Abstract
Pre-implantation development requires the specification and organization of embryonic and extra-embryonic lineages. The separation of these lineages takes place when asymmetric divisions generate inside and outside cells that differ in polarity, position and fate. Here we assess the global transcriptional identities of these precursor cells to gain insight into the molecular mechanisms regulating lineage segregation. Unexpectedly, this reveals that complementary components of the BMP signalling pathway are already differentially expressed after the first wave of asymmetric divisions. We investigate the role of BMP signalling by expressing dominant negative forms of Smad4 and Bmpr2, by down-regulating the pathway using RNAi against BMP ligands and by applying three different BMP inhibitors at distinct stages. This reveals that BMP signalling regulates the correct development of both extra-embryonic lineages, primitive endoderm and trophectoderm, but not the embryonic lineage, prior to implantation. Together these findings indicate multiple roles of BMP signalling in the early mouse embryo.
Keywords: embryo, mouse, BMP signalling, development, pluripotency, differentiation
Introduction
Pre-implantation development results in the formation of a blastocyst comprising three distinct cell lineages: the trophectoderm (TE), primitive endoderm (PE) and epiblast (EPI). The specification of these lineages is initiated when a subset of cells are directed to the inside of the embryo during two major waves of asymmetric divisions at the eight- to 16-cell and 16- to 32-cell transitions 1. Cells on the outside of the embryo are destined to differentiate into extra-embryonic TE, while inside cells form the pluripotent inner cell mass (ICM). Cells of the ICM will become either the pluripotent EPI that will give rise to the foetus or the PE that contributes predominantly to extra-embryonic lineages. Understanding of the molecular events underlying pre-implantation development has been significantly improved by several recent single-cell microarray and RNA sequencing studies 2-4. However, the transcriptional identities of the first progenitors of the TE (outside cells) and the ICM (inside cells) currently remain unknown. Early asymmetries in ligand/receptor expression could indicate involvement in later cell fate decisions, as has been shown recently for differential expression of Fgfr2 and Fgf4 5,6, but the involvement of other key signalling pathways in lineage segregation is unclear 7. For example, the BMP signalling pathway is critical for development and patterning in many developmental systems 8,9. In the mouse, BMP signalling is known to regulate several aspects of early post-implantation development, including germ cell differentiation and anterior-posterior patterning 10-15, and to drive TE differentiation in vitro 16. However, studies showing that mice lacking core BMP signalling pathway components do not arrest embryogenesis until after implantation suggested that BMP signalling might be dispensable for pre-implantation development 10,11,17,18. Here, with an aim to investigate the mechanisms involved in the first steps leading to lineage segregation in the mouse embryo, we used deep mRNA sequencing to identify the transcripts expressed in inside and outside cells as they first become set apart at the 16-cell stage. Analysis of this dataset pointed to an unexpected role of BMP signalling in pre-implantation development. To test this hypothesis, we inhibited BMP signalling in the embryo using six distinct and independent approaches. Our results indicate that BMP signalling is important much earlier in development than previously expected and that it is key for the development of both pre-implantation extra-embryonic lineages.
Results
Expression of BMP pathway components at the 16-cell stage
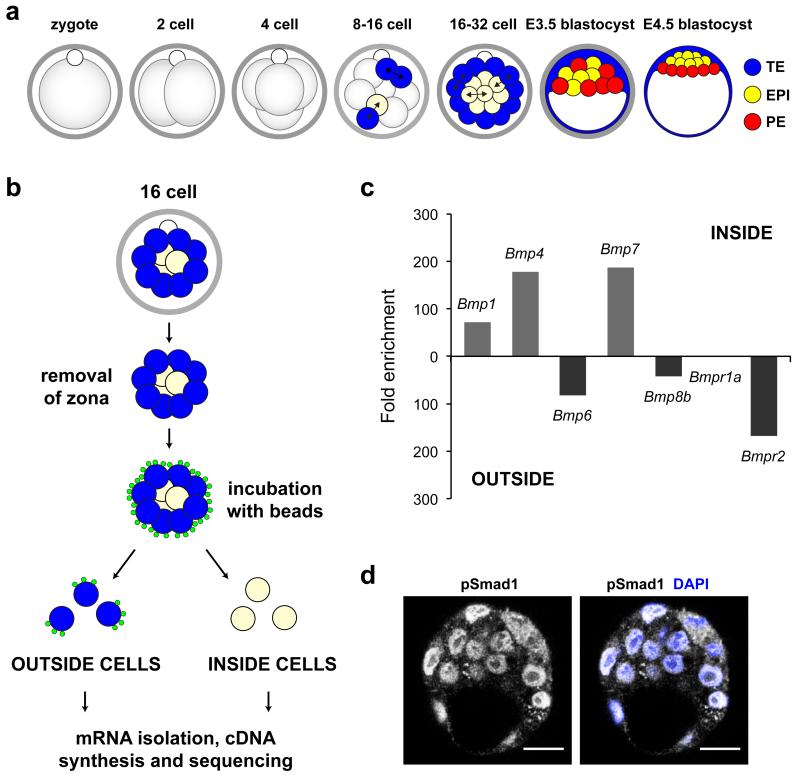
In order to investigate the first steps leading to lineage segregation in the mouse embryo, we first wished to determine global differences in gene expression between inside and outside cells after the first wave of asymmetric cell divisions (Fig. 1a). To isolate these cells, we incubated early 16-cell stage embryos in a suspension of 0.2 μm fluorescent beads (Supplementary Fig. 1) and then used gentle pipetting to separate inside (non-fluorescent) and outside (fluorescent) cells (Fig. 1b). We combined 30-40 cells of the same identity and performed mRNA deep sequencing on the SOLiD platform, using the protocol developed for low cell number samples 19. Colour-space reads obtained were mapped to the mouse genome and all reads that mapped to particular genes in either cell type were summed. We applied the GFOLD (generalized fold change) algorithm 20 to obtain biologically meaningful rankings of differentially expressed genes (Supplementary Fig. 2 and Supplementary Data 1,2). We found that more than 11,000 transcripts from the mouse RefSeq database were expressed in either cell type. Transcripts from about 10% of these genes were enriched more than five-fold (GFOLD ≤ 0.01) in one cell type (Supplementary Table 1). To verify our findings we assessed the expression levels of Id2 and Sox2, genes reported to be early markers of outside and inside cells respectively 2, and found that they were enriched in the expected cell type, with Id2 enriched four-fold more in outside cells (outside:inside = 613:149), and Sox2 enriched 39-fold more in inside cells (9:355). We also quantified relative expression levels between inside and outside cells for 46 genes by qRT-PCR (Supplementary Fig. 3), which further validated the differential expression. We wished to determine whether other genes involved in maintaining pluripotency in the ICM, or driving differentiation in the TE and PE, in addition to Sox2 and Id2, are already differentially expressed after the first wave of asymmetric divisions. We found that transcripts for Cdx2 (748:413) 21,22 and Gata3 (5746:1086) 23 are enriched in outside cells, while Fgf4 (3:322) 24,25 is expressed 100-fold more in inside cells.
Figure 1. Transcriptome analysis of inside and outside cells at the 16-cell stage.
(a) Schematic representation of pre-implantation development. Briefly, two waves of asymmetric divisions at the 8-16 and 16-32 cell transitions set apart the outside progenitors of the TE (blue) and the inside progenitors of the ICM (pale yellow). Cells of the ICM will further segregate into the EPI and PE in the late blastocyst. (b) Isolation and deep sequencing of outside and inside cells from the 16-cell embryo. (c) BMP ligands and receptors differentially expressed at the 16-cell stage. (d) pSmad1 expression in the early blastocyst. Scale bars = 40 μm.
It has recently been established that differential expression of Fgf4 and Fgfr2 at the 16-cell stage is important for regulating segregation of the EPI and PE lineages within the ICM 5,6. This prompted us to determine whether ligands or receptors of any other signalling pathways might also show differential expression at the 16-cell stage and led us to focus on the BMP pathway (Fig. 1c). We found that while inside cells express Bmp4 (0:89) and Bmp7 (0:94), outside cells express Bmp6 (41:0) and Bmp8b (393:9). Bmpr1a, which encodes the receptor that binds Bmp4 and 7, is expressed in both inside and outside cells at similar levels (566:546). However mRNA for Bmpr2, necessary for signal transduction, is expressed exclusively in outside cells (84:0), suggesting that the outside cells are the predominant recipients of the signal. These early differential expression patterns correlate with the expression of Bmpr2 and Bmp4/Bmp7 at later developmental stages in the same reciprocal outside/inside pattern in TE and ICM cells of the blastocyst 3. BMP signalling can occur via both Smad-dependent and -independent pathways 26 and we detected mRNAs for components of both downstream signalling routes in outside and inside cells, including Smad4 (1172:936), Smurf1 (809:826) and Tak1 (556:636). To confirm that the BMP signalling pathway is active in the pre-implantation embryo, we determined the localization of pSmad1 at the early blastocyst stage (Fig. 1d). We found that nuclear pSmad1 was present at this stage, in agreement with previous work showing nuclear pSmad1 in the TE and PE of the late blastocyst 27. The differential expression of BMP pathway ligands and receptors at the 16-cell stage prompted us to investigate whether BMP signalling might play a role in development prior to embryo implantation.
Inhibition of Smad4 and Bmpr2 in the pre-implantation embryo
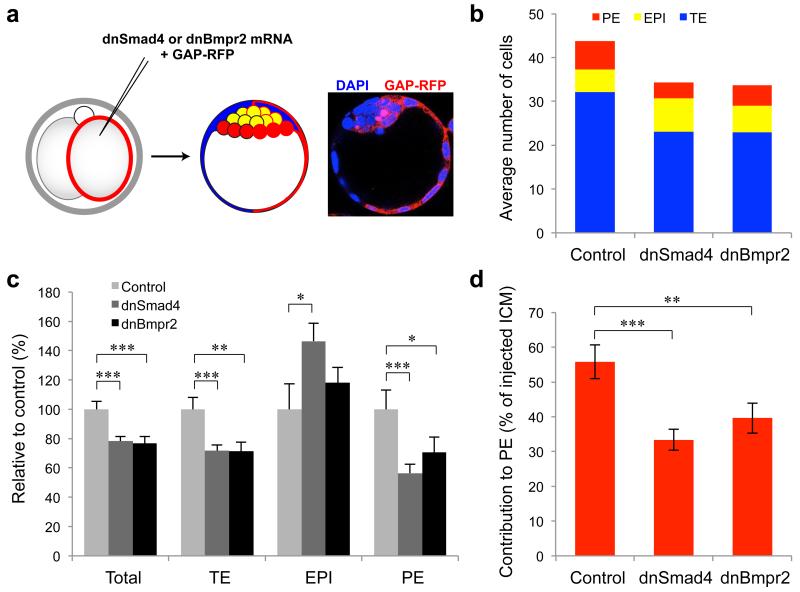
In order to determine whether BMP signalling is involved in pre-implantation development, we first used dominant negative forms of Smad4 (dnSmad4) and Bmpr2 (dnBmpr2) to inhibit the BMP/Smad4 pathway in vivo. We injected one blastomere of late two-cell stage embryos with mRNA for either dnSmad4 or dnBmpr2, along with mRNA for membrane-bound RFP (Gap43-RFP) as a lineage marker of the injected clone of cells. The half of the embryo developing from the non-injected blastomere provided an internal control. An additional group of control embryos were injected with Gap43-RFP alone. The embryos were then cultured for three days to the late blastocyst stage (E4.5), and the contribution of Gap43-RFP-expressing cells to each lineage assessed by immunofluorescence for lineage markers of the TE (Cdx2) and PE (Sox17) (Fig. 2a). As the red channel was required for visualising the Gap43-RFP expression, EPI cells were counted as those that were negative for both Cdx2 and Sox17. We found that expression of both dominant negatives resulted in significantly fewer cells in the injected half of the embryo, by 22% for dnSmad4 (p < 0.001, Student’s t-test) and by 23% for dnBmpr1 (p < 0.001, Student’s t-test), compared with controls (Fig. 2b,c). This reduction in total cell number could be entirely attributed to a reduced number of TE and PE cells, with the number of EPI cells either increased for dnSmad4 (by 47%, p < 0.05, Student’s t-test) or not significantly affected for dnBmpr2. Expression of dnSmad4 resulted in a 28% decrease in TE cells (p < 0.001, Student’s t-test) and a 44% decrease in PE cells (p < 0.001, Student’s t-test), while expression of dnBmpr2 reduced TE cells by 29% (p < 0.001, Student’s t-test) and PE cells by 29% (p < 0.05, Student’s t-test). While control injected ICM cells contributed roughly equally to the EPI and PE (56% PE), ICM cells with dnSmad4 (33% PE, p < 0.001, Student’s t-test) or dnBmpr2 (40% PE, p < 0.01, Student’s t-test) showed reduced contribution to the PE lineage (Fig. 2d). These results suggest an important involvement of BMP signalling in pre-implantation development and specifically in the development of the extra-embryonic lineages: the TE and PE.
Figure 2. Expression of dominant negative forms of Smad4 and Bmpr2 affects TE and PE development.
(a) One blastomere of two-cell embryos was injected with dnSmad4 (n = 21), dnBmpr2 (n = 22) or Gap43-RFP only (controls n = 20) and cultured to E4.5. (b) Total cell numbers for each lineage in the injected (Gap43-RFP-positive) half of the embryo. (c) Total cell numbers and lineage contributions relative to control. (d) Contribution of injected ICM cells to PE. * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001 (Student’s t-test). Error bars represent s.e.m.
BMP inhibition impairs development of the TE and PE lineages
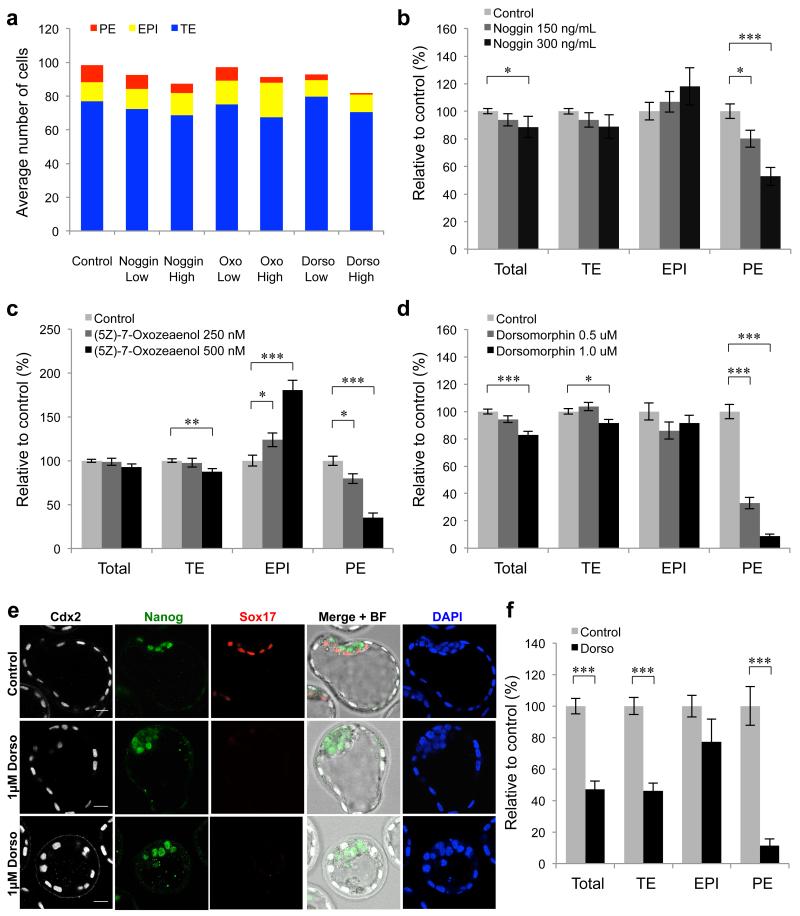
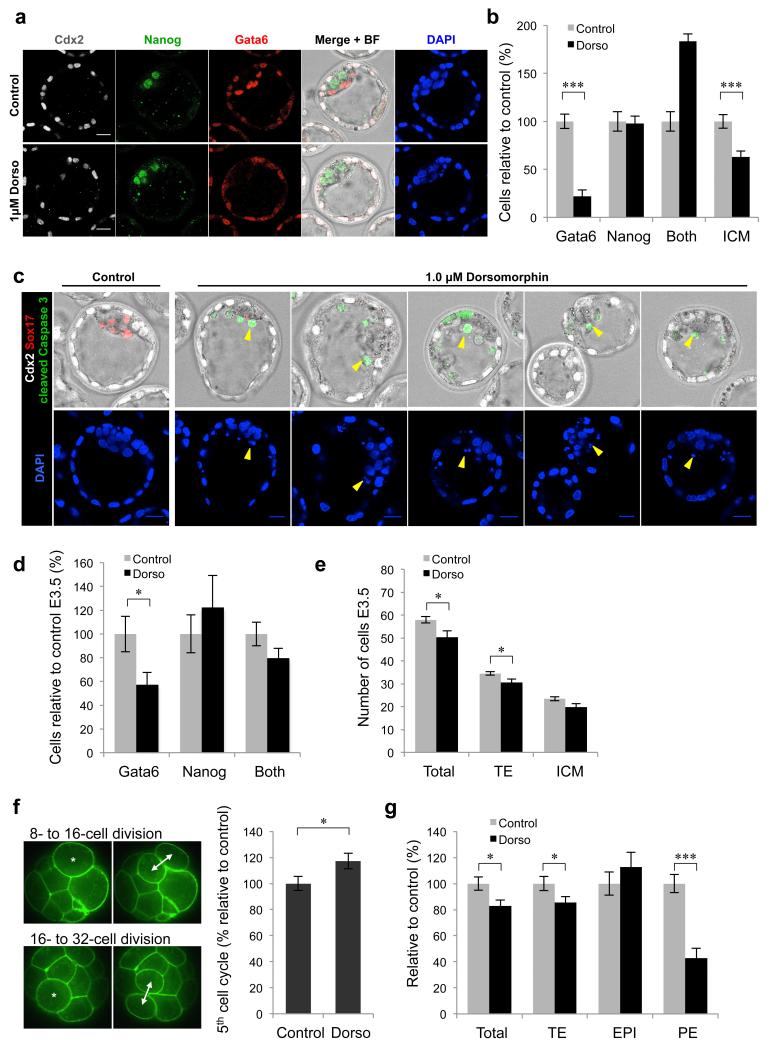
Blocking the activity of Smad4 and Bmpr2 suggested that BMP signalling might play a role in the development of the extra-embryonic lineages before implantation. We sought to confirm this finding by alternative methods of BMP inhibition. To do this, we used three specific inhibitors to block either the Smad-dependent or -independent BMP pathways in vivo: Noggin, a BMP ligand antagonist 28; Dorsomorphin, an inhibitor of Bmpr1 that leads to inhibition of Smad1 phosphorylation 29 and the Tak1 kinase inhibitor (5Z)-7-Oxozeaenol that inhibits the Smad-independent response to BMPs 30. Embryos were cultured in the presence of the inhibitors from the eight-cell stage (E2.5) to the late blastocyst stage (E4.5) and assessed for the expression of markers of the TE (Cdx2), EPI (Nanog) and PE (Sox17) (Fig. 3a). Each inhibitor was used at two different concentrations to determine if any effects on development were dose-dependent. We found that all three inhibitors caused a significant reduction in the number of Sox17-positive PE cells compared with control embryos, in a dose-dependent manner (Noggin 150 ng ml−1, 20% less PE, p < 0.05, Student’s t-test; Noggin 300 ng ml−1, 47% less PE, p < 0.001, Student’s t-test; (5Z)-7-Oxozeaenol 250 nM, 20% less PE, p < 0.05, Student’s t-test; (5Z)-7-Oxozeaenol 500 nM, 5% less PE, p < 0.001, Student’s t-test; Dorsomorphin 0.5 μM, 67% less PE, p < 0.001, Student’s t-test; Dorsomorphin 1.0 μM, 91% less PE, p <0.001, Student’s t-test) (Fig. 3b-e). A decrease in the total number of cells in the embryos compared with controls (98.5 cells) was observed for the higher concentrations of Noggin (by 11%, 87.3 cells, p < 0.05, Student’s t-test) and Dorsomorphin (by 17%, 81.7 cells, p < 0.001, Student’s t-test), however (5Z)-7-Oxozeaenol treatment did not significantly affect total cell number. All higher concentration treatments resulted in a decrease in TE cells, by 11% for Noggin (p = 0.07, Student’s t-test), by 13% for (5Z)-7-Oxozeaenol (p < 0.01, Student’s t-test) and by 9% for Dorsomorphin (p < 0.05, Student’s t-test). Both Noggin and Dorsomorphin had an effect only on the development of the TE and PE lineages, but not on the EPI lineage. The only inhibitor to have a significant effect on the number of Nanog-positive EPI cells was (5Z)-7-Oxozeaenol, which caused a significant increase in Nanog-positive cells in a dose-dependent fashion, by 24% at 250 nM (p < 0.05, Student’s t-test) and by 81% at 500 nM (p < 0.001, Student’s t-test).
Figure 3. BMP inhibitor treatments reduce the number of extra-embryonic cells at E4.5.
(a-e) Embryos were incubated in the presence of BMP inhibitors for 48 hours from E2.5-E4.5. Controls n = 37, 150 ng ml−1 Noggin n = 23, 300 ng ml−1 Noggin n = 12, 250 nM (5Z)-7-Oxozeaenol n = 27, 500 nM (5Z)-7-Oxozeaenol n = 16, 0.5 μM Dorsomorphin n = 25, 1.0 μM Dorsomorphin n = 47. (a) Total cell numbers for each lineage at E4.5. (b-d) Total cell numbers and lineage contributions relative to control embryos. (e) Immunofluorescence for markers of the TE (Cdx2), EPI (Nanog) and PE (Sox17) in embryos treated with 1.0 μM Dorsomorphin. Scale bars = 20 μm. (f) Relative number of cells in each lineage compared with controls (n = 10) in embryos treated with 1.0 μM Dorsomorphin (n = 10) for 72 hours from E1.5 to E4.5. * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001 (Student’s t-test). Error bars represent s.e.m.
Inhibition of BMP signalling through the use of specific inhibitors again indicated a role of the BMP pathway in the development of both extra-embryonic cell lineages, however we noticed that targeting the activity of Smad4 and Bmpr2 had a greater effect on the TE lineage than the inhibitors. To determine whether this greater effect on the TE was due to the different start points of each experiment, from late two-cell stage in the case of dnSmad4 and dnBmpr2 and from the eight-cell stage in case of inhibitor treatments, we next cultured embryos in 1.0 μM Dorsomorphin from the late two-cell stage for 72 hours and analysed lineage-specific gene expression at E4.5, compared with controls (Fig. 3f). This longer Dorsomorphin treatment had a much more severe effect on the number of total (reduced by 53%, p < 0.001, Student’s t-test) and TE (reduced by 54%, p < 0.001, Student’s t-test) cells than the previous 48 hour treatment. The number of EPI cells was not significantly different to controls and the PE was reduced by 89% (p < 0.001, Student’s t-test), consistent with previous experiments. Together these results demonstrate that inhibition of BMP signalling causes a defect specifically in the development of the extra-embryonic lineages of the blastocyst.
Cell death in BMP inhibitor-treated embryos
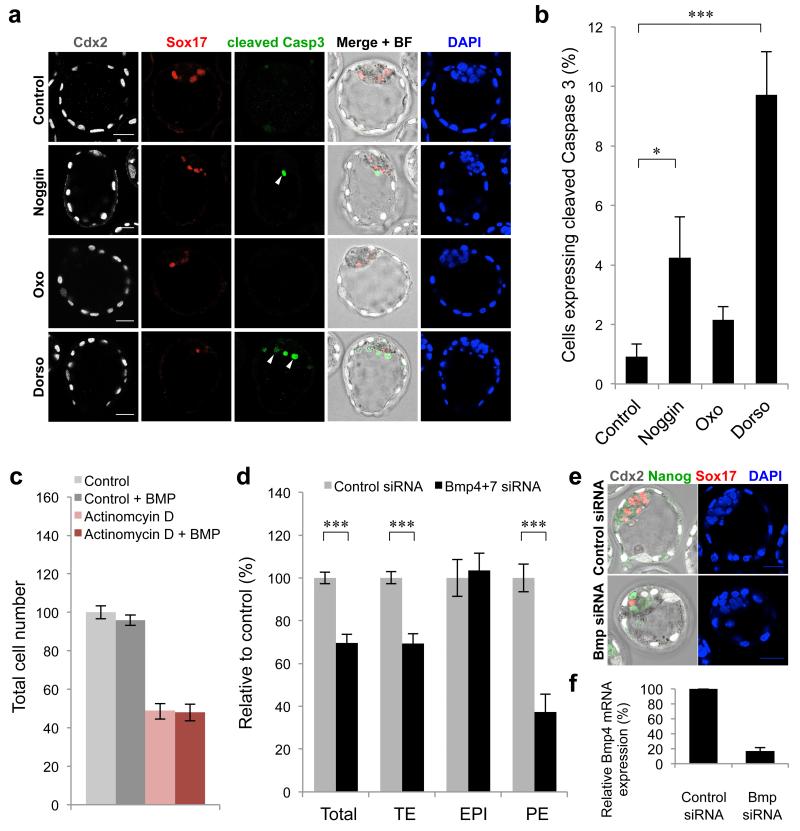
To gain insight into the mechanism by which inhibition of BMP signalling prevents normal development of extra-embryonic lineages, we assessed whether programmed cell death might play a role. Embryos were treated with 300 ng ml−1 Noggin, 500 nM (5Z)-7-Oxozeaenol or 1.0 μM Dorsomorphin from E2.5 to E4.5 and examined for expression of cleaved Caspase 3 (Fig. 4a,b). We found that the average percentage of cells expressing cleaved Caspase 3 in each embryo was significantly increased by 4.6-fold for Noggin (p < 0.05, Student’s t-test) and by 10.6-fold for Dorsomorphin (p < 0.001, Student’s t-test), compared with control embryos. Embryos treated with (5Z)-7-Oxozeaenol showed a slight, but not quite significant (p = 0.05, Student’s t-test) increase in the percentage of cells expressing cleaved Caspase 3, suggesting that in these embryos another mechanism may be responsible for the decrease in extra-embryonic cells. The increased presence of apoptotic cells in embryos treated with Noggin or Dorsomorphin could indicate a pro-survival role of BMP signalling during pre-implantation development. To investigate whether BMP signalling inhibited apoptosis, we assessed whether BMP4 treatment could inhibit Actinomycin D-induced cell death in pre-implantation embryos 31. Eight-cell stage embryos were treated with a low concentration of Actinomycin D (2 ng ml−1) for six hours, then washed in normal media and cultured for 48 hours to E4.5. Throughout the experiment, half of the embryos were cultured in the presence of exogenous BMP4 to determine whether this would affect cell survival (Fig. 4c). We found that the addition of BMP4 did not increase the number of cells in Actinomycin D-treated embryos, suggesting that BMP signalling does not inhibit apoptosis and that the cell death observed in Dorsomorphin and Noggin-treated embryos is most likely secondary to a developmental defect.
Figure 4. Mechanisms of extra-embryonic cell loss in embryos with impaired BMP signalling.
(a) Examples of cleaved Caspase 3 expression in embryos treated with 300 ng ml−1 Noggin (n = 12), 500 nM (5Z)-7-Oxozeaenol (n = 15) or 1.0 μM Dorsomorphin (n = 15) for 48 hours from E2.5-E4.5 (controls n = 14). White arrows indicate examples of apoptotic cells. (b) Percentage of cells per embryo that express cleaved Caspase 3. (c) Total cell number relative to controls (n = 12) in E4.5 stage embryos treated with BMP4 (n = 12), Actinomycin D (n = 17) or Actinomycin D + BMP4 (n = 17). (d) Number of cells in each lineage at E4.5 in embryos injected with Bmp4+7 siRNA (n = 13), relative to controls (n = 12). (e) Examples of E4.5 embryos, 72 hours after microinjection with siRNA. (f) Confirmation of Bmp4 knockdown by qRT-PCR 48 hours after siRNA injection. Bmp siRNA n = 50, controls n = 50 (3 experiments). Scale bars = 30 μm. * denotes p < 0.05, *** denotes p < 0.001 (Student’s t-test). Error bars represent s.e.m.
Source of BMP signals in the pre-implantation embryo
Our finding that both inside and outside cells express Bmp ligands led us to investigate which cells are the source of the BMP signals important for the development of the TE and PE. To do this, we used siRNA to knockdown the expression of Bmp4 and Bmp7 as these two ligands are exclusively produced by inside cells at the 16-cell stage and by the ICM in the blastocyst 2,3,32. Both blastomeres of two-cell stage embryos were injected with Bmp4+7 siRNA or control siRNA and the embryos cultured for 72 hours to the late blastocyst stage (Fig. 4d,e). We found that the Bmp4+7 siRNA-injected embryos had 30% less total cells than control siRNA-injected embryos (p < 0.001, Student’s t-test) and that this was due to a decrease in the number of TE cells (by 31%, p < 0.001, Student’s t-test) and PE cells (by 63%, p < 0.001, Student’s t-test). The number of EPI cells did not differ between the two groups of embryos. To confirm the knockdown of Bmp4 and Bmp7, both blastomeres of two-cell stage embryos were injected with Bmp4+7 siRNA or control siRNA and the levels of Bmp4 and 7 mRNA analysed 48 hours later, at E3.5 (Fig. 4f). Transcripts for Bmp4 were depleted by 83% compared with controls, however Bmp7 could not be detected, most likely due to its low expression at this stage 33. These results indicate that BMP signals produced by the pluripotent inside cells are important for the development of the extra-embryonic TE and PE lineages.
Mechanism of PE cell loss in Dorsomorphin-treated embryos
Dorsomorphin treatment had the strongest effect on Sox17-positive PE cell number therefore we checked the expression of other PE-specific genes in embryos treated with 1.0 μM Dorsomorphin from E2.5 to E4.5 (Supplementary Fig. 4). We found that the expression of two other PE markers, Gata6 and Gata4, was markedly reduced compared with controls, confirming compromised development of PE. To further investigate this we analysed the expression of early markers of PE and EPI (Gata6 and Nanog, respectively) in E4.5 blastocysts treated with 1.0 μM Dorsomorphin from E2.5, compared with controls (Fig. 5a). We found that the number of Gata6 positive cells was reduced by 78% (p < 0.001, Student’s t-test) and the overall number of ICM cells was reduced by 37% (p < 0.001, Student’s t-test), however the number of cells expressing Nanog was not significantly affected (Fig. 5b). This suggests that PE precursor cells are either eliminated by cell death or are never specified. Quantification of the cleaved Caspase 3 staining indicates that there is a dramatically increased occurrence of cell death in Dorsomorphin-treated embryos (with almost 10% of cells in the blastocyst expressing cleaved Caspase 3; Fig. 4b) and the presence of dying cells on the surface of the ICM supports the hypothesis that PE cells are actively eliminated (Fig. 5c). To determine whether PE precursor cells are specified in the early ICMs of Dorsomorphin-treated embryos, we assessed Gata6 and Nanog expression at E3.5 in embryos treated with 1.0 μM Dorsomorphin from E2.5 (Fig. 5d). We found that at this stage around half of ICM cells expressed both Gata6 and Nanog in both controls and Dorsomorphin-treated embryos. There was a reduction in the number of ICM cells expressing only Gata6 in Dorsomorphin-treated embryos (by 43%, 2.4 cells compared with 4.3 cells, p < 0.05, Student’s t-test; Fig. 5d), however this did not significantly affect the total number of ICM cells (19.9 cells compared with 23.4 cells, p = 0.05, Student’s t-test; Fig. 5e). This reduction of Gata6-positive cells at E3.5 is therefore unlikely to be sufficient to account for the almost complete loss of PE cells at E4.5.
Figure 5. Mechanism of PE cell loss in Dorsomorphin-treated embryos.
(a-c) Embryos were treated with 1.0 μM Dorsomorphin (n = 15) for 48 hours from E2.5 to E4.5 (controls n = 15). (a) Expression of Cdx2, Gata6 and Nanog at E4.5. (b) Number of cells relative to controls at E4.5. Both = positive for Gata6 and Nanog. (c) Examples of dying cells on surface of ICM (identified by cleaved Caspase 3 expression and DNA fragments, yellow arrows) at E4.5. (d-e) Embryos were cultured in the presence of 1.0 μM Dorsomorphin (n = 16) for 24 hours from E2.5 to E3.5 (controls n = 16). (d) Number of cells relative to controls at E3.5. (e) Number of cells in each lineage at E3.5. (f) Time taken for individual cells to complete the fifth cell cycle in embryos cultured with 1.0 μM Dorsomorphin (n = 17 cells, 6 embryos) for 48 hours from E2.5, relative to controls (n = 23 cells, 5 embryos). (g) Number of cells in each lineage relative to controls at E4.5 following treatment with 1.0 μM Dorsomorphin for 24 hours from E3.5. Scale bars = 20 μm. * denotes p < 0.05, *** denotes p < 0.001 (Student’s t-test). Error bars represent s.e.m. See also Supplementary Fig. 4.
The number of total (50.4 compared with 58.0, p < 0.05, Student’s t-test) and TE (30.6 compared with 34.5, p < 0.05, Student’s t-test) cells were both significantly decreased by Dorsomorphin treatment (Fig. 5e), indicating that the development of these embryos might be delayed. To determine whether Dorsomorphin treatment indeed causes a developmental delay, we cultured embryos expressing a GFP-tagged membrane protein (GFP-GPI) from E2.5 to E3.5 in 1.0 μM Dorsomorphin and used time-lapse microscopy to film developmental progression. To establish whether Dorsomorphin treatment affects cell cycle time, we measured the time taken for individual cells to undertake the fifth cell cycle (from the eight- to 16-cell division to the 16- to 32-cell division) (Fig. 5f). We found a significant increase in the length of this cell cycle (by 17%, p < 0.05, Student’s t-test), compared with control embryos. Dorsomorphin treatment therefore causes a developmental delay that contributes to decreased cell numbers in the extra-embryonic lineages by E4.5. Importantly, our analyses revealed that the embryonic EPI cells are able to escape the effects of this delay as their numbers are not significantly decreased by any of the three inhibitors.
Finally, we sought to determine the extent to which the inhibition of BMP signalling between E2.5 and E3.5 contributes to the loss of PE cells observed following 48 hours of Dorsomorphin treatment, by only adding the inhibitor after this period. We treated embryos with 1.0 μM Dorsomorphin from E3.5 to E4.5 and analysed lineage-specific gene expression compared with controls (Fig. 5g). This 24 hour treatment resulted in a similar decrease in the number of total (by 17%, p < 0.05, Student’s t-test) and TE (by 15%, p < 0.05, Student’s t-test) cells as in embryos treated for 48 hours. PE cell number was reduced by 57% (p < 0.001, Student’s t-test), representing around 60% of the reduction seen with the 48 hour treatment. Together, these results suggest that inhibition of the Smad-dependent BMP pathway by Dorsomorphin impairs PE development by two mechanisms. First by reducing the number of PE precursor cells in the ICM as the early blastocyst forms (E2.5-E3.5), and second by eliminating Gata6-positive cells by apoptosis in the maturing blastocyst (E3.5-E4.5).
(5Z)-7-Oxozeaenol treatment impairs PE cell differentiation
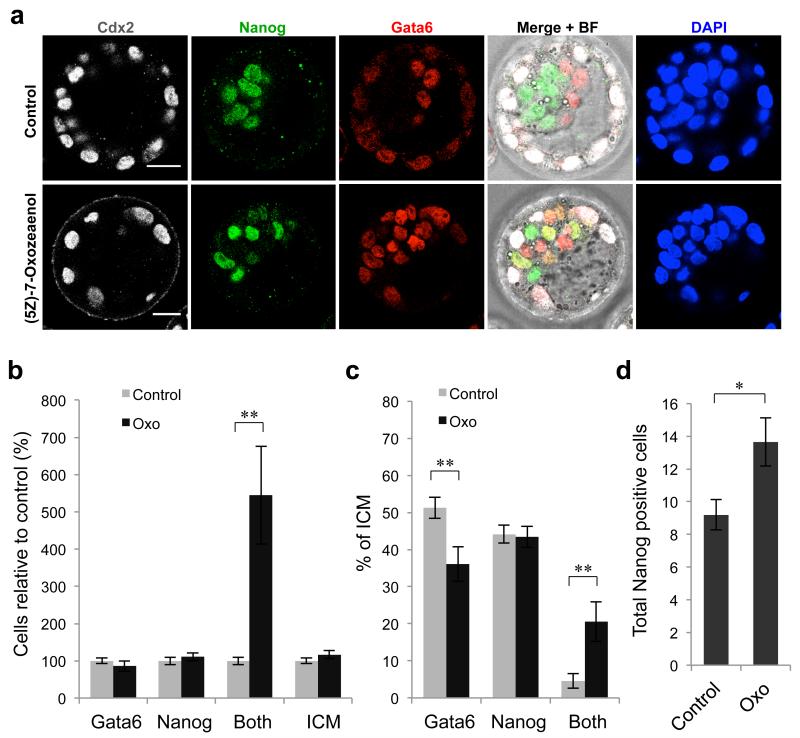
The strongest effects on PE development were observed with Dorsomorphin and (5Z)-7-Oxozeaenol, however our results suggested that these inhibitors might be acting in different ways to reduce PE cell number. To further investigate this hypothesis, we analysed the expression of Gata6 and Nanog in E4.5 embryos treated with 500 nM (5Z)-7-Oxozeaenol from E2.5, compared with controls (Fig. 6a-c). Control embryos at this stage had well-sorted PE and EPI cells, with only 5% of ICM cells co-expressing both Gata6 and Nanog. In contrast, 21% of ICM cells in embryos treated with (5Z)-7-Oxozeaenol expressed both genes (p < 0.01, Student’s t-test) (Fig. 6c). The percentage of ICM cells expressing only Gata6 was reduced compared with controls (36% compared with 51%, p < 0.01, Student’s t-test), while the percentage of ICM cells expressing only Nanog was unchanged (43% compared with 44%). These results suggest that (5Z)-7-Oxozeaenol does not prevent Gata6-positive PE precursor cell specification, but rather prevents efficient down-regulation of Nanog and up-regulation of later PE genes such as Sox17. The total number of Nanog-positive cells (including those that are also positive for Gata6) in (5Z)-7-Oxozeaenol-treated embryos at E4.5 is therefore significantly increased compared with control embryos (13.6 cells compared with 9.2 cells, p < 0.05, Student’s t-test) (Fig. 6d), as observed earlier. This indicates that inhibition of the Smad-independent BMP pathway by (5Z)-7-Oxozeaenol treatment leads to a reduction in the amount of PE formed, not because of an ICM cell fate switch from PE to EPI, but because of a failure of the PE fate program.
Figure 6. (5Z)-7-Oxozeaenol treatment impairs PE cell differentiation.
(a-d) Embryos were incubated in the presence of 500 nM (5Z)-7-Oxozeaenol (n = 15) for 48 hours from E2.5 to E4.5 (controls n = 15). (a) Expression of Cdx2, Gata6 and Nanog at E4.5. Scale bars = 20 μm. (b) Number of cells relative to controls at E4.5. Both = positive for Gata6 and Nanog. (c) Percentage of ICM at E4.5. (d) Number of Nanog-positive cells at E4.5 (including those also positive for Gata6). * denotes p < 0.05, ** denotes p < 0.01 (Student’s t-test). Error bars represent s.e.m.
Discussion
The segregation of cell lineages in the pre-implantation mouse embryo occurs through asymmetric cell divisions that generate outside and inside cells. By analysing the complete transcriptomes of 16-cell stage inside and outside cells we have identified that components of the BMP signalling pathway are already differentially expressed at this early stage. Most notably, inside cells express the ligands Bmp4 and Bmp7 and outside cells express the receptor Bmpr2. The differential expression of these genes so early in development was unexpected as the BMP signalling pathway has not been thought to play a role in pre-implantation development. Indeed, knock-out mice that lack individual components of the BMP pathway have been reported to develop beyond implantation 10,11,17,18. Differential expression of BMP ligands and receptors in different pre-implantation cell lineages has however been observed before, with Bmp4 and Bmp7 found in the ICM and Bmpr2 in the TE at the blastocyst stage 2,3,32. Our data therefore shows the same pattern of differential expression in the first distinct precursors of these lineages. Gene expression alone does not mean that BMP signalling is active at these stages, however we show here that nuclear pSmad1, which indicates activity of the Smad-dependent BMP pathway, is present already at the early blastocyst stage (Fig. 1), in agreement with a previous report 27. To determine whether active BMP signalling is important for pre-implantation development we used dominant negative forms of Smad4 and Bmpr2. Microinjection of dnSmad4 or dnBmpr2 into one blastomere at the late two-cell stage, thereby inhibiting BMP signalling in half of the embryo from the four-cell stage, resulted in a significant reduction in the number of TE and PE cells in the injected clone at E4.5, but did not affect the embryonic EPI (Fig. 2), suggesting that BMP signalling is important for the development of these two extra-embryonic lineages. Smad4 and Bmpr2 knock-out embryos are able to develop past implantation, however they exhibit abnormalities in extra-embryonic tissues and fail to gastrulate 10,11,17,18.
To confirm that BMP signalling is important for the pre-implantation development of the TE and PE lineages we next cultured embryos in the presence of three different BMP inhibitors as these lineages are first set apart, from the eight-cell stage onwards (Fig. 3). Treatment with all of the inhibitors caused a dose-dependent decrease in the number of PE cells, with the Bmpr1 inhibitor Dorsomorphin having the most severe phenotype, causing a reduction in PE cells by 91% at 1.0 μM concentration. All three inhibitors also impaired development of the TE, although to a lesser extent than dominant negative inhibition of Smad4 or Bmpr2. Because the dominant negative mRNAs were injected into the embryos a day earlier than the start of the inhibitor treatments, we hypothesised that their greater effect on the TE may be due to an early effect before the eight-cell stage. Incubation of embryos with Dorsomorphin from the late two-cell stage confirmed that this was indeed the case as TE cell number was reduced by 54% relative to controls, compared with only 9% when the inhibitor was added at E2.5 (Fig. 3). The effect of Dorsomorphin treatment on the PE lineage was not influenced by the longer incubation, with both treatment times resulting in a reduction of PE cells of around 90%. It is therefore likely that the reduction of PE cells represents a slightly later phenotype of BMP inhibition than that of TE cells, probably because the PE lineage is specified later in development. The observed effects on these more differentiated lineages could potentially result from an impaired differentiation process. BMP4 has been shown to be able to induce the differentiation of ES cells into TE and PE in vitro 16,34. While the differentiation of ES cells is not a direct model for the differentiation of pluripotent blastomeres, it is possible that similar mechanisms could be involved. The Bmp4-induced Smad pathway activates expression of the TE transcriptional program in ES cells through the interaction of pSmad1 with an evolutionary conserved enhancer region in intron 1 of Cdx2 16. The expression of Bmpr2 in outside cells at the 16-cell stage could relate to a role of BMP signalling in the development of the extra-embryonic lineages because outside cells at the 16-cell stage are progenitors of both the TE and PE. Indeed, the inside daughters of 16-cell stage outside cells are biased towards a PE fate 6,35. An important question is why these pre-implantation phenotypes have not been observed when studying knock-out mice that lack BMP signalling pathway components. Many inhibitor-treated embryos, despite severely compromised PE and TE lineages, were able to hatch and therefore would most likely be able to implant. An early effect of BMP signalling inhibition on blastocyst development could therefore have been missed in knock-out embryos if the cells in each lineage were not counted prior to implantation. In addition, it is also possible that an early phenotype may have been masked by the influence of maternal factors in the oviduct and uterus.
To investigate how the number of extra-embryonic cells becomes decreased following BMP inhibitor treatment we examined the embryos for apoptotic cells (Fig. 4). We found evidence of increased levels of apoptosis in embryos treated with Noggin and Dorsomorphin, indicating that programmed cell death is responsible, at least in part, for the decrease in TE and PE cells. Interestingly, treatment with (5Z)-7-Oxozeaenol, which inhibits an alternative Smad-independent downstream pathway, was not associated with an increased incidence of cell death. Moreover, treatment with (5Z)-7-Oxozeaenol also resulted in an increase in EPI cells, while the other two inhibitors did not, and it was the only inhibitor not to reduce total cell number in the blastocyst suggesting that it impairs pre-implantation development by an alternative mechanism to Noggin and Dorsomorphin.
To determine whether the BMP signals important for the development of the extra-embryonic lineages are generated by inside or outside cells, we knocked-down expression of Bmp4 and Bmp7 (Fig. 4) as these ligands are expressed exclusively by inside cells at the 16-cell stage and by ICM cells in the blastocyst 2,3,32. Depletion of these ligands alone resulted in a decrease in the number of TE and PE cells, consistent with the inhibition of BMP signalling. This suggests that BMP signals produced by the pluripotent inside cells are critical for regulating the development of the extra-embryonic TE and PE. Outside cells also express Bmp ligands, so an involvement of autocrine BMP signalling in their development cannot be excluded, however the dramatic reduction of extra-embryonic cells in Bmp4+7 siRNA embryos suggests that these inside-produced signalling molecules are the predominant ligands involved.
The effect of BMP inhibition on the PE lineage is particularly interesting as the earliest phenotype reported in Smad4 knock-out mice is abnormal visceral endoderm, a tissue derived from the pre-implantation PE 17. These embryos are reduced in size, have secondary defects in gastrulation and die by E7.5. In embryos treated with Dorsomorphin Sox17-positive PE cells were almost completely absent. To determine whether this is the result of a failure of PE cell specification, a defect in PE differentiation or specific elimination of PE precursor cells, we analysed the expression of Gata6 after Dorsomorphin treatment at both the early (E3.5) and late (E4.5) blastocyst stages (Fig. 5). Gata6 is the earliest marker of PE precursor cells 36,37 and therefore the presence of cells in the E4.5 ICM that are positive for Gata6 but negative for Sox17 would imply a fault in the PE transcriptional program. We found that Gata6-positive cells were reduced by 78% in Dorsomorphin-treated embryos at E4.5, indicating a lack of PE cells rather than an effect on PE cell differentiation. At E3.5, the number of Gata6-positive cells was significantly less than in controls, although this could possibly be due to a slight developmental delay in these embryos.
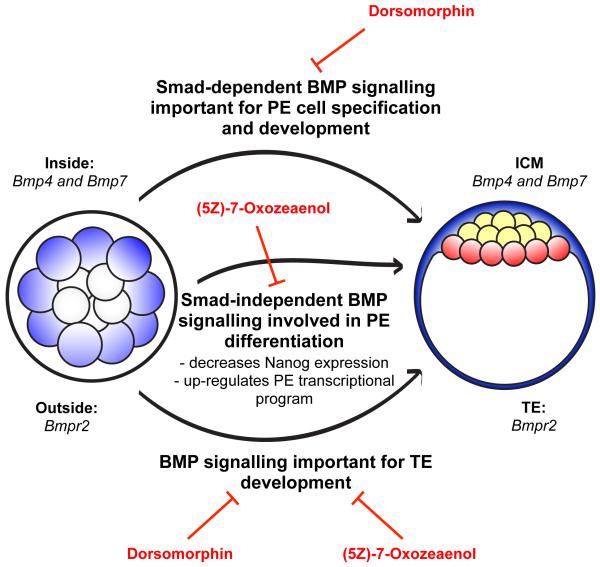
These results, along with the presence of dead and dying cells on the surface of the ICM at E4.5 (Fig. 5), suggest that Dorsomorphin treatment reduces the number of PE cells in the pre-implantation embryo primarily through apoptosis of PE precursors during blastocyst maturation from E3.5 to E4.5, but also reduces the number of PE precursors specified in the early ICM. The greater effect of Dorsomorphin treatment on PE cell number compared with injection of dnSmad4 or dnBmpr2 most likely relates to degradation of the injected mRNAs over time so that they had a lesser effect in later pre-implantation stages. It is also relevant that the dominant negatives were expressed only in half of the embryo and therefore a potential influence of the non-injected half of the embryo on the injected clone of cells cannot be excluded. Embryos treated with (5Z)-7-Oxozeaenol had an increased number of Nanog-positive cells at E4.5 and did not have an increased incidence of apoptosis, suggesting that (5Z)-7-Oxozeaenol impairs development of the PE by a different mechanism to Dorsomorphin. By analysing Gata6 and Nanog expression in (5Z)-7-Oxozeaenol-treated embryos we discovered that in these embryos PE precursor cells fail to down-regulate Nanog and activate the later PE fate transcriptional program (Fig. 6), indicating that BMP signalling is important for multiple aspects of PE cell development that are possibly mediated by different downstream pathways (Fig. 7). In conclusion, through the use of chemical inhibitors, Bmp-specific siRNAs and the expression of dominant negative components of the BMP pathway we have uncovered an unexpected role of BMP signalling in the pre-implantation development of extra-embryonic cell lineages.
Figure 7. Roles of BMP signalling in pre-implantation development.
BMP signalling is important for the development of both extra-embryonic cell lineages, the TE and PE. Inhibitors of both the Smad-dependent (Dorsomorphin) and Smad-independent ((5Z)-7-Oxozeaenol) BMP pathways impair development of the TE but have different effects on the PE. Blocking the Smad-dependent pathway results in the elimination of PE precursor cells, while blocking the Smad-independent pathway compromises the differentiation of PE cells.
Methods
Embryo culture and inhibitor treatments
Animals were maintained in the Gurdon Institute Animal Facility and all experiments were conducted in compliance with Home Office regulations. Mouse embryos were collected from 4- to 6-week-old F1 (C57Bl6 × CBA) females superovulated with 7.5 IU of pregnant mares’ serum gonadotropin (PMSG; Intervet) and 7.5 IU human chorionic gonadotropin (hCG; Intervet) 48 hours later, and mated with F1 males. Embryos were isolated in M2 medium containing 4 mg ml−1 BSA and cultured in KSOM medium as described previously 21. The following inhibitors were used: Mouse recombinant Noggin (ProSpec), Dorsomorphin (Tocris Bioscience) (5Z)-7-Oxozeaenol (Tocris Bioscience) and Actinomcyin D (Sigma). All inhibitors were dissolved in DMSO. DMSO diluted in KSOM to an equivalent volume to the highest concentration of inhibitor was used for controls. Human recombinant BMP4 (Sigma) was added to the medium where indicated at a concentration of 100 ng ml−1.
Isolation of cells for mRNA-sequencing
Embryos were collected into M2 medium at the four-cell stage and their progression to the eight-cell stage was monitored. Embryos were grouped based on the time they entered the eight-cell stage and embryos in the same group were observed to have undertaken eight- to 16-cell divisions before being incubated in M2 with a fluorescently labelled 0.2 μm microsphere suspension (Polysciences, Inc.) diluted to 1:50 for 30 seconds. This brief incubation ensures that only outside cells are sufficiently bound by the beads. Embryos were subsequently disaggregated in divalent cations-free M2 medium with gentle pipetting. Outside (strongly fluorescent) and inside (non-fluorescent) cells were isolated, released into cell lysis buffer (PicoPure RNA isolation kit, Arcturus) and frozen on dry ice.
SOLiD sequencing and data pre-processing
Purified RNA was subjected to the mRNA-seq protocol as described in detail elsewhere 19. Briefly, oligo(dT)-primed cDNA was generated and amplified representing approximately 1 kb coverage at the 3′ end of all polyadenylated transcripts. SOLiD 4 machine (Applied Biosystems) was used to produce 50-base colour-space reads. Greater than 90 million reads for each of the two libraries was generated and greater than 35 million of each uniquely mapped to the genome. Reads were mapped to the mouse genome (v. NCBI137/mm9) using Bioscope software (Applied Biosystems). Normalisation was carried out for sequencing depth (the total number of uniquely aligned reads).
qRT-PCR of inside and outside cells
For analysis of gene expression at the 16-cell stage, quantitative RT-PCR was performed on the Fluidigm platform following RNA amplification as described earlier 2 using RNA isolated from 3-4 cells of the same kind (inside or outside from 16-cell stage embryos, isolated as above). Expression of Actin B mRNA was used for normalization. Relative expression in inside (9 replicates) and outside (14 replicates) cells was calculated using ΔΔCt method.
Immunofluorescence
Immunofluorescence was carried out as described previously 38. Briefly, embryos were fixed in 4% paraformaldehyde, washed in PBS-T (PBS containing 0.1% Tween) and permeabilized with 0.5% Triton X-100 for 20 minutes. Embryos were incubated with primary antibodies in 3% BSA (Sigma) in PBS-T overnight at 4°C. The embryos were then washed twice in PBS-T and incubated with secondary antibodies (Invitrogen, AlexFluor; 1:400 in 3% BSA) for 2 hours before final washes in PBS-T and M2 and imaging in drops of M2 on glass-bottomed dishes. Primary antibodies used: goat anti-Sox17 (R&D Systems), mouse anti-Cdx2 (Biogenex), rabbit anti-Nanog (2B Scientific), goat anti-Gata4 (Santa Cruz), goat anti-Gata6 (R&D Systems), mouse anti-Oct4 (Santa Cruz), rabbit anti-pSmad1 (Cell Signaling) and rabbit anti-cleaved Caspase 3 (R&D Systems). DAPI was used as the nuclear counterstain in all experiments and all primary antibodies were used at a dilution of 1:200. Multi-channel images were acquired for multiple sections using a Leica SP5. Image processing and cell counting were performed with Fiji software (http://fiji.sc).
Microinjection of pre-implantation embryos
A dominant negative form of Smad4 (dnSmad4; 39) was cloned downstream of the TagRFP protein in pRN3P as described previously 40. A dominant negative form of Bmpr2 (dnBmpr2; 41) was cloned upstream of the Ruby protein. To express the dominant negative proteins, one blastomere of two-cell stage embryos was injected with dnSmad4 or dnBmpr2 mRNA (500 ng μl−1) and Gap43-RFP mRNA (400 ng μl−1) as a marker of injection. Control embryos were injected with Gap43-RFP mRNA alone. Embryos were fixed at E4.5 and the lineage contributions of Gap43-RFP-expressing cells assessed by immunofluorescence. To deplete expression of Bmp4 and Bmp7 in the embryo, two-cell stage embryos were injected into both blastomeres with a combination of four siRNAs (two each targeting Bmp4 and Bmp7) at a total concentration of 16 μM. Controls were injected with 16 μM AllStars Negative Control siRNA (Qiagen) and all embryos were co-injected with 100 ng μl−1 Gap43-GFP mRNA as an injection control. The embryos were fixed at E4.5 and the number of cells in each lineage assessed by immunofluorescence. The siRNA sequences are as follows: Bmp4 siRNA 1: GCGGTCCAGGAAGAAGAATAA, Bmp4 siRNA 2:
AGGAGTTTCCATCACGAAGAA, Bmp7 siRNA 1:
CGGGAGATCCTGTCCATCTTA, Bmp7 siRNA 2:
CAGCTCTAATGTCATCCTGAA.
qRT-PCR for analysis of siRNA efficiency
For confirmation of knockdown, embryos were collected 48 hours after siRNA injections (E3.5). Total RNA was extracted using the Arcturus PicoPure RNA Isolation Kit and qRT-PCR was performed using the Power SYBR Green RNA-to-CT 1-Step Kit (Life Technologies) and a StepOne Plus Real-time PCR machine (Applied Biosystems). Relative levels of transcript expression were assessed by the ΔΔCt method, with Gapdh as an endogenous control. The following primers were used:
Bmp4 5′ -TCTAGAGGTCCCCAGAAGCA-3′, 5′ -
AGGAATCATGGTGTCTTGACAGA-3′ and Gapdh 5′ -
AGAGACGGCCGCATCTTC-3′, 5′ -CCCAATACGGCCAAATCCGT-3′.
Time-lapse imaging
Eight-cell stage embryos from F1 × GFP-GPI 42 matings were cultured in KSOM with or without Dorsomorphin for 48 hours. Green fluorescence and transmitted light multi-section images were acquired every 20 minutes using a spinning disk confocal microscopy system and analysed using SlideBook software (3i Intelligent Imaging Innovations).
Supplementary Material
Acknowledgments
We are grateful to Ivan Bedzhov for the pSmad1 staining and the members of our group and David Glover for helpful discussions. This work was supported by a Wellcome Trust programme grant to MZG and an Agency for Science, Technology and Research (A*Star) core research budget to PR.
Footnotes
Competing financial interests: The authors declare no competing financial interests.
Accession codes: NCBI BioSample accession numbers are SAMN03098868 (outside cells) and SAMN03098869 (inside cells). Sequencing data has been deposited in the NCBI short read archive under BioProject ID PRJNA263313.
References
- 1.Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- 2.Guo G, et al. Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell. 2010;18:675–685. doi: 10.1016/j.devcel.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Tang F, et al. Deterministic and stochastic allele specific gene expression in single mouse blastomeres. PLoS One. 2011;6:e21208. doi: 10.1371/journal.pone.0021208. doi:10.1371/journal.pone.0021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohnishi Y, et al. Cell-to-cell expression variability followed by signal reinforcement progressively segregates early mouse lineages. Nat Cell Biol. 2014;16:27–37. doi: 10.1038/ncb2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris SA, Graham SJL, Jedrusik A, Zernicka-Goetz M. The differential response to Fgf signalling in cells internalized at different times influences lineage segregation in preimplantation mouse embryos. Open Biology. 2013;3 doi: 10.1098/rsob.130104. doi:10.1098/rsob.130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krupa M, et al. Allocation of inner cells to epiblast vs primitive endoderm in the mouse embryo is biased but not determined by the round of asymmetric divisions (8→16- and 16→32-cells) Developmental Biology. 2014;385:136–148. doi: 10.1016/j.ydbio.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Ozair MZ, Kintner C, Brivanlou AH. Neural induction and early patterning in vertebrates. Wiley Interdisciplinary Reviews: Developmental Biology. 2013;2:479–498. doi: 10.1002/wdev.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes and Development. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 9.De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of spemann’s organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beppu H, et al. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- 11.Chu GC, Dunn NR, Anderson DC, Oxburgh L, Robertson EJ. Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. doi: 10.1242/dev.01248. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara T, Dehart DB, Sulik KK, Hogan BLM. Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development. 2002;129:4685–4696. doi: 10.1242/dev.129.20.4685. [DOI] [PubMed] [Google Scholar]
- 13.Lawson KA, et al. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes and Development. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares ML, et al. Functional studies of signaling pathways in peri-implantation development of the mouse embryo by RNAi. BMC Dev Biol. 2005;5:28. doi: 10.1186/1471-213X-5-28. doi:1471-213X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes and Development. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi Y, et al. BMP4 induction of trophoblast from mouse embryonic stem cells in defined culture conditions on laminin. In Vitro Cell Dev Biol Anim. 2010;46:416–430. doi: 10.1007/s11626-009-9266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirard C, et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Li C, Xu X, Deng C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci U S A. 1998;95:3667–3672. doi: 10.1073/pnas.95.7.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang F, et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- 20.Feng J, et al. GFOLD: a generalized fold change for ranking differentially expressed genes from RNA-seq data. Bioinformatics. 2012;28:2782–2788. doi: 10.1093/bioinformatics/bts515. [DOI] [PubMed] [Google Scholar]
- 21.Jedrusik A, et al. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22:2692–2706. doi: 10.1101/gad.486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skamagki M, Wicher KB, Jedrusik A, Ganguly S, Zernicka-Goetz M. Asymmetric localization of Cdx2 mRNA during the first cell-fate decision in early mouse development. Cell Rep. 2013;3:442–457. doi: 10.1016/j.celrep.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ralston A, et al. Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development. 2010;137:395–403. doi: 10.1242/dev.038828. [DOI] [PubMed] [Google Scholar]
- 24.Frankenberg S, et al. Primitive endoderm differentiates via a three-step mechanism involving Nanog and RTK signaling. Dev Cell. 2011;21:1005–1013. doi: 10.1016/j.devcel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–724. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- 26.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, et al. Antagonism between Smad1 and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. J Cell Biol. 2009;184:323–334. doi: 10.1083/jcb.200808044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh DW, Godson C, Brazil DP, Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends Cell Biol. 2010;20:244–256. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Yu PB, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ninomiya-Tsuji J, et al. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 31.Fabian D, et al. Inhibitory effect of IGF-I on induced apoptosis in mouse preimplantation embryos cultured in vitro. Theriogenology. 2004;61:745–755. doi: 10.1016/s0093-691x(03)00254-1. [DOI] [PubMed] [Google Scholar]
- 32.Coucouvanis E, Martin GR. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- 33.Wang QT, et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6:133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi M, Takada T, Takahashi K, Noda Y, Torii R. BMP4 induces primitive endoderm but not trophectoderm in monkey embryonic stem cells. Cloning Stem Cells. 2008;10:495–502. doi: 10.1089/clo.2008.0030. [DOI] [PubMed] [Google Scholar]
- 35.Morris SA, et al. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proc Natl Acad Sci U S A. 2010;107:6364–6369. doi: 10.1073/pnas.0915063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- 37.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Jedrusik A, et al. Maternally and zygotically provided Cdx2 have novel and critical roles for early development of the mouse embryo. Dev Biol. 2010;344:66–78. doi: 10.1016/j.ydbio.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- 40.Zernicka-Goetz M, et al. Following cell fate in the living mouse embryo. Development. 1997;124:1133–1137. doi: 10.1242/dev.124.6.1133. [DOI] [PubMed] [Google Scholar]
- 41.Pouliot F, Blais A, Labrie C. Overexpression of a dominant negative type II bone morphogenetic protein receptor inhibits the growth of human breast cancer cells. Cancer Res. 2003;63:277–281. [PubMed] [Google Scholar]
- 42.Rhee JM, et al. In vivo imaging and differential localization of lipid-modified GFP-variant fusions in embryonic stem cells and mice. genesis. 2006;44:202–218. doi: 10.1002/dvg.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.