Abstract
Vegetation is a key driver of ecosystem functioning (e.g. productivity and stability) and of the maintenance of biodiversity (e.g. creating habitats for other species groups). While vegetation sensitivity to climate change has been widely investgated, its spatio-temporally response to the dual efects of land management and climate change has been ignored at landscape scale. Here we use a dynamic vegetation model called FATE-HD, which describes the dominant vegetation dynamics and associated functional diversity, in order to anticipate vegetation response to climate and land-use changes in both short and long-term perspectives. Using three contrasted management scenarios for the Ecrins National Park (French Alps) developed in collaboration with the park managers, and one regional climate change scenario, we tracked the dynamics of vegetation structure (forest expansion) and functional diversity over 100 years of climate change and a further 400 additional years of stabilization. As expected, we observed a slow upward shift in forest cover distribution, which appears to be severely impacted by pasture management (i.e. maintenance or abandonment). The tme lag before observing changes in vegetation cover was the result of demographic and seed dispersal processes. However, plant diversity response to environmental changes was rapid. Afer land abandonment, local diversity increased and spatial turnover was reduced, whereas local diversity decreased following land use intensification. Interestingly, in the long term, as both climate and management scenarios interacted, the regional diversity declined. Our innovative spatio-temporally explicit framework demonstrates that the vegetation may have contrasting responses to changes in the short and the long term. Moreover, climate and land-abandonment interact extensively leading to a decrease in both regional diversity and turnover in the long term. Based on our simulations we therefore suggest a continuing moderate intensity pasturing to maintain high levels of plant diversity in this system.
Introduction
Vegetation distribution and diversity are vital for humans, as they are not only playing a direct role in ecosystem services such as fodder or wood production, but also involved in key ecosystem functions (de Bello et al. 2010). Dominant plant species drive ecosystem stability (Smith and Knapp 2003; Sasaki and Lauenroth 2011) and more generally ecosystem functioning (Grime 1998). Plant species richness is known to influence forest productivity (Paquette and Messier 2011) but there is a growing consensus that it is functional diversity rather than species numbers per se that affects ecosystem functions (Diaz and Cabido 2001; Cadotte et al. 2011). For instance, in a savanna biome in South Africa, the loss of entire plant functional groups has been shown to affect biomass and long-term vegetation stability leading to desertification, which is a major concern for wood production and biological conservation (Lechmere-Oertel et al. 2005). The construction of robust scenarios anticipating not only the dynamic of the vegetation structure but also its functional diversity dynamic in response to environmental changes is therefore of major importance (Pereira et al. 2010; Bellard et al. 2012).
Amongst the five main drivers of changes in biodiversity (climate, land use change, nitrogen deposition, atmospheric CO2, biotic exchange, Sala et al. 2000, Thuiller 2007), climate and land use are expected to be the most influential in temperate regions in the near future. Climate, which has been recognized as a primary factor determining species diversity (O’Brien et al. 2000; Whittaker et al. 2001), is expected to drastically change over the next century (IPCC 2007). On-going climate change has already been shown to impact on species distributions and diversity (Parmesan 2006; Lenoir et al. 2010; Gottfried et al. 2012) and notable to cause a shift in the tree line at the highest elevations (Gehrig-Fasel et al. 2007, Randin et al. 2009).
Changes in land uses are also known to heavily influence vegetation structure and diversity (Foley et al. 2005). This is particularly true in rural landscapes and mountain systems where over the centuries past land use has shaped the current landscapes (Quétier et al. 2007). In Europe, two main trends in land use changes have been observed in the past decades. On the one hand, the agricultural crisis has led to land abandonment, resulting in shrub and tree encroachments into old pastures and cultivated lands (Gehrig-Fasel et al. 2007). On the other hand, lowland areas have seen a dramatic increase in intensive agriculture and farming leading to unprecedented rates of local species extinctions (Hodgson et al. 2005). There is therefore a crucial need to investigate the interplay between climate and land use changes and its impact on biodiversity at regional scales where biodiversity management and protected areas are implemented (e.g. NATURA 2000, Thuiller et al. 2013).
Ecological modeling is a tool of choice for building biodiversity change scenarios as it makes it possible to quantify changes and explore large time scales and spatial extents. Recent model developments based on species coexistence theories and which aim to represent plant diversity offer new opportunities for simulating the spatio-temporal biodiversity response to environmental changes (Boulangeat et al. 2012, Boulangeat et al. in press). The dynamic vegetation model FATE-HD (Boulangeat et al. in press) integrates the most important mechanisms driving plant species coexistence, such as the biotic interactions, the seed dispersal, the abiotic filtering, and the response to disturbances. Besides, it is capable of modelling the vegetation dynamics in enough detail to represent the vegetation structure (height strata) and plant functional diversity at high spatial resolution (100-300m) and intermediate spatial extent (e.g. a national park of 250,000 hectares). The whole plant community dynamics over time and space, explicitly modeled in FATE-HD, make it possible to analyze the transient dynamics of the vegetation and diversity.
Using FATE-HD, we analyzed the short and long term consequences of combined land use and climate changes as well as their interactions on the regional plant diversity of the Ecrins National Park (PNE) in the French Alps. The PNE harbors a heterogeneous landscape due to a complex topography and half of its surface is moderately to intensively grazed by domestic animals. We developed three land use change scenarios in partnership with the PNE managers: no change, domestic grazing intensification, and domestic grazing abandonment. Our expectations were that species would shift their distributions upward under the effects of climate change (Jump et al. 2012), and that forest would colonize higher elevations (Randin et al. 2009). We further expected that the abandonment of domestic grazing would have a similar effect, namely the colonization of abandoned pastures by forest. An intensification of grazing was expected to maintain open environments and compensate, to a certain degree, the effect of the climate. Finally, species diversity was expected to decrease in the short term, in both the land use abandonment and the land use intensification scenarios (Niedrist et al. 2009). Functional diversity was expected to be more resilient to global changes than species richness (Ponce Campos et al. 2013) until a critical threshold is reached, as they do not highly correlate (Hirota et al. 2011). However, the response of functional diversity to environmental changes has never been analyzed using a spatially and temporally explicit dynamic approach.
Material and methods
(a) The study area
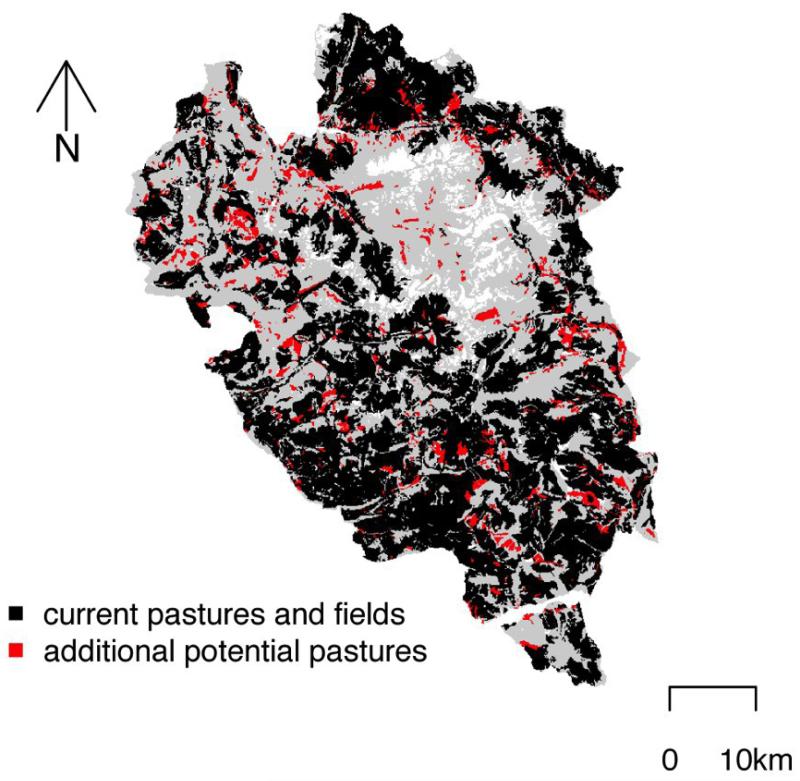
The Ecrins National Park hosts a high level of plant diversity (2000 species) over 270,000 hectares in the French Alps, and an elevation range of between 669 and 4103 m a.s.l. It is located at the crossroads between the continental and Mediterranean climate zones. Two-thirds of its area is open habitats which is almost all exploited by extensive grazing (80%) and/or mowing (25%) (Fig. 1). Forests constitute 25% of the surface of which the most accessible are exploited (40%) although this was neglected in the following simulations.
Fig. 1. Grazing intensification zones.
Grazed and mown areas under current management and under the intensification scenario. The grey background represents the study area that excludes glaciers. Current managed areas are shown in black, and additional pastures considered in the intensification scenario are shown in red.
(b) The dynamic vegetation model
FATE-HD is a dynamic vegetation model that simulates the spatial and temporal dynamics of plant functional groups (PFGs) in annual time-steps. It is built on past developments of model combinations as described e.g. in Albert et al. (2008) or BioMove (Midgley et al. 2010). The PFG life cycle is modeled in each grid-cell. PFG abundances are structured into cohorts by age, and each cohort is attributed to a height stratum according to the growth parameters. Four sub-models affect this cycle at various levels. (1) The PFG’s demography and the process of shading are modeled independently in each grid-cell of the study area. The amount of light available in each stratum positively or negatively affects PFG germination, recruitment and survival depending on the PFG’s preferences. (2) Maps of PFG habitat suitability determine PFG recruitment and the seed production in each grid-cell, with inter-annual stochasticity. (3) A stochastic seed dispersal model connects the grid cells laterally. The quantity of dispersed seeds depends on the amount of seeds produced by mature plants in the source pixel. (4) Several disturbance models simulate the effects of grazing and mowing, affecting juveniles and mature plants differentially depending on each PFG’s the sensitivity and response to the intensity and frequency of the disturbances (see Appendix A1a for details). The region of interest was represented as a regular grid of 100m resolution.
PFGs were constructed using an emergent clustering approach based on the dominant species and six plant characteristics, with the aim of representing the vegetation diversity, and modeling its dynamic response to environmental changes (Boulangeat et al. 2012). They were determined using plant characteristics related to the mechanisms implemented in FATE-HD (competition for resources, tolerance to abiotic conditions, dispersal, and response to disturbance). In a previous study (Boulangeat et al. 2012), we compared species-based and PFG-based functional diversity indices and community weighted mean of selected traits and demonstrated the capability of PFGs to represent species-based diversity and community means.
Spatial habitat suitability maps for each PFG were modeled using traditional species distribution models with the ensemble platform biomod2 (Thuiller et al. 2009). Recorded occurrences of representative species for each PFG were pulled together and related to seven topo-climatic variables including slope, percentage of calcareous soil, and five climatic variables (Appendix A1).
Grazing and mowing were considered at an annual time step and we considered the three grazing intensity levels for grazing described in the PNE database. All the parameters were set based on expert knowledge and the available databases (Appendix A1).
(c) Simulations
A first simulation phase aimed to reconstruct the current vegetation as an initial state for the scenario simulations. This initialization followed four steps: (1) Seeds from all PFGs were added every year over an average tree life cycle (300 years), which was enough for the PFG distributions to reach quasi equilibrium. (2) The seeding was stopped for the next 500 years in order for the PFG demography to stabilize. At this stage, the forest had settled in all locations where the abiotic environment was suitable for trees. During the last 200 years of this step, trees were removed from all currently managed areas and the annual disturbances (grazing and mowing) were applied. At this point, tree demography had stabilized across the study area. (4) Current disturbances were not able to maintain open grasslands in the long term. It is known that there are regular human intervention in the pastures to prevent tree colonization. In order to represent the current vegetation distribution, which is not in equilibrium with the disturbance regime, all trees that had settled in the grasslands during the step 3 were cut again and only 50 years of stabilization were run. The final vegetation state was shown to be fairly similar to observations (Boulangeat et al. in press).
After this initialization, the environmental change scenarios were run for 500 years. The climate changed every 15 years over a period of 90 years and remained the same for the following 410 years. Land use changes were applied in the fifth year and remained constant thereafter.
(d) Climate and land use change scenarios
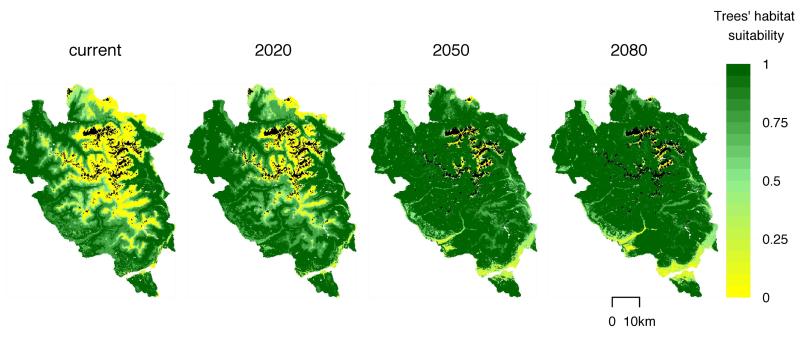
The effect of climate change was modeled based on its influence on the PFGs habitat suitability maps. For future climate, we decided to use the A1B scenario, as the variations between scenarios at our resolution and spatial scale were negligible. Climate change was simulated for the years 2020, 2050 and 2080 by the regional climate model (RCM) RCA3 (Samuelsson et al. 2011) fed by the global circulation model (GCM) CCSM3, which was derived from the ENSEMBLES EU project output. The RCM data was further downscaled to a 100×100m resolution using the change factor method (Diaz-Nieto and Wilby 2005). Habitat suitability maps were then projected into the future using these climate forecasts and the calibrated statistical relationship between topo-climatic variables and PFG distributions (Fig. 2 and Appendix A1). Finally, the habitat suitability of the PFGs was interpolated linearly between the time steps 2020, 2050 and 2080 in order to obtain a gradual change every 15 years during the first 90 years of the simulation. After 90 years of climate change, we kept climate constant and the habitat suitability maps remained unchanged for the rest of the simulations.
Fig. 2. Change in tree habitat suitability.
Maximum habitat suitability among tree PFGs obtained from projections of PFGs’ probability of presence varying from 0 to 1. From left to right: under current climate and future climate forecasts for the years 2020, 2050 and 2080. Glaciers are shown in black.
We applied three different land use management scenarios. The baseline management scenario consisted of keeping the current grazing and mowing conditions constant (with annual frequency). The second management scenario simulated land use with all grazing activities abandoned at year five. This scenario is based on a hypothetical withdrawal of European agricultural subsidies. A contrasting, third land use scenario reflected an extreme case in which all potential pastures were used intensively during the simulation. According to the PNE managers, this scenario is likely to occur if repeat droughts were to take place in southern France, which would ultimately increase the demand for the use of the PNE pastures in the summer season at high altitudes. For this third scenario, we worked with the PNE managers to determine where the potential new pastures would be located and we removed all trees in these locations prior to applying the annual disturbances (Fig.1). We hypothesized that our local land use change scenarios would not be constrained by global socio-economic conditions. Each land use scenario simulation was repeated six times with a stable climate and six times with climate change, making a total of six scenarios and 36 simulations. The repetitions made it possible to account for stochasticity in the dispersal process and annual habitat suitability. We limited the number of repetitions to six because the variations due to stochasticity had strictly local effects, and therefore did not affect the main patterns and thus our conclusions.
(e) Biodiversity measures
We analyzed the dynamics of two aspects of the vegetation. Firstly, in order to ensure our results could be compared with previous studies, the evolution of tree cover (above 1.5m) was examined for all scenarios according to simulation time and elevation. Secondly, since PFGs represent the vegetation diversity, we analyzed the evolution of the regional and local vegetation diversity. We used the “true diversity” of order two (Jost 2007; Tuomisto 2010) as a common framework for all diversity indices.
| (Eqn. 1) |
where pi and pj are the relative abundances of PFG i and j, dij is the dissimilarity between i and j, and S is the total number of PFGs. Gamma diversity (i.e. regional diversity) was calculated following Eqn. 1 with pi and pj being the relative abundance of PFGs in the whole study area. dij was equal to 0 if i=j and 1 if not. The alpha diversity (i.e. local diversity), the diversity measured at the 100m pixel level, was also calculated following Eqn. 1 so that pi and pj were the local (pixel) abundances of the PFGs and S the total number of PFGs. dij was also equal to 0 if i=j and 1 if not. The beta diversity (i.e. spatial turnover) was calculated by pixel for an ensemble of 9 pixels composed of the focal pixel and the eight adjacent pixels, and using a multiplicative decomposition (gamma / average alpha). For the functional diversity, we applied the same equations but with dij being the Euclidean distance between PFGs based on their height differences. In order to distinguish between cases where the beta diversity was the result of a change in species composition from cases where it was only a result of rearranging PFG abundances we computed two components of beta diversity. The first, the compositional diversity, was calculated with all pi and pj set as equal to 1/S. The second, the evenness component, was calculated as the ratio between the compositional diversity and the functional beta diversity (Jost 2010).
Results
The variation in outputs between repetitions (for each scenario) were limited to local changes only and did not affect the range change trends (Supplementary Material Appendix A2), nor the regional metrics (coefficient of variation <1% for tree cover, <0.5% for gamma diversity and <0.1% for beta diversity), and the structural patterns along the elevation gradient. The following statistics were therefore calculated from the average amongst the repetitions and the figures were drawn from one single repetition.
Land use change scenarios without climate change
Effects of land use intensification
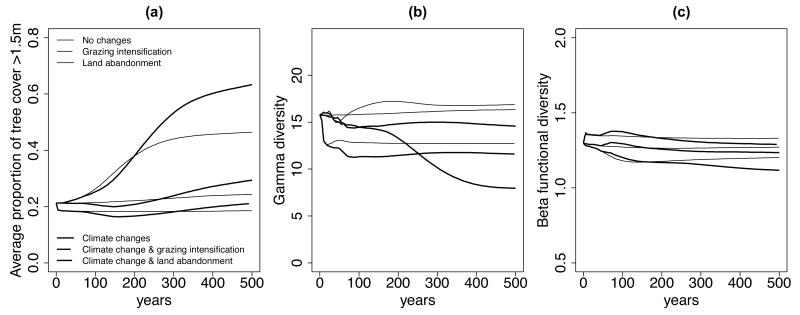
The main effect of creating new pastures and increasing grazing intensity (Fig. 1) was an immediate decrease in regional forest cover (Fig. 3), a reduction in range size for all palatable PFGs (Appendix S2), and mainly confined to mountainous to subalpine areas (Fig. 4a). In addition, the grazing intensification impeded the long-term tree colonization in pastures, which was observed under the baseline scenario of no change in management intensity (Fig. 3). As a consequence of the exclusion of palatable herbaceous species and tree PFGs in intensively grazed pastures, regional gamma diversity decreased (Fig. 3) as did local alpha diversity (Fig. 4a). The resulting landscape, a mosaic of very contrasting PFG assemblages (forest and pasture), therefore had a higher functional beta diversity than the current landscape (Fig. 3). Locally, the heterogeneity was due both to changes in PFG types (compositional component) and rearrangements of PFG abundances (evenness component) (see arrows a in Fig. 4).
Fig. 3. Dynamic response of the vegetation structure and diversity to environmental changes.
The figure shows the change in three metrics during the 500 years simulations for different scenarios. (a) The evolution of tree cover, (g) the evolution of gamma diversity, (c) the evolution of beta functional diversity.
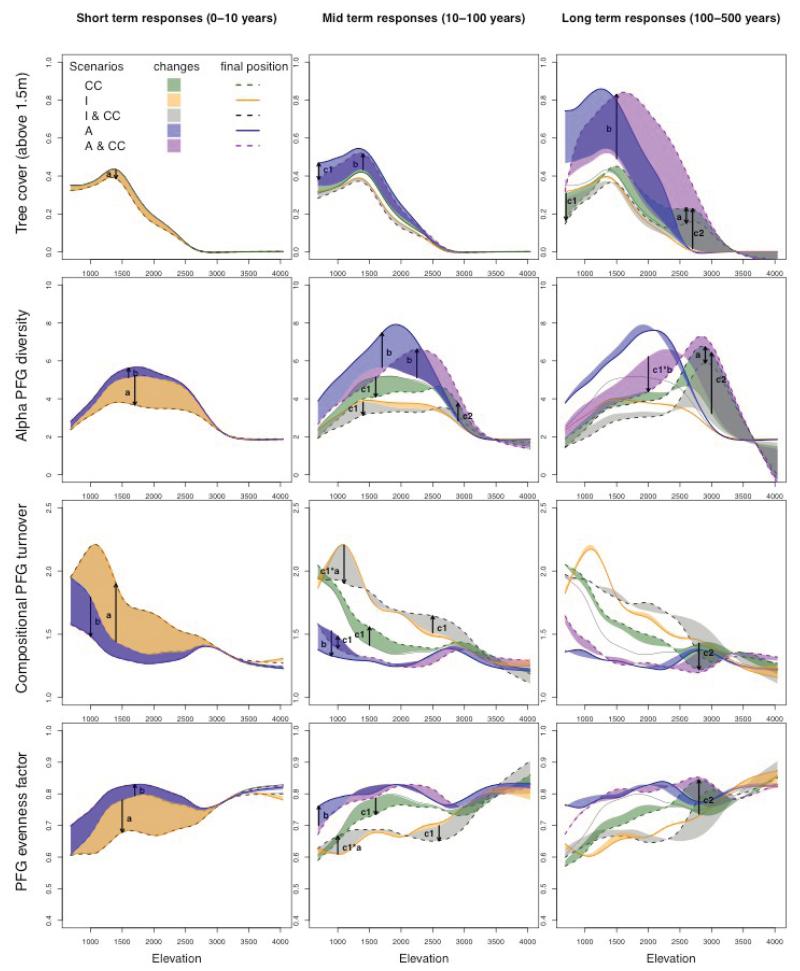
Fig. 4. Changes in vegetation structure and diversity changes along the elevation gradient.
For each scenario, the change in vegetation pattern along the elevation gradient is shown for three simulation time intervals: short-term response (first ten years), mid-term response (between year 10 and year 100), long-term response (between year 100 and year 500). We used generalized additive models to draw the trend from all pixels. Four vegetation measures are presented (from top to bottom): tree cover, alpha diversity, compositional PFG turnover, and PFG evenness factor. The arrows point to the main effects of (a) grazing intensification, (b) land abandonment, (c1) the climate change effects at low elevations, (c2) the climate change effects at high elevations.
Effects of land use abandonment
Tree colonization after land abandonment was relatively fast and stabilized after an average tree life cycle (~250 years) (Fig. 3). Land use abandonment led to a temporary decrease in regional gamma diversity that later recovered rapidly (Fig. 3). In the very short term, grazed herbaceous PFGs began colonizing pastures and increased local evenness (Fig.4) but these species were soon excluded in some areas by pioneer trees (mid term, Fig. 4, and Appendix A2). This competitive exclusion explains the initial drop and then recovery in gamma diversity. Shade intolerant herbaceous PFG and pioneer trees coexisted in old pastures where the forest was not yet settled. In the long-term, this coexistence only persisted in cold sites unsuitable for forests leading to a slight decrease in, and stabilization of, the gamma diversity. Conversely, the effect of land use abandonment on functional beta diversity was negative in the short and mid-term due to a local decrease in PFG turnover and increase in evenness (Fig.4). In the long term, once tree cover had stabilized and the transient dynamic had ended, functional beta diversity slightly increased.
Scenarios accounting for both land use and climate change
Climate change and management as usual
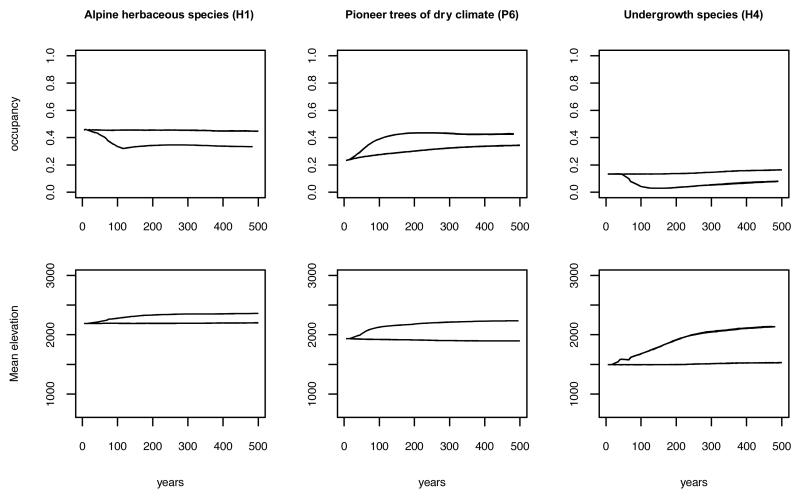
The simulated long-term consequences of climate change resulted in an upslope migration of the treeline (tree cover threshold of 60%) from 1800m a.s.l. to 2400m a.s.l (Fig. 4). The general migration of understory species (Fig. 5) also indicated a 600m upward shift. Vegetation diversity began to respond to climate change in the midterm, in contrast to vegetation structure which lagged behind climate change (Figs. 3 and 4). This immediate response was a decrease in gamma diversity (Fig. 3). This is the result of PFG range changes and increased asymmetry in PFG distribution, originating from a segregation between more narrow-range PFGs and those more widely distributed. Some PFGs did indeed become rarer in the region (e.g. alpine species, Fig. 5) while others expanded their ranges (e.g. pioneer trees, Fig. 5). At low elevations, the heterogeneity increased due to changes in both composition and evenness (Fig. 4).
Fig. 5. Area of occupancy and mean elevation over time for three PFGs.
All six repetitions are superimposed in the graphs. The solid lines correspond to the current management scenario without climate change and the dotted lines to the same scenario with climate change taken into account.
There was a lengthy time lag before the effects of climate change on vegetation structure and the forest cover response could be observed (Fig. 3). There was indeed very little variation in tree cover at landscape scale due to climate change during the simulated changes in climate (years 0-100). New developed mature forests only appeared in a second phase almost 100 years later. The distribution of tree cover along the elevation gradient showed that the decrease in forest cover was localized at low elevations (c1 in Fig. 4) where the habitat was no longer suitable for trees, leading to limited seed production and recruitment (Fig. 2). The specific PFG responsible for this effect was P8 (Betula alba), which prefers cold climates and cannot regenerate in a warming climate at its lowest elevation limit (Appendix A2). However, at landscape scale this decrease in tree cover is probably an artifact of these low elevation areas being situated at the edges of the PNE. Other tree species from the lowlands outside of the park might colonize these vanishing forests if the study area was enlarged. After the observed time lag (most PFGs need 50 years to reach new habitats, Appendix A2) the highest elevations were finally colonized by trees (c2 in Fig. 4). The time lag was a result of both dispersal limitation (to access new favorable environments) and demography (time required for trees to regenerate and grow) (Supplementary Material Appendix A3). In the long term, functional beta diversity continued to decrease slowly, reflecting homogenization at landscapes scale due to increased forest cover at high elevations (c2 in Fig. 4).
Intensification of grazing under climate change
Combined grazing intensification and climate change had mainly additive effects on the vegetation dynamics (Fig. 3) because intensification impacted the vegetation immediately, while climate change affected the vegetation with a time lag at higher elevation. However, grazing intensification slightly constrained the tree colonization of new suitable habitats (Fig. 3), which can be attributed to the decrease in the number of propagules as regional tree abundance decreased. In some valleys, the intensively grazed pastures may also have formed a dispersal barrier preventing tree seeds from accessing some parts of new suitable habitats (see example in Appendix A3). In the long term, the expected compensation between the effects of land use intensification and climate change was observed for the regional tree cover and beta functional diversity, but not for the gamma diversity (Fig. 3). Interestingly, intensification and climate change had interacting effects on local beta functional diversity at low elevations (c1*a in Fig. 4).
Land use abandonment under climate change
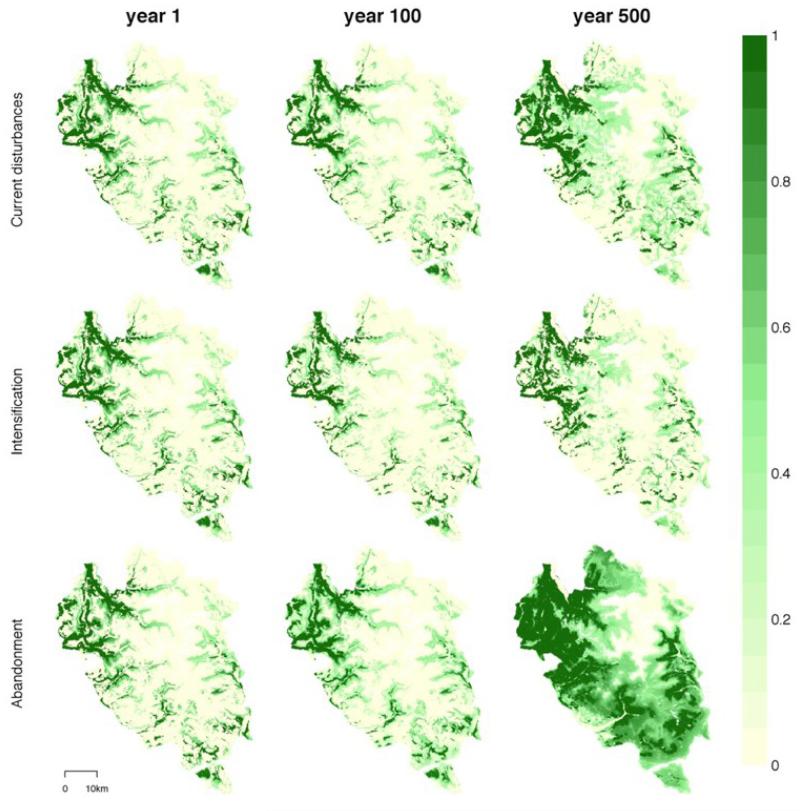
In the scenario in which climate change and land abandonment interacted, the colonization of new habitats by trees was accelerated (Fig. 3) although population dynamics and dispersal limitations still played an important role in restricting the colonization speed (Appendix A3). Locally, the consequence of this enhanced tree colonization is a significant decrease in local alpha diversity at low and intermediate elevations (c1*b in Fig. 4). Two phenomena explain this interaction: (1) as the number of trees increased, more propagules were dispersed throughout the landscape (i.e. mass effect, Leibold 2004), and (2) the rapid colonization of the low-lying former subalpine pastures (Fig. 6) increased the proximity of the forest sources to new suitable habitats (Appendix A3). This last point again emphasizes the importance of the time lag before the effects of climate changes are seen, and how this can interfere with what happens before. As a result of the simulated landscape homogenization and the exclusion of open habitats with their representative species, the regional vegetation diversity (gamma and beta) continuously decreased.
Fig. 6. Sequence of forest dynamics under climate change and various management scenarios.
For each scenario, the tree cover (strata above 1.5m) is shown for the initial simulation state (end of initialization, year 1) and years 10 and 500. Tree cover varies from 0 (no vegetation above 1.5m) to 1 (closed forest).
Discussion
The simulated vegetation dynamic in a spatially and temporally explicit model of vegetation (Fate-HD) under both climate and/or land use change scenarios showed interesting trends that concur with previous studies and expectations (Carlson et al. 2013). As the climate becomes more suitable at higher elevations, subalpine PFGs are likely to shift their distribution upward (Appendix A2) as suggested by Jump et al. (2012) and forest colonizes higher elevations (Randin et al. 2009). Concerning diversity changes, we demonstrated that regional gamma and beta diversity may at least initially decrease as a consequence of land use changes, which is consistent with previous studies predicting a decline in species richness under either domestic grazing intensification or land use abandonment (Niedrist et al. 2009). Moreover, our simulations demonstrated that a moderate intensity grazing disturbance (current management) favors vegetation diversity, which corresponds to the intermediate disturbance hypothesis explaining plant species diversity (Connell 1978).
In addition, our results reveal a different time frame for reaching equilibrium between scenarios. After the abandonment of grazing and mowing, forest colonized herbaceous ecosystems relatively rapidly (Fig. 3). This phenomenon has already been observed as an ongoing, though slow, process during the last few decades in the Alps (Gehrig-Fasel et al. 2007). The simulated vegetation dynamics under climate change were of similar dimensions to other studies but slower, suggesting an significant time lag usually not considered by studies using statistical habitat shift modeling (see also Dullinger et al. 2012, Meier et al. 2012). This time lag probably originates from two different processes, namely: (1) “migration limitation”, which refers to the time required for a species to colonize new suitable habitats, and (2) “time-delayed extinction”, which refers to the species’ persistence in locations where the habitat is no longer suitable (Schurr et al. 2012). Under the three land use change scenarios including climate change, tree colonization was much faster when the dispersal distance limitation and seed production limitation were ignored (Appendix A3). Nonetheless, even when seeding compensated for these constraints, the colonization took time to reach equilibrium, which demonstrates the importance of accounting for species demography. The simulated time lag was therefore the result of multiple factors including species demography, dispersal distance, barriers to accessing new suitable sites (Appendix A3), and propagule pressure, which in turn is determined by the complex interplay between species population dynamics, habitat suitability, vegetation as a propagule source, and the component species’ dispersal capability.
The combination of land use intensification and climate change has additive effects with little interaction, possibly due to a spatial and temporal decoupling of the vegetation response to these two drivers. Grazing intensification immediately impacted the vegetation, mostly in montane and subalpine habitats, whereas climate change affected the vegetation mainly at higher elevations and later in time (Fig. 4). Conversely, the combined simulation of climate change and land use abandonment showed a significant interacting effect. Here, the colonization of herbaceous ecosystems by trees was little constrained. Following the abandonment grazing and mowing, trees rapidly colonized all suitable areas, and forest occupied a large proportion of the area after the simulation. As soon as the climate became suitable at higher elevations, the model simulated a relatively fast upward shift in forests. Indeed, once the trees had colonized neighboring habitats, the dispersal distance to new suitable habitats was reduced, and consequently, the colonization of sub-alpine and alpine belts was faster (Appendix A3). In our simulations, the interaction between climate change and land abandonment also affected the evolution of diversity patterns (Figs. 3 and 4). In the absence of climate change, vegetation diversity was fairly resilient to land use abandonment. Climate change slowly modified this dynamic over the long term, resulting in a sharp decrease in both gamma and beta diversity.
Our model takes into account the most important determinants of vegetation distribution and its dynamics, notably biotic interactions (Svenning et al. 2013). However, other factors may also be important. For instance, natural disturbances such as snow avalanches and rock falls were neglected, despite the fact that they can maintain open patches in woodland allowing the co-occurrence of forests and herbaceous communities (Bebi et al. 2009). Our simulations therefore probably underestimated the diversity. However, all our scenarios have the same bias, which makes them comparable and our conclusions relevant. A second aspect neglected in our model is the interaction between soils and vegetation. Even if the climate and light conditions are suitable for the recruitment of a PFG, soil properties other than those used in the model (slope and percentage of calcareous soil) may influence the vegetation dynamics (Macia-Fauria et al. 2013). The time until soils above treeline become favorable to trees can be expected to further delay the colonization process (Carlson et al. 2013). Our model may also overestimate the migration of trees into dense abandoned grasslands above the treeline, which may not always offer easy colonization at the climatic edge of the tree life form. Gehrig-Fasel et al. (2007) only observed very slow upward shifts above historical treelines in the wake of recent climate change. Finally, the abiotic conditions might indirectly affect vegetation via for instance the soil biota (Defossez et al. 2011), the distribution of pathogens and pollinators (Colwell et al. 2012), dispersal vectors (Beckman et al. 2012) and herbivores (Suzuki et al. 2012). Given that these interactions with vegetation are complex and still poorly described and understood, including them in a time-space modeling context in order to analyze their effects on vegetation and diversity dynamics is a major challenge.
Conclusions
Using a spatially and temporally explicit, dynamic vegetation model, we showed that taking into account the time required for ecosystems to respond to environmental changes is crucial. Indeed, even if no or little effect is detected during the continuous change in climate, significant modifications in the vegetation structure and diversity might be observed when tracking the changes over 400 years, when the new vegetation equilibrium with climate and land use is reached. The short and long term effects are intertwined and depend on the specific combination of drivers. Our simulations based on climate alone, land use change alone, and then interactions between the two drivers allowed us to disentangle their respective effects and demonstrated the importance of making realistic projections even within a simplified modeling exercise. Notably, we observed very different responses and time lags in intensification vs. abandonment of land use scenarios.
The simulations highlight the general importance of land use as a human-induced disturbance in maintaining open habitats and conserving important vegetation diversity. Maintaining regular disturbances such as grazing and mowing is vital to biodiversity conservation. This is particularly true under climate change, because large areas will be suitable for the dominant trees in the European Alps, and forests will colonize most grasslands in the absence of human land use.
Supplementary Material
ACKNOWLEDGEMENTS
The research leading to these results was funded by the European Research Council under the European Community’s Seven Framework Programme FP7/2007-2013 Grant Agreement no. 281422 (TEEMBIO). IB was funded by the French “Agence Nationale de la Recherche” under the SCION (ANR-08-PEXT-03) project. The research was hosted by the Laboratory of Alpine Ecology (LECA), which is part of Labex OSUG@2020 (ANR10 LABX56). The author are grateful to the anonymous reviewers who contributed to the improvement of this publication from the first version.
REFERENCES
- Albert CH, et al. Land-use change and subalpine tree dynamics: colonization of Larix decidua in French subalpine grasslands. J. Appl. Ecol. 2008;45:659–669. [Google Scholar]
- Bebi P, et al. Snow avalanche disturbances in forest ecosystems—State of research and implications for management. For. Ecol. Manage. 2009;257:1883–1892. [Google Scholar]
- Beckman NG, et al. The interacting effects of clumped seed dispersal and distance- and density-dependent mortality on seedling recruitment patterns. J. Ecol. 2012:862–873. [Google Scholar]
- Bellard C, et al. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012;15:365–377. doi: 10.1111/j.1461-0248.2011.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulangeat I, et al. Improving plant functional groups for dynamic models of biodiversity: at the crossroads between functional and community ecology. Glob. Chang. Biol. 2012;18:3464–3475. doi: 10.1111/j.1365-2486.2012.02783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulangeat I, et al. FATE-HD: a spatially and temporally explicit integrated model for predicting vegetation structure and diversity at regional scale. Glob. Chang. Biol. doi: 10.1111/gcb.12466. doi: 10.1111/gcb.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadotte MW, et al. Beyond species: functional diversity and the maintenance of eco logical processes and services. J. Appl. Ecol. 2011;48:1079–1087. [Google Scholar]
- Carlson BZ, et al. Working toward integrated models of alpine plant distribution. Alp. Bot. 2013 doi: 10.1007/s00035-013-0117-4. doi 10.1007/s00035-013-0117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RK, et al. Coextinction and Persistence of Dependent Species in a Changing World. Annu. Rev. Ecol. Evol. Syst. 2012;43:183–203. [Google Scholar]
- Connell JH. Diversity in Tropical Rain Forests and Coral Reefs. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- De Bello F, et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010;19:2873–2893. [Google Scholar]
- Defossez E, et al. Do interactions between plant and soil biota change with elevation? A study on Fagus sylvatica. Biol. Lett. 2011;7:699–701. doi: 10.1098/rsbl.2011.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S, Cabido M. Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution. 2001;16:646–655. [Google Scholar]
- Diaz-nieto J, Wilby RL. A comparison of statistical downscaling and climate change factor methods: impacts on low flows in the river thames, United Kingdom. Climate Change. 2005;69:245–268. [Google Scholar]
- Dullinger S, et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Chang. 2012;2:619–622. [Google Scholar]
- Foley JA, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Gallien L, et al. Predicting potential distributions of invasive species: where to go from here? Diversity and Distributions. 2010;16:331–342. [Google Scholar]
- Gehrig-Fasel J, et al. Tree line shifts in the Swiss Alps: Climate change or land abandonment? Journal of Vegetation Science. 2007;18:571–582. [Google Scholar]
- Gottfried M, et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012;2:111–115. [Google Scholar]
- Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 1998;86:902–910. [Google Scholar]
- Hirota M, et al. Global resilience of tropical forest and savanna to critical transitions. Science. 2011;334:232–235. doi: 10.1126/science.1210657. [DOI] [PubMed] [Google Scholar]
- Hodgson JG, et al. The impacts of agricultural change (1963-2003) on the grassland flora of Central England: processes and prospects. Basic and Applied Ecology. 2005;6:107–118. [Google Scholar]
- IPCC . Contribution of Working Group I to the Fourth Assessment Report of the IPCC. Cambridge University Press; Cambridge, UK: 2007. Climate Change: The Physical Science Basis. [Google Scholar]
- Jost L. Partitioning diversity into independent alpha and beta components. Ecology. 2007;88:2427–2439. doi: 10.1890/06-1736.1. [DOI] [PubMed] [Google Scholar]
- Jost L. The Relation between Evenness and Diversity. Diversity. 2010;2:207–232. [Google Scholar]
- Jump AS, et al. Rapid altitudinal migration of mountain plants in Taiwan and its implications for high altitude biodiversity. Ecography. 2012;35:204–210. [Google Scholar]
- Lechmere-Oertel RG, et al. Patterns and implications of transformation in semi-arid succulent thicket, South Africa. J. Arid. Environ. 2005;62:459–474. [Google Scholar]
- Lenoir J, et al. Forest plant community changes during 1989-2007 in response to cli mate warming in the Jura Mountains (France and Switzerland) Journal of Vegetation Science. 2010;21:949–964. [Google Scholar]
- Macias-Fauria M, Johnson EA. Warming-induced upslope advance of subalpine forest is severely limited by geomorphic processes. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1221278110. doi. 10.1073/pnas.1221278110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier ES, et al. Climate, competition and connectivity affect future migration and ranges of European trees. Global Ecology and Biogeography. 2012;21:164–178. [Google Scholar]
- Midgley GF, et al. BioMove - an integrated platform simulating the dynamic response of species to environmental change. Ecography. 2010;33:612–616. [Google Scholar]
- Niedrist G, et al. Plant diversity declines with recent land use changes in European Alps. Plant Ecology. 2009;202:195–210. [Google Scholar]
- O’Brien EM, et al. Climatic gradients in woody plant (tree and shrub) diversity:water-energy dynamics, residual variation, and topography. Oikos. 2000;89:588–600. [Google Scholar]
- Paquette A, Messier C. The effect of biodiversity on tree productivity: from temperate to boreal forests. Global Ecology and Biogeography. 2011;20:170–180. [Google Scholar]
- Parmesan C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006;37:637–669. [Google Scholar]
- Pereira HM, et al. Scenarios for global biodiversity in the 21st century. Science. 2010;330:1496–501. doi: 10.1126/science.1196624. [DOI] [PubMed] [Google Scholar]
- Ponce Campos GE, et al. Ecosystem resilience despite large-scale altered hydroclimatic conditions. Nature. 2013;494:349–52. doi: 10.1038/nature11836. [DOI] [PubMed] [Google Scholar]
- Quétier F, et al. Plant-trait-based modeling assessment of ecosystem-service sensitivity to land-use change. Ecological Applications. 2007;17:2377–2386. doi: 10.1890/06-0750.1. [DOI] [PubMed] [Google Scholar]
- Randin CF, et al. Climate change and plant distribution: local models predict high-elevation persistence. Glob. Chang. Biol. 2009;15:1557–1569. [Google Scholar]
- Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Samuelsson P, et al. The Rossby Centre Regional Climate model RCA3: model description and performance. Tellus. 2011;63A:4–23. [Google Scholar]
- Sasaki T, Lauenroth WK. Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia. 2011;166:761–768. doi: 10.1007/s00442-011-1916-1. [DOI] [PubMed] [Google Scholar]
- Schurr FM, et al. How to understand species’ niches and range dynamics: a demographic research agenda for biogeography. J. Biogeogr. 2012;39:2146–2162. [Google Scholar]
- Smith MD, Knapp AK. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003;6:509–517. [Google Scholar]
- Suzuki M, et al. Deer herbivory as an important driver of divergence of ground vegetation communities in temperate forests. Oikos. 2012;122:104–110. [Google Scholar]
- Svenning JC, et al. The influence of biotic interactions on species range expansion rates. Ecography. 2013;(this issue) doi: 10.1111/j.1600-0587.2013.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W. Biodiversity - Climate change and the ecologist. Nature. 2007;448:550–552. doi: 10.1038/448550a. [DOI] [PubMed] [Google Scholar]
- Thuiller W, et al. BIOMOD - a platform for ensemble forecasting of species distributions. Ecography. 2009;32:369–373. [Google Scholar]
- Thuiller W, et al. A road map for integrating eco-evolutionary processes into biodiversity models. Ecol. Lett. 2013;16:94–105. doi: 10.1111/ele.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33:2–22. [Google Scholar]
- Whittaker RJ, et al. Scale and species richness: towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001;28:453–470. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.