Abstract
Background and Purpose
In acute ischemic stroke, the Hyperdense Artery Sign (HAS) on non-contrast CT is thought to represent intra-luminal thrombus and therefore is a surrogate of arterial obstruction. We sought to assess the accuracy of HAS as a marker of arterial obstruction by thrombus.
Methods
The Third International Stroke Trial (IST-3) was a randomized controlled trial testing use of intravenous thrombolysis for acute ischemic stroke in patients who did not clearly meet the prevailing license criteria. Some participating IST-3 centers routinely performed CT or MR angiography (CTA and MRA, respectively) at baseline. One reader assessed all relevant scans independently, blinded to all other data; we checked observer reliability. We combined IST-3 data with a systematic review and meta-analysis of all studies that assessed the accuracy of HAS using angiography (any modality).
Results
IST-3 had 273 patients with baseline CTA or MRA and was the largest study of HAS accuracy. The meta-analysis (n=902+273=1175, including IST-3) found sensitivity and specificity of HAS for arterial obstruction on angiography to be 52% and 95%, respectively. HAS was more commonly identified in proximal than distal arteries (47% versus 37%, p=0.015), and its sensitivity increased with thinner CT slices (r=−0.73, p=0.001). Neither extent of obstruction nor time after stroke influenced HAS accuracy.
Conclusions
When present in acute ischemic stroke, HAS indicates a high likelihood of arterial obstruction, but its absence indicates only a 50/50 chance of normal arterial patency. Thin-slice CT improves sensitivity of HAS detection.
Keywords: Hyperdense artery, Stroke, Angiography, Meta-analysis
INTRODUCTION
Non-contrast CT remains the primary imaging modality for hyperacute assessment of stroke in most centers1. Identifying features of acute ischemic stroke on CT therefore remains important for routine practice. Hyper-attenuation of a cerebral artery on non-contrast CT in acute ischemic stroke is thought to represent acute thrombus or embolus; the presence of the Hyperdense Artery Sign (HAS) therefore is a surrogate of arterial obstruction and may provide useful confirmation of the diagnosis of acute ischemic stroke. The sign has been defined as any artery that subjectively appears transiently denser than adjacent or equivalent contralateral vessels2,3 although objective measures have also been applied4. Compared with angiography, previous studies have shown that the HAS is a specific (although false positives are described5) but not very sensitive indicator of arterial obstruction6,7. To our knowledge, no systematic review and meta-analysis of HAS sensitivity and specificity has been published.
The Third International Stroke Trial (IST-3) was a multi-center, randomized controlled trial which tested intravenous thrombolysis (Alteplase) given within 6 hours of ischemic stroke8. Baseline (pre-randomization) and follow-up (within 48 hours) brain imaging (predominantly non-contrast CT) was performed for all IST-3 patients (n=3035). In some centers, CT or MR angiography (CTA and MRA, respectively) were also routinely obtained pre-randomization as part of their local stroke imaging protocol9.
In a prespecified analysis, we investigated the diagnostic accuracy of HAS for arterial obstruction detected with CTA or MRA and assessed if characteristics of the non-contrast CT scan (slice thickness), the corresponding angiographic obstruction (location, extent), or the patient (time from stroke onset) affected the accuracy of HAS. We examined data from IST-3 and performed a systematic review and meta-analysis of previous studies.
METHODS
Third International Stroke Trial
IST-3 was an international, multi-center, prospective, randomized, open, blinded endpoint (PROBE) trial of intravenous rt-PA (recombinant tissue plasminogen activator) in acute ischemic stroke. Ethical approval, enrolment and data collection were described elsewhere10. Briefly, patients with acute stroke of any severity, with no upper age limit, were eligible for trial inclusion if, in the opinion of the responsible physician the patient might benefit from rt-PA and there was no clear indication for or contraindication to rt-PA, if intravenous rt-PA could be started within 6 hours of stroke onset and CT/MR imaging had reliably excluded both intracranial hemorrhage and any structural stroke mimic. In other words, patients who definitely met the prevailing strict license criteria, or who had definite contraindications to rt-PA were not eligible. Many patients fell outside the strict license criteria and did not have definite contraindications and therefore could be randomized in the trial. Stroke severity prior to randomization was assessed with the National Institutes of Health Stroke Scale (NIHSS). Patients were randomized to receive intravenous rt-PA (0.9mg/Kg) or control. No intra-arterial therapy was used. Functional status was assessed at six-months with the Oxford Handicap Scale (OHS). IST-3 is registered, ISRCTN25765518.
The imaging protocol required that non-contrast CT scans extend from the foramen magnum to vertex, with maximum slice thickness 4-5mm through the posterior fossa and 8-10mm for the cerebral hemispheres. There was no pre-defined requirement for thin-slice sections but all acquired data including spiral volumes were accepted.
CTA or MRA data were also collected if available; the protocol for the IST-3 angiography substudy specified minimum acquisition standards11. Only IST-3 patients who had CTA or MRA performed concurrently with baseline non-contrast CT are included in this present analysis.
All centers had to submit test imaging to the IST-3 central office for quality assessment prior to being certified to join the trial.
Image analysis
A single neuroradiologist evaluated all relevant IST-3 images analysing first the non-contrast CT followed by CTA or MRA sequentially and independently, blinded to any subsequent imaging or other scan reads, clinical and treatment data, using a validated, pre-specified rating proforma (see: www.sbirc.ed.ac.uk/research/imageanalysis.html) that recorded presence, location, extent of HAS and any angiographic obstruction9,11.
Standard brain window settings (center 40 Hounsfield Units [HU], width 80 HU) were used for non-contrast CT analysis but these could be altered as required. We identified HAS on non-contrast CT if the lumen of any intracranial artery appeared more dense than adjacent or equivalent contralateral arteries but non-calcified. Thin-slice sections were used where possible to minimize volume averaging of any arterial wall calcification. We classified the internal carotid, mainstem of middle cerebral, vertebral and basilar arteries as ‘proximal’ and the sylvian branches of middle cerebral and any part of the anterior or posterior cerebral arteries as ‘distal’ for analysis purposes. We classified arterial obstruction on CTA/MRA using a modified 4 point Thrombolysis in Cerebral Infarction (TICI) score9,11.
To assess intra-observer reliability of HAS and CTA/MRA, the single neuroradiologist repeated ratings of 15 randomly selected patients at least 2 months later. To assess inter-observer reliability, we compared the single neuroradiologist to the ratings performed by the IST-3 expert image reading panel performed separately using the same analysis method11 (details of expert panel are provided in online Appendix II; these expert panel reads were not otherwise used in this present analysis).
Systematic Review and Meta-Analysis
We performed the systematic review and meta-analysis according to the PRISMA 2009 checklist12.
Search Strategy
We searched Embase and Medline (see online Table I for full strategy) between 1980 and September 2013, since HAS was first described in the early 1980s2, including hand searching references of returned papers.
Inclusion/exclusion criteria and data extraction
We screened abstracts for more in-depth assessment and included only peer-reviewed original articles, published in English, that contained data on ischemic stroke patients assessed for HAS who underwent invasive or non-invasive angiography.
We assessed study quality for secondary eligibility criteria, using a modified STARD checklist13 (online Table II). We excluded articles if imaging was performed >24 hours after stroke onset (limit chosen to include articles assessing posterior fossa HAS) or if <20 patients underwent CT-, MR-, or digital subtraction angiography.
Two observers independently extracted data to calculate true and false positive and negative rates. We only meta-analysed papers where sensitivity or specificity (ideally both) could be calculated. We also recorded time from stroke onset to imaging, location and/or extent of angiographic obstruction. Disagreements were resolved by consensus.
Statistics
We compared clinical characteristics of the IST-3 patients with angiography to all IST-3 patients using t-tests, Mann-Whitney U tests, or Chi-squared tests as appropriate. We assessed observer reliability using the Kappa statistic. We used Spearman’s rank correlation coefficient to assess correlations between normally distributed continuous data and t-tests to compare ratios of patients with and without HAS in the systematic review.
For simplicity in the present analysis and to harmonise angiographic scoring between papers, we dichotomised angiography as ‘normal’ or ‘obstructed’ (i.e. any luminal narrowing or occlusion). We compared the angiography location of arterial obstruction with the HAS location, noting false positives and negatives.
We calculated sensitivity (true positives/[true positives plus false negatives]), specificity (true negatives/[true negatives plus false positives]) in individual studies. We meta-analysed sensitivity and specificity with a random effects model in R2.8.1 (http://cran.r-project.org/), using the DiagMeta function, modelling within-study variation as a binomial proportion14 (joint meta-analysis of sensitivity and specificity was not possible due to estimation problems).
Unless stated otherwise, all analyses were performed using SPSS Statistics software, version 20.0 (IBM Corporation, NY, USA) and a p-value <0.05 was considered significant.
RESULTS
In total, 273 IST-3 patients (9% of the total of 3035) had baseline CTA (n=269) or MRA (n=4). Patients with (versus without) angiography had very similar baseline characteristics, but less severe strokes (median NIHSS 10 versus 11, p=0.020) and better 6-month outcomes (median OHS 3 versus 4, p=0.002); online Table III.
Of the 273 IST-3 patients with angiography, 114 (42%) had some degree of luminal obstruction on angiography while 69 (25%) had a HAS.
Inter- and intra-observer reliability (Kappa) for identification of HAS was 0.59 and 0.58, respectively; for any versus no obstruction on angiography was 0.59 and 0.82, respectively.
Reliability of HAS versus angiography in IST-3
In IST-3, HAS correctly identified arterial obstruction in 62, was falsely positive in 7 and falsely negative in 52, giving a sensitivity of 54% (95% CI 45-64%) and a specificity of 96% (92-99%).
Sensitivity, but not specificity, improved with thinner baseline non-contrast CT scan slices: ≤3mm slices, n=162, sensitivity 62%, specificity 98%; versus >3mm slices, n=108, sensitivity 41%, specificity 92%, (p=0.031, p=0.089, respectively). There was no difference in the prevalence of HAS by location of arterial obstruction: proximal n=91, sensitivity 55% versus distal, n=23, sensitivity 52% (p=0.814). More extensive angiographic obstruction, i.e. involving more than one named artery (n=48) versus obstruction of one named artery (n=66), did not influence sensitivity of HAS (58% versus 52%, p=0.475). Time from stroke onset did not alter the accuracy of HAS: patients scanned ≤180 minutes (n=151) sensitivity 49%, specificity 97% versus patients scanned >180 minutes (n=122), sensitivity 61% (p=0.221), specificity 94% (p=0.500).
Systematic review, results of search
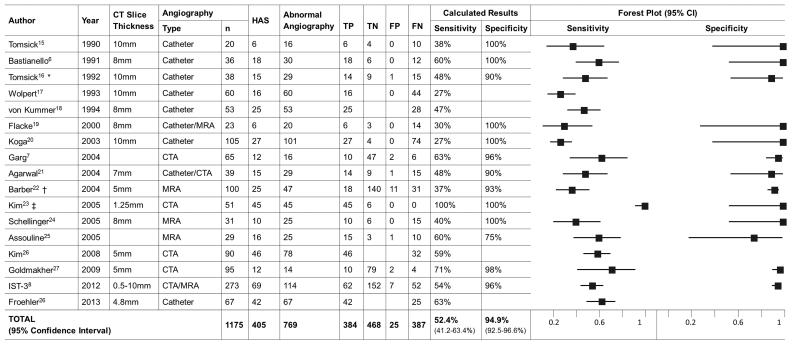
We identified 326 papers by database search: 75% discussed non-intracranial HAS; 10% were published only in abstract; 10% were review articles or non-English language; online Figure I. Thirty one articles underwent more in-depth assessment plus 5 further articles were found in reference lists, giving a total of 36 articles for full review. After secondary exclusion criteria, 16/36 original articles (n=902, Figure 1) remained for meta-analysis6,7,15-28. Twenty articles were excluded: 7 provided insufficient raw data; 6 had <20 patients with angiography; 2 failed essential quality criteria; 2 were duplicates; 2 included patients imaged >24 hours after stroke; 1 included non-ischemic strokes.
Figure 1.
Systematic review data for the 16 selected articles and IST-3
Footnote: Data from individual studies was only included if at least sensitivity or specificity could be calculated. Unless stated otherwise, CT slice-thickness refers to the thickest slices used. CTA = CT angiography. MRA = MR angiography. HAS = Hyperdense Artery Sign. TP = True positive. TN = True negative. FP = False positive. FN = False negative. CI = Confidence Interval.
* One patient had both a false positive HAS and a true occlusion without HAS (FN) in contralateral arteries; 39 results are therefore reported from 38 angiograms.
† Data for proximal and distal middle cerebral artery are presented separately providing assessment of 200 arterial segments from 100 angiograms.
‡ Thin-slice CT data are presented. Thick-slice (5mm) data for the same angiography is also available.
Quality assessment
The 16 papers identified in systematic review (n=902, not including IST-3) had a median of 52 patients (range 20-105); most (14/16, 88%) were prospective, only 7 (44%) provided specific inclusion and/or exclusion criteria and none included data from a randomized controlled trial.
Most papers provided scan parameters and time from stroke onset to scan (15/16, 94% in both cases). Catheter angiography was the commonest technique (9/16, 56%); CTA and MRA were equally common (5/16, 31%, 4/16, 25%, respectively) and used almost exclusively since 2003. Most articles declared the experience or professional position of those analysing images (14/16, 88%); with 24 neuroradiologists and 9 neurologists in the range of 1-6 observers per article (median 2). Image assessors were blinded to other data in 11/16 (69%) papers, 12/16 (75%) papers used a standardized definition for HAS, only 4 papers assessed reproducibility of HAS (median kappa-statistic for HAS detection 0.85, range 0.53-0.91) and no papers assessed reproducibility of angiography.
Meta-analysis
Amongst a total of 1175 patients with angiography including IST-3, 769 had arterial obstruction, and 405 had a HAS (Figure 1). The random effects summary estimate of sensitivity, based on 771 patients (384 true positive plus 387 false negative), was 52.4% (95% CI 41.2-63.4%). The random effects summary estimate of specificity, based on 493 patients, (468 true negative plus 25 false positive), was 94.9% (92.5-96.6%). Four studies with missing data were omitted from specificity analysis.
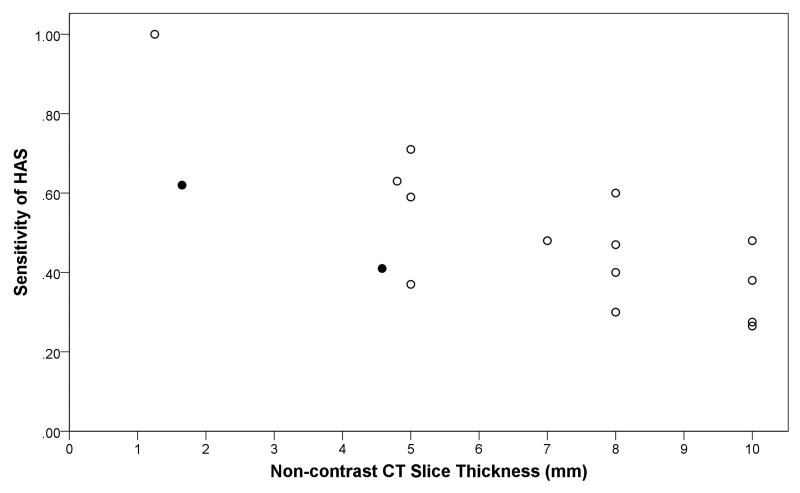
HAS was more common with angiographic obstruction in proximal arteries than distal (47% versus 37%, p=0.015), Table 1. CT slice thickness was significantly associated with sensitivity (Figure 2, r=−0.72, p=0.002) but not specificity of HAS, and was inversely proportional to the year of article publication (r=−0.80, p=0.001, Figure 1). The number of obstructed arterial segments (59% had HAS if ≥2 segments obstructed versus 49% if 1 segment obstructed, p=0.160) and time from stroke onset to scan (27% had HAS if ≤180 minutes from stroke onset versus 25% if >180 minutes, p=0.682), were not associated with HAS prevalence. Three studies with missing data were omitted from analyses of thrombus characteristics and time from stroke onset.
Table 1.
Systematic review data assessing how characteristics of arterial obstruction (location and extent) and time from stroke onset affect hyperdense artery sign (HAS) prevalence
| Author | Year | Angiography n | Location of Arterial Obstruction | Number of Obstructed Arterial Segments | Time from Stroke Onset to Scan (minutes) | |||
|---|---|---|---|---|---|---|---|---|
| Proximal | Distal | 1 | ≥2 | ≤180 | ≥180 | |||
| Assouline25 | 2005 | 39 | 8/16(50) | 6/7(86) | 11/17(65) | 4/8(50) | ||
| Barber22 | 2004 | 100 | 7/25(28) | 11/24(46) | ||||
| Bastianello6 | 1991 | 36 | 12/17(71) | 6/13(46) | ||||
| Flacke19 | 2000 | 23 | 6/10(60) | 0/10(0) | ||||
| Froehler28 * | 2013 | 67 | 15/20(75) | 23/43(53) | ||||
| Garg7 | 2004 | 65 | 1/8(13) | 8/57(14) | ||||
| Kim23 † | 2005 | 51 | 11/38(29) | 7/31(23) | ||||
| Kim26 | 2008 | 78 | 36/56(64) | 10/22(45) | ||||
| Koga20 | 2003 | 105 | 21/63(33) | 6/38(16) | ||||
| Tomsick15 | 1990 | 20 | 4/9(44) | 2/7(29) | 3/10(30) | 3/6(50) | ||
| Tomsick16 | 1992 | 38 | 7/14(50) | 5/12(42) | 8/22(36) | 6/7(86) | ||
| von Kummer18 | 1994 | 53 | 20/43(47) | 5/10(50) | ||||
| Wolpert17 | 1993 | 60 | 12/43(28) | 4/17(24) | ||||
| IST-38 | 2012 | 273 | 50/91(55) | 12/23(52) | 34/66(52) | 28/48(58) | 34/151(23) | 35/122(29) |
| TOTAL | 189/402(47) | 92/247(37) | 56/115(49) | 41/69(59) | 55/202(27) | 48/189(25) | ||
| p-value for difference | 0.015 | 0.160 | 0.682 | |||||
Results represent number of HAS within each total (%). Data were only included when results were available for both sides of the equation (e.g. proximal and distal); 3 articles with incomplete data are not included.
Unless otherwise stated, proximal arterial locations include internal carotid artery, main-stem of the middle cerebral artery (MCA), vertebral and basilar arteries. Distal arterial locations include sylvian branches of the MCA, and anterior and posterior cerebral arteries.
Proximal and distal arteries are defined here as internal carotid and middle cerebral arteries, respectively.
Thick-slice (5mm) CT data are presented here. Thin-slice data are also available.
Figure 2.
Relationship between the sensitivity of a hyperdense artery sign (HAS) for arterial obstruction and non-contrast CT slice thickness
Footnote: Closed dots represent IST-3 data (thin-slice ≤3mm, mean 1.65mm; thick-slice >3mm, mean 4.5mm). Open dots represent results from articles identified on systematic review. Two dots have been fractionally altered to reveal identical results (sensitivity = 0.27, slice thickness = 10mm). Correlation is r=−0.73, p=0.001.
DISCUSSION
We provide this first meta-analysis assessing the accuracy of HAS as a non-contrast CT marker of arterial obstruction in acute ischemic stroke and confirm using large patient numbers, that HAS is highly specific and moderately sensitive for angiographically demonstrated arterial obstruction with overall specificity 95% and sensitivity 52%. IST-3, as the largest individual study of HAS sensitivity and specificity, contributes 30% more data (273/902 patients, new total 1175) than previously available. Our results are widely applicable and in situations where angiographic imaging is not currently available, can enable those performing non-contrast CT to make the best use of all available imaging information; the presence of HAS provides substantial confidence that there is a very high likelihood of the diagnosis of acute ischemic stroke and of arterial obstruction. However, absence of HAS does not predict normal arterial patency; in acute ischemic stroke patients without HAS, approximately half will have arterial obstruction on angiography. It remains to be seen whether the presence (or absence) of acute arterial obstruction is important for intravenous thrombolysis treatment decisions; but in that context, or indeed in centers looking to perform appropriate endovascular therapy, this limitation of a ‘negative’ HAS might encourage centers performing acute ischemic stroke imaging to consider providing baseline CTA or MRA in all cases.
Our meta-analysis confirmed that HAS prevalence increases with thinner CT slices, but thinner slices have no effect on HAS specificity, perhaps as HAS is already highly specific for obstruction23. The mean diameter of intracranial arteries is less than 3mm. A slice thickness above this value, used in most of the included studies, may impair HAS sensitivity (especially in smaller arteries) by averaging intra-luminal thrombus and surrounding CSF space. Volumetric thin-slice CT is now widely available, as suggested by the highly significant inverse relationship between year of publication and CT slice thickness in the systematic review. Allowing for the rising availability of volumetric CT, sensitivity rates in current routine clinical practice are therefore likely to be on the high side of those we report here.
Meta-analysis also confirmed that HAS is more likely to be identified in proximal than distal arteries, probably reflecting the larger calibre of proximal arteries and greater volumes of thrombus required for obstruction26. Other factors such as extent of angiographic obstruction and time after stroke onset were not significantly related to HAS prevalence.
Strengths and limitations
IST-3 was conducted in many centers, so inevitably includes variability in scan parameters and protocols. However, IST-3 represents ‘real world practice’ and, combined with the systematic review, provides results that are widely applicable to centers assessing acute stroke with a range of CT scanners. Angiography in IST-3 was performed in about 10% of centers and may have been influenced by local practice, so has limitations. Nevertheless, IST-3 angiography is the largest complete dataset of its kind, the only one performed in the standardized context of a randomized trial, and increases the available data by almost one-third. We found only one larger dataset29 but it only included patients with a HAS precluding assessment of sensitivity and/or specificity.
We used a qualitative measure to identify HAS in IST-3 which reflects routine practice. Further work is ongoing to assess whether measuring intra-arterial thrombus density quantitatively improves accuracy for arterial obstruction or interacts with treatment response.
Our method of dichotomizing angiography results may have included some patients with chronic atheroma in the ‘obstructed’ group. This could erroneously raise the number of false negative HAS cases and thereby appear to reduce the sensitivity of HAS. However this is a general problem in acute stroke, no other studies that we identified in the literature had addressed this point, and we decided that the opposite approach (to only consider patients with occluded arteries as abnormal) would have been less accurate by having the same effect on HAS specificity but also by excluding patients with genuine non-dense thrombus from our analyses entirely.
By using PRISMA and STARD, we maintained a high quality systematic review and meta-analysis of HAS. We identified many papers, most not relevant, but we assert that evaluating several hundred abstracts was preferable to missing relevant work. Excluding abstract-only and non-English publications may have reduced the completeness and led to publication bias, but abstract-only publication provides insufficient raw data for our analyses.
The final 16 articles retained for meta-analysis were of moderate to high quality according to our criteria. In particular, most of the data were prospective and the methods were detailed enough to be replicated. More standard definitions for HAS and more consistent reporting of factors such as blinding of image assessment would improve future research30.
Conclusions
The high specificity of HAS provides confidence for its use as a surrogate marker of angiographic obstruction and to confirm the diagnosis of acute ischemic stroke. The moderate sensitivity means that absence of HAS cannot be used alone to indicate that angiography will be normal; those performing acute stroke imaging might therefore consider undertaking angiography in this context. Sensitivity of HAS is significantly improved with thin-slice volumetric CT.
Supplementary Material
ACKNOWLEDGEMENTS
The IST-3 collaborative group thanks all patients who participated in the study. The authors gratefully acknowledge the members of the angiography reading panel, non-contrast scan reading panel, trial steering committee, and national coordinators (online Appendix II).
SOURCES OF FUNDING
The IST-3 main trial was funded from many sources detailed in online Appendix III. The angiography study was funded by the National Institutes of Health Research (NIHR) Efficacy and Mechanisms Evaluation Panel (EME 08-43-52). The views are those of the authors and not of the NIHR.
Footnotes
DISCLOSURES
Grant Mair None
Elena Boyd None
Francesca Chappell None
Rüdiger von Kummer Lundbeck, Penumbra, Covidien, Brainsgate, Boehringer Ingelheim
Richard Lindley Boehringer Ingelheim, Covidien
Peter Sandercock Boehringer Ingelheim
Joanna Wardlaw None
Clinical Trial Registration Information URL: http://www.controlled-trials.com/ISRCTN25765518 Registration number: ISRCTN25765518
REFERENCES
- (1).Mair G, Wardlaw JM. Imaging of acute stroke prior to treatment: Current practice and evolving techniques. Br J Radiol. 2014:20140216. doi: 10.1259/bjr.20140216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gacs G, Fox AJ, Barnett HJ, Vinuela F. CT visualization of intracranial arterial thromboembolism. Stroke. 1983;14:756–62. doi: 10.1161/01.str.14.5.756. [DOI] [PubMed] [Google Scholar]
- (3).Pressman BD, Tourje EJ, Thompson JR. An early CT sign of ischaemic infarction: increased density in a cerebral artery. AJNR Am J Neuroradiol. 1987;8:645–8. doi: 10.2214/ajr.149.3.583. [DOI] [PubMed] [Google Scholar]
- (4).Koo CK, Teasdale E, Muir KW. What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis. 2000;10:419–23. doi: 10.1159/000016101. [DOI] [PubMed] [Google Scholar]
- (5).Rauch RA, Bazan CI, Larsson EM, Jinkins JR. Hyperdense middle cerebral arteries identified on CT as a false sign of vascular occlusion. AJNR Am J Neuroradiol. 1993;14:669–73. [PMC free article] [PubMed] [Google Scholar]
- (6).Bastianello S, Pierallini A, Colonnese C, Brughitta G, Angeloni U, Antonelli M, et al. Hyperdense middle cerebral artery CT sign. Comparison with angiography in the acute phase of ischemic supratentorial infarction. Neuroradiology. 1991;33:207–11. doi: 10.1007/BF00588219. [DOI] [PubMed] [Google Scholar]
- (7).Garg N, Eshkar N, Tanenbaum L, Cohen B, Sen S. Computed tomography angiographic correlates of early computed tomography signs in acute ischemic stroke. J Neuroimaging. 2004;14:242–5. doi: 10.1177/1051228404264938. [DOI] [PubMed] [Google Scholar]
- (8).The IST-3 Collaborative Group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–63. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wardlaw JM, Carpenter T, Sakka E, Mair G, Cohen G, Shuler K, et al. Imaging perfusion deficits, arterial patency and thrombolysis safety and efficacy in acute ischaemic stroke. An observational study of the effect of advanced imaging methods in The Third International Stroke Trial (IST-3), a randomised controlled trial. Efficacy Mech Eval. 2014:1. [PubMed] [Google Scholar]
- (10).Sandercock P, Lindley R, Wardlaw J, Dennis M, Lewis S, Venables G, et al. Third international stroke trial (IST-3) of thrombolysis for acute ischaemic stroke. Trials. 2008;9:37. doi: 10.1186/1745-6215-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wardlaw JM, von Kummer R, Carpenter T, Parsons M, Lindley R, Cohen G, et al. [Accessed 15 Oct 2014];Protocol for the perfusion and angiography imaging sub-study of the Third International Stroke Trial (IST-3) of alteplase treatment within six hours of acute ischemic stroke. [published online ahead of print 22 Jan 2013] Int J Stroke. 2013 doi: 10.1111/j.1747-4949.2012.00946.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1747-4949.2012.00946.x/abstract. [DOI] [PubMed]
- (12).Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD initiative. Clin Radiol. 2003;58:575–80. doi: 10.1016/s0009-9260(03)00258-7. [DOI] [PubMed] [Google Scholar]
- (14).Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat Med. 2009;28:2653–68. doi: 10.1002/sim.3631. [DOI] [PubMed] [Google Scholar]
- (15).Tomsick TA, Brott TG, Chambers AA, Fox AJ, Gaskill MF, Lukin RR, et al. Hyperdense middle cerebral artery sign on CT: efficacy in detecting middle cerebral artery thrombosis. AJNR Am J Neuroradiol. 1990;11:473–7. [PMC free article] [PubMed] [Google Scholar]
- (16).Tomsick T, Brott T, Barsan W, Broderick J, Haley EC, Spilker J. Thrombus localization with emergency cerebral CT. AJNR Am J Neuroradiol. 1992;13:257–63. [PMC free article] [PubMed] [Google Scholar]
- (17).Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- (18).von Kummer R, Meyding-Lamade U, Forsting M, Rosin L, Rieke K, Hacke W, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol. 1994;15:9–15. [PMC free article] [PubMed] [Google Scholar]
- (19).Flacke S, Urbach H, Keller E, Traber F, Hartmann A, Textor J, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology. 2000;215:476–82. doi: 10.1148/radiology.215.2.r00ma09476. [DOI] [PubMed] [Google Scholar]
- (20).Koga M, Saku Y, Toyoda K, Takaba H, Ibayashi S, Iida M. Reappraisal of early CT signs to predict the arterial occlusion site in acute embolic stroke. J Neurol Neurosurg Psychiatry. 2003;74:649–53. doi: 10.1136/jnnp.74.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Agarwal P, Kumar S, Hariharan S, Eshkar N, Verro P, Cohen B, et al. Hyperdense middle cerebral artery sign: can it be used to select intra-arterial versus intravenous thrombolysis in acute ischemic stroke? Cerebrovasc Dis. 2004;17:182–90. doi: 10.1159/000075789. [DOI] [PubMed] [Google Scholar]
- (22).Barber PA, Demchuk AM, Hill MD, Pexman JH, Hudon ME, Frayne R, et al. The probability of middle cerebral artery MRA flow signal abnormality with quantified CT ischaemic change: targets for future therapeutic studies. J Neurol Neurosurg Psychiatry. 2004;75:1426–30. doi: 10.1136/jnnp.2003.029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kim EY, Lee SK, Kim DJ, Suh SH, Kim J, Heo JH, et al. Detection of thrombus in acute ischemic stroke: value of thin-section noncontrast-computed tomography. Stroke. 2005;36:2745–7. doi: 10.1161/01.STR.0000185720.03803.41. [DOI] [PubMed] [Google Scholar]
- (24).Schellinger PD, Chalela JA, Kang DW, Latour LL, Warach S. Diagnostic and prognostic value of early MR Imaging vessel signs in hyperacute stroke patients imaged <3 hours and treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2005;26:618–24. [PMC free article] [PubMed] [Google Scholar]
- (25).Assouline E, Benziane K, Reizine D, Guichard JP, Pico F, Merland JJ, et al. Intra-arterial thrombus visualized on T2* gradient echo imaging in acute ischemic stroke. Cerebrovasc Dis. 2005;20:6–11. doi: 10.1159/000086120. [DOI] [PubMed] [Google Scholar]
- (26).Kim EY, Yoo E, Choi HY, Lee JW, Heo JH. Thrombus volume comparison between patients with and without hyperattenuated artery sign on CT. AJNR Am J Neuroradiol. 2008;29:359–62. doi: 10.3174/ajnr.A0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Goldmakher GV, Camargo EC, Furie KL, Singhal AB, Roccatagliata L, Halpern EF, et al. Hyperdense basilar artery sign on unenhanced CT predicts thrombus and outcome in acute posterior circulation stroke. Stroke. 2009;40:134–9. doi: 10.1161/STROKEAHA.108.516690. [DOI] [PubMed] [Google Scholar]
- (28).Froehler MT, Tateshima S, Duckwiler G, Jahan R, Gonzalez N, Vinuela F, et al. The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. J Neurointerv Surg. 2013;5:289–93. doi: 10.1136/neurintsurg-2012-010313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kharitonova T, Ahmed N, Thorén M, Wardlaw JM, von Kummer R, Glahn J, et al. Hyperdense middle cerebral artery sign on admission CT scan - prognostic significance for ischaemic stroke patients treated with intravenous thrombolysis in the Safe Implementation of Thrombolysis in Stroke International Stroke Thrombolysis Register. Cerebrovasc Dis. 2009;27:51–9. doi: 10.1159/000172634. [DOI] [PubMed] [Google Scholar]
- (30).Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Acute Stroke Imaging Research Roadmap II. Stroke. 2013;44:2628–39. doi: 10.1161/STROKEAHA.113.002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.