | 28,994 | 130,417 | 29,613 | 88,197 | 33,264 | 76,827 | 33,025 | 104,481 | 39,939 | |
| Class II obese | 139,193 | 37,370 | 119,860 | 36,135 | 137,159 | 35,918 | 86,170 | 33,296 | 74,388 | 31,457 | 102,067 | 39,311 | |
| Class III obese | 104,837 | 31,498 | 89,136 | 29,116 | 104,104 | 31,277 | 133,672 | 80,908 | 113,148 | 69,682 | 157,507 | 92,647 | |
| 40–49 | Normal weight | 96,031 | 23,347 | 83,397 | 23,484 | 99,795 | 23,987 | 80,697 | 30,540 | 68,017 | 29,674 | 100,526 | 38,544 |
| Overweight | 98,659 | 23,478 | 85,248 | 24,083 | 101,501 | 24,530 | 84,872 | 30,226 | 72,319 | 29,009 | 104,059 | 37,700 | |
| Class I obese | 115,304 | 29,231 | 98,859 | 28,003 | 117,731 | 29,698 | 89,661 | 35,315 | 75,903 | 34,401 | 109,106 | 43,529 | |
| Class II obese | 120,696 | 36,400 | 102,491 | 35,061 | 122,828 | 36,118 | 86,678 | 35,516 | 72,921 | 32,924 | 105,581 | 43,008 | |
| Class III obese | 86,952 | 30,109 | 73,140 | 27,791 | 89,255 | 31,007 | 131,207 | 85,410 | 108,156 | 72,255 | 159,380 | 100,428 | |
| 50–59 | Normal weight | 75,536 | 21,848 | 64,125 | 21,845 | 81,449 | 23,259 | 75,005 | 30,885 | 61,027 | 29,074 | 96,456 | 39,899 |
| Overweight | 78,705 | 22,162 | 66,552 | 22,537 | 84,036 | 23,964 | 80,890 | 31,022 | 66,455 | 28,899 | 102,246 | 39,577 | |
| Class I obese | 91,845 | 27,552 | 77,122 | 26,277 | 97,425 | 29,062 | 84,443 | 36,077 | 68,850 | 34,065 | 106,202 | 45,674 | |
| Class II obese | 94,672 | 34,452 | 78,578 | 32,878 | 100,201 | 35,261 | 80,493 | 36,477 | 65,410 | 32,796 | 101,474 | 45,292 | |
| Class III obese | 63,973 | 27,638 | 52,800 | 25,339 | 68,449 | 29,596 | 118,104 | 85,106 | 94,411 | 70,062 | 148,817 | 103,097 | |
| 60–69 | Normal weight | 48,061 | 18,662 | 38,602 | 18,578 | 55,930 | 21,079 | 60,342 | 28,543 | 45,134 | 25,695 | 82,277 | 38,739 |
| Overweight | 51,451 | 18,990 | 41,312 | 19,154 | 59,093 | 21,769 | 68,207 | 29,340 | 51,972 | 26,357 | 90,716 | 39,199 | |
| Class I obese | 59,638 | 23,829 | 47,518 | 22,595 | 68,207 | 26,699 | 69,427 | 34,002 | 52,078 | 30,768 | 92,445 | 45,273 | |
| Class II obese | 59,159 | 29,814 | 46,106 | 28,061 | 67,959 | 32,058 | 64,168 | 34,175 | 47,780 | 29,497 | 86,146 | 44,591 | |
| Class III obese | 33,364 | 22,901 | 25,405 | 20,688 | 39,996 | 26,271 | 86,628 | 75,475 | 62,322 | 58,890 | 118,050 | 96,609 | |
| 70+ | Normal weight | 28,449 | 15,443 | 21,239 | 15,414 | 36,405 | 18,508 | 47,603 | 25,210 | 33,241 | 22,030 | 68,081 | 35,514 |
| Overweight | 31,488 | 15,797 | 23,674 | 15,928 | 39,473 | 19,225 | 55,878 | 26,320 | 40,094 | 23,075 | 77,514 | 36,487 | |
| Class I obese | 36,132 | 20,094 | 26,880 | 19,028 | 45,258 | 23,856 | 55,788 | 30,606 | 39,118 | 26,882 | 77,883 | 42,384 | |
| Class II obese | 33,971 | 25,442 | 24,128 | 23,718 | 43,315 | 28,599 | 50,337 | 31,047 | 34,865 | 26,025 | 71,190 | 41,930 | |
| Class III obese | 14,168 | 19,046 | 8,946 | 17,022 | 20,764 | 23,166 | 63,462 | 65,640 | 41,448 | 49,297 | 92,515 | 87,517 | |
Health care cost differentials between patients with diabetes and those without diabetes.
Age refers to the age of patients entering into the Markov model.
Figure 1.
Life years lost associated with diabetes for insured population.
In terms of BMI, the life years lost associated with diabetes demonstrated an inverted U shape across BMI for most age-race-sex-BMI classification groups, with the largest number of life years lost in the overweight group. On average, those of normal weight lost 12.44 years (from 4.94 to 18.10 years); the overweight lost 12.70 years (from 5.85 to 18.13 years); and the class I, II, and III obese lost 12.09 years (from 5.22 to 17.92 years), 11.45 years (from 4.59 to 17.19 years), and 9.84 years (from 3.30 to 15.20 years), respectively. The life years lost associated with diabetes decreased with age. The average life years lost for the 20–29 age-group was 11.92 years, and the average years lost for age 70+ was 8.94 years. Uninsured individuals showed a similar pattern in life years lost compared with the insured. In general, people with insurance had around two times higher health care expenditure differentials than people without insurance (see Supplementary Table 17 for bootstrapped means of life years lost associated with diabetes for uninsured individuals).
Health Care Expenditure Differentials Associated With Diabetes
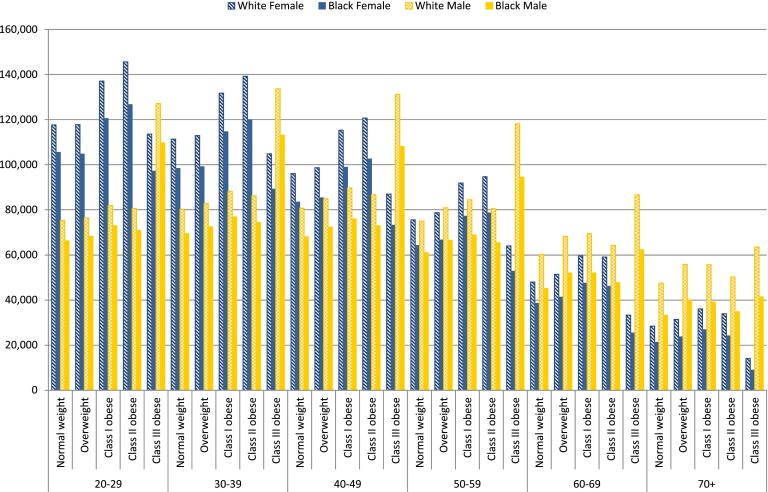
Figure 2 demonstrates the lifetime health care expenditures with and without diabetes for individuals by age-race-sex-BMI classification groups. Lifetime health care expenditure differentials were higher for whites than blacks and for females than males. For example, the average health care cost differentials for white females were $85,001 compared with $81,545 for white males across age and BMI groups. The average health care expenditure differentials were $72,045 for black females and $66,515 for black males.
Figure 2.
Lifetime health care expenditure differentials for insured population.
On average, the health care expenditure differentials were $74,623 (from $21,239 to $117,651) for the normal weight, $77,954 (from $23,674 to $117,794) for the overweight, $85,782 for the class I obese (from $26,880 to $137,074), $85,451 for the class II obese (from $24,128 to $145,620), and $89,087 for the class III obese (from $8,946 to $159,380). The uninsured population showed similar patterns of expenditure differentials across BMI classifications (see Supplementary Table 18 for details of expenditure differentials for uninsured population). ANOVA tests showed that the differences between the means of predicted life years lost are significant across age-race-sex-BMI classification groups. For expenditure differentials, the differences between the means of predicted values are significant across age and race groups (see details in Supplementary Table 20).
Life years lost and lifetime economic costs are two widely accepted measures of population health impact (32,33). We estimated both measures for individuals with and without diabetes by age-race-sex-BMI classification groups that were not previously reported. The results showed that the life years lost and health care expenditures associated with diabetes were substantial, which suggest that diabetes imposes a persistent economic burden over a life span and highlight the potential economic return of diabetes prevention.
We found that diabetes decreased life years by 3.30 to 18.74 years depending on age, sex, race, and BMI. Our findings showed that the life years lost associated with diabetes was greater for females than males and whites than blacks. Life years lost also declined with age. Similarly, Narayan et al. (9) showed that females lost more life years compared with males and life years lost diminished with age; on the other hand, they found that non-Hispanic blacks lost more life years compared with non-Hispanic whites. Using individuals of age 50 years as an example, they found that the life years lost associated with diabetes was 11.8 years for non-Hispanic white females and 13.6 years for non-Hispanic black females, whereas the life years lost was 8.8 years for non-Hispanic white males and 10.1 years for non-Hispanic black males. However, their study differs from ours in several ways: 1) Narayan et al. (9) compared differences in life years lost across age, sex, and race but not BMI; they eliminated obesity as an important risk factor for diabetes; and 2) they classified race as non-Hispanic white, non-Hispanic black, Hispanic, and other, whereas our study classified race as white, black, and racial groups other than white and black.
We also observed that life years lost was about two times higher for females than males. This disparity can be explained by the larger differences in probabilities of death between males and females observed for patients with diabetes relative to those without diabetes. This finding is consistent with the finding in Gregg et al. (34), who used data from the NHIS and demonstrated that the age-adjusted difference in mortality rates between patients with diabetes and without diabetes was higher in men than in women.
Diabetes increased lifetime health care expenditures by $8,946 to $159,380 depending on age, sex, race, and BMI. Our results are comparable with the estimates from previous studies. Zhuo et al. (16) estimated the lifetime direct medical costs of managing type 2 diabetes and treating diabetes complications. They reported that the age-sex weighted average of the lifetime medical costs was $85,200. A recent study by Zhuo et al. (19) estimated the discounted excess lifetime medical spending for people with diabetes ranged from $35,900 to $124,600 depending on the age at diagnosis. Similar to our results, they found that the excess lifetime medical spending associated with diabetes was higher for females than males. However, their estimates were consistently larger in comparison with ours as we modeled disease transition using a Markov model, which allowed individuals without diabetes to develop diabetes in future ages. Additionally, a previous study (7) showed that people with diabetes had medical expenditures ∼2.3 times higher on average than those without diabetes, whereas our estimates showed that remaining lifetime health care expenditures for patients with diabetes were 1.19–2.75 times higher than for those without diabetes, depending on age, sex, race, and BMI.
Our results are consistent with the literature that women have a longer life expectancy, and there is an inverted U-shaped relationship between BMI and life years lost, with the largest life years lost in the overweight for most of the age, sex, and race groups (9). This relationship was also found between BMI and life years lost associated with diabetes, demonstrating that life years lost was the most for the overweight group. Class III obese individuals had the least number of life years lost associated with diabetes, possibly because people with the highest degree of obesity have increased risk of other obesity-related comorbidities in addition to diabetes, like coronary heart disease, hypertension, and stroke, which lowers the life expectancy for class III obese individuals without diabetes (35,36). This leads to smaller life years lost between patients with diabetes and those without diabetes for class III obese individuals. However, on average, class III obese individuals had fewer life years (77.1 years) than those of other BMI groups (80.1 years for the normal weight, 84.9 years for the overweight, and 83.8 and 81.5 years for the class I and II obese).
It should be noted that our conclusions are applicable to the U.S. general population in 1997–2000. This population was chosen because the NHIS Linked Mortality Public-use Files provide the most up-to-date mortality follow-up data from the NHIS interview through 31 December 2006, allowing us to have 6–10 years of mortality follow-up data to estimate the probability of death for patients with diabetes and those without diabetes (23). In addition, all risk and cost estimates were estimated from the same source of nationally representative data and were used to populate a Markov model to predict life years lost and total lifetime health care expenditures by age, race, sex, and BMI. None of these estimates were adapted from other existing studies. Therefore, no additional assumptions were made for the use of published data in populating our model.
The prevalence of diabetes has increased over the last few decades and has shown signs of leveling off in the past few years. As reported by Geiss et al. (4), the prevalence per 100 persons was 3.5 in 1990, 7.9 in 2008, and 8.3 in 2012. It has also been documented that the mortality rate of diabetes has been decreasing over time. Using NHIS 1997–2004, Gregg et al. (34) showed that the excess all-cause 3-year mortality rate associated with diabetes declined by 44% (from 10.8 to 6.1 deaths per 1,000). These trends have led to ambiguous effects on both life years lost associated with diabetes and lifetime health care expenditure differentials. Further investigation using more recent data are needed to analyze the impact of the change in time trends of the risk and probability of death of diabetes on the lifetime cost of diabetes.
Our analysis has several strengths. First, our study focused on the estimation of life years lost and total lifetime medical expenditures associated with diabetes by age, race, sex, and BMI. Our results can inform policy makers on which demographic groups in the population are subject to the greatest impact of life years lost and lifetime health care expenditures associated with diabetes. Second, lifetime burden is provided in our study rather than risk or cost estimates at one point in time. Third, the use of Markov model allowed us to infer the life expectancy and the lifetime health care expenditures over cohorts of different age-race-sex-BMI classification groups, which had not been reported before. Last, instead of adapting estimates from existing studies, which requires stringent assumptions, our model was populated by probabilities that were consistently estimated from the same sources of nationally representative data. Our analysis has several limitations. First, the data from NHIS are self-reported, which may be subject to reporting errors, particularly with respect to BMI, diabetes status, and age of diagnosis. In our study, we used BMI category rather than BMI level, which may mitigate the reporting error in the BMI, although self-reported and measured BMI have been found to be highly correlated (0.9–0.95) and sufficient for epidemiological studies (37). Moreover, there is evidence demonstrating that the accuracy of self-reporting data for diabetes is reasonably high in population surveys (38). Second, literature suggests that average BMI among U.S. adults increases with age, but our analysis could not capture BMI change throughout an individual’s lifetime (39). This might lead to an underestimation of the life years lost and cost differentials of diabetes as the weight of individuals in younger cohorts is likely to increase over time. Third, we did not separately consider type 1 and 2 diabetes, because the data did not differentiate between them. However, previous studies have shown that the majority (95%) of diabetes in the U.S. is type 2 (40). Finally, given changes in secular trends of risk and mortality rates of diabetes, our results cannot be extended to the current period.
Conclusions
We predicted the life years lost and the total health care expenditures associated with diabetes over the life course of individuals by age, race, sex, and BMI in the U.S. general population, 1997–2000. Our results showed that diabetes is associated with large decreases in life expectancy and large increases in lifetime health care expenditures. Diabetes decreased life expectancy by 3.3–18.7 years and increased lifetime health expenditures by $8,946 to $159,380, depending on age-race-sex-BMI classification groups. Life years lost associated with diabetes was greater for females than for males and whites than blacks. For a given age, race, and sex, overweight individuals with diabetes had, on average, lost the most life years, and the class II obese individuals had the largest increase in lifetime health care expenditures.
Our results provide evidence that the health and economic burden of diabetes is substantial over the life span. We find that certain population groups are most susceptible to the health and economic consequences of diabetes over the life course. Policy makers can implement diabetes prevention and intervention policies that target these populations to decrease life years lost and reduce the economic burden of diabetes more effectively.
Article Information
Funding. Funding from the Foundation for Barnes-Jewish Hospital supported this research. G.A.C. is supported in part by U54-CA-155496, the Breast Cancer Research Foundation, and the Foundation for Barnes-Jewish Hospital. S.-H.C. is supported by grants K01-HS-022330 through the Agency for Healthcare Research and Quality and U54-CA-155496 through the National Cancer Institute at the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.-Y.M.L. conceived and designed the study; provided administrative, technical, or material support; analyzed and interpreted data; provided statistical expertise; and drafted and critically revised the manuscript. L.M.P. drafted and critically revised the manuscript. G.A.C. obtained funding; provided administrative, technical, or material support; analyzed and interpreted data; critically revised the manuscript; and supervised the study. S.-H.C. obtained funding; conceived and designed the study; provided administrative, technical, or material support; analyzed and interpreted data; provided statistical expertise; drafted and critically revised the manuscript; and supervised the study. S.-H.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. An earlier version of the information contained in this article was presented as an abstract at the 6th Annual Conference of the Institute for Public Health, Washington University in St. Louis, St. Louis, MO, 15–16 October 2013. The abstract of this article was also presented at the 19th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research, Montreal, QC, Canada, 31 May–4 June 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-1453/-/DC1.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Crude and age-adjusted rate per 100 civilian, noninstitutionalized adults with diagnosed diabetes, United States, 1980-2011 [Internet]. Available from http://www.cdc.gov/diabetes/statistics/prev/national/figageadult.htm. Accessed 25 February 2014
- 3.Centers for Disease Control and Prevention. Number (in millions) of civilian, noninstitutionalized persons with diagnosed diabetes, United States, 1980-2011 [Internet]. Available from http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm. Accessed 25 February 2014
- 4.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014;312:1218–1226 [DOI] [PubMed] [Google Scholar]
- 5.Sherry LMB, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports, Vol. 61, no. 4. Hyattsville, MD, National Center for Health Statistics, 2010 [Google Scholar]
- 6.Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by type of service: United States, 2011. Medical Expenditure Panel Survey Household Component data. Generated interactively. Rockville, MD, Agency for Healthcare Research and Quality, 2011
- 7.American Diabetes Association . Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76–79 [DOI] [PubMed] [Google Scholar]
- 9.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007;30:1562–1566 [DOI] [PubMed]
- 10.Thompson D, Edelsberg J, Colditz GA, Bird AP, Oster G. Lifetime health and economic consequences of obesity. Arch Intern Med 1999;159:2177–2183 [DOI] [PubMed] [Google Scholar]
- 11.Chang SH, Pollack LM, Colditz GA. Life years lost associated with obesity-related diseases for U.S. non-smoking adults. PLoS ONE 2013;8:e66550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 [DOI] [PubMed] [Google Scholar]
- 13.Jönsson B. The economic impact of diabetes. Diabetes Care 1998;21(Suppl. 3):C7–C10 [DOI] [PubMed] [Google Scholar]
- 14.Morgan CL, Currie CJ, Peters JR. Relationship between diabetes and mortality: a population study using record linkage. Diabetes Care 2000;23:1103–1107 [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein EA, Brown DS, Wrage LA, Allaire BT, Hoerger TJ. Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring) 2010;18:333–339 [DOI] [PubMed] [Google Scholar]
- 16.Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med 2013;45:253–261 [DOI] [PubMed] [Google Scholar]
- 17.Østbye T, Stroo M, Eisenstein EL, Peterson B, Dement J. Is overweight and class I obesity associated with increased health claims costs? Obesity 2014;22:1179–1186 [DOI] [PubMed]
- 18.Birnbaum H, Leong S, Kabra A. Lifetime medical costs for women: cardiovascular disease, diabetes, and stress urinary incontinence. Womens Health Issues 2003;13:204–213 [DOI] [PubMed] [Google Scholar]
- 19.Zhuo X, Zhang P, Barker L, Albright A, Thompson TJ, Gregg E. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes Care 2014;37:2557–2564 [DOI] [PubMed] [Google Scholar]
- 20.Daviglus ML, Liu K, Yan LL, et al. Relation of body mass index in young adulthood and middle age to Medicare expenditures in older age. JAMA 2004;292:2743–2749 [DOI] [PubMed] [Google Scholar]
- 21.Lakdawalla DN, Goldman DP, Shang B. The health and cost consequences of obesity among the future elderly. Health Aff (Millwood) 2005;24(Suppl. 2):W5R30–W5R41 [DOI] [PubMed]
- 22.Centers for Disease Control and Prevention. National Health Interview Survey: The Principal Source of Information on the Health of the U.S. Population. Atlanta, GA, Centers for Disease Control and Prevention, 2012
- 23.Centers for Disease Control and Prevention. National Health Interview Survey (NHIS) Public-use Linked Mortality Files: Survey Years 1986-2004. Atlanta, GA, Centers for Disease Control and Prevention, 2010
- 24.Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey (MEPS). Rockville, MD, Agency for Healthcare Research and Quality, September 2012
- 25.Botman SL, Jack SS. Combining National Health Interview Survey Datasets: issues and approaches. Stat Med 1995;14:669–677 [DOI] [PubMed] [Google Scholar]
- 26.Chang SH, Pollack LM, Colditz GA. Obesity, mortality, and life years lost associated with breast cancer in nonsmoking US Women, National Health Interview Survey, 1997-2000. Prev Chronic Dis 2013;10:E186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorogood M, Appleby PN, Key TJ, Mann J. Relation between body mass index and mortality in an unusually slim cohort. J Epidemiol Community Health 2003;57:130–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i–xii, 1–253 [PubMed] [Google Scholar]
- 29.Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ 2001;20:461–494 [DOI] [PubMed] [Google Scholar]
- 31.Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013;258:628–636; discussion 636–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray CJ, Salomon JA, Mathers C. A critical examination of summary measures of population health. Bull World Health Organ 2000;78:981–994 [PMC free article] [PubMed] [Google Scholar]
- 33.Alemayehu B, Warner KE. The lifetime distribution of health care costs. Health Serv Res 2004;39:627–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregg EW, Cheng YJ, Saydah S, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care 2012;35:1252–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–1529 [DOI] [PubMed] [Google Scholar]
- 36.National Institutes of Health . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res 1998;6(Suppl. 2):51S–209S [PubMed] [Google Scholar]
- 37.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring) 2007;15:188–196 [DOI] [PubMed] [Google Scholar]
- 38.O’Connor PJ, Rush WA, Pronk NP, Cherney LM. Identifying diabetes mellitus or heart disease among health maintenance organization members: sensitivity, specificity, predictive value, and cost of survey and database methods. Am J Manag Care 1998;4:335–342 [PubMed] [Google Scholar]
- 39.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA 1994;272:205–211 [DOI] [PubMed] [Google Scholar]
- 40.National Diabetes Data Group (U.S.), National Institute of Diabetes and Digestive and Kidney Diseases (U.S.), National Institutes of Health (U.S.). Diabetes in America. Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 1995 [Google Scholar]