Abstract
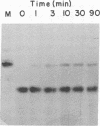
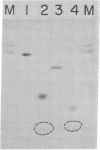
RNA ligase from T4-phage-infected Escherichia coli cells catalyzes the covalent joining of two polynucleotides that are partially hydrogen-bonded to each other. Two polynucleotide fragments derived from yeast tRNAPhe and consisting of residues 1-36 and 38-74 are covalently joined by the enzyme. The product of the reaction lacks residue Y37 and has an anticodon loop with six nucleotide residues, whereas all tRNA species whose sequences have so far been determined have seven nucleotides in this loop.
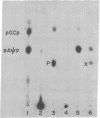
Evidence is also presented for the formation of a polynucleotide-adenylylate intermediate in the joining reaction, in which a pyrophosphate bond links the 5′-phosphoryl terminus of the polynucleotide and the phosphoryl group of AMP.
Keywords: polyribonucleotide synthesis, anticodon loop, adenylylated polyribonucleotides
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Harvey C. L., Gabriel T. F., Wilt E. M., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. IX. Synthesis and properties of the deoxyribonucleic acid adenylate in the phage T4 ligase reaction. J Biol Chem. 1971 Jul 25;246(14):4523–4530. [PubMed] [Google Scholar]
- Olivera B. M., Hall Z. W., Lehman I. R. Enzymatic joining of polynucleotides, V. A DNA-adenylate intermediate in the polynucleotide-joining reaction. Proc Natl Acad Sci U S A. 1968 Sep;61(1):237–244. doi: 10.1073/pnas.61.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RajBhandary U. L., Chang S. H. Studies on polynucleotides. LXXXII. Yeast phenylalanine transfer ribonucleic acid: partial digestion with ribonuclease T-1 and derivation of the total primary structure. J Biol Chem. 1968 Feb 10;243(3):598–608. [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermeyer W., Thiebe R., Zachau H. G. Amin-katalysierte Spaltung von Phenylalanin-spezifischer tRNA nach Baseneliminierung. Hoppe Seylers Z Physiol Chem. 1972 Oct;353(10):1625–1632. [PubMed] [Google Scholar]