Abstract
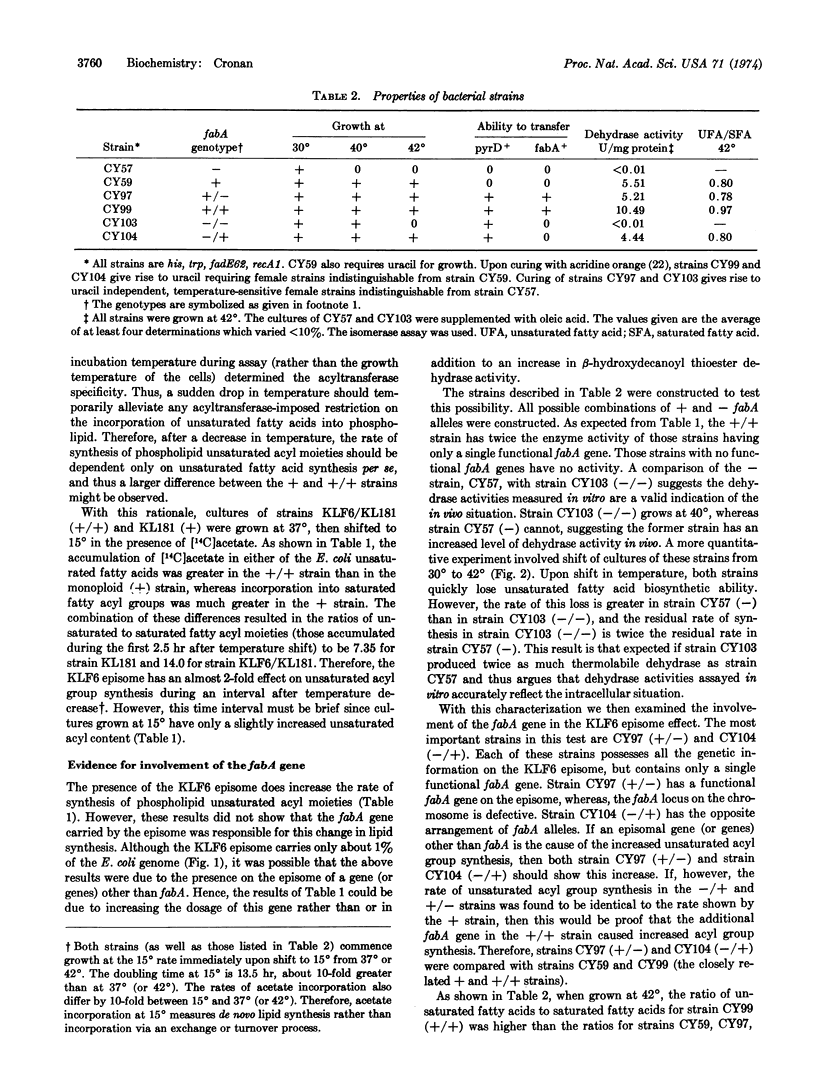
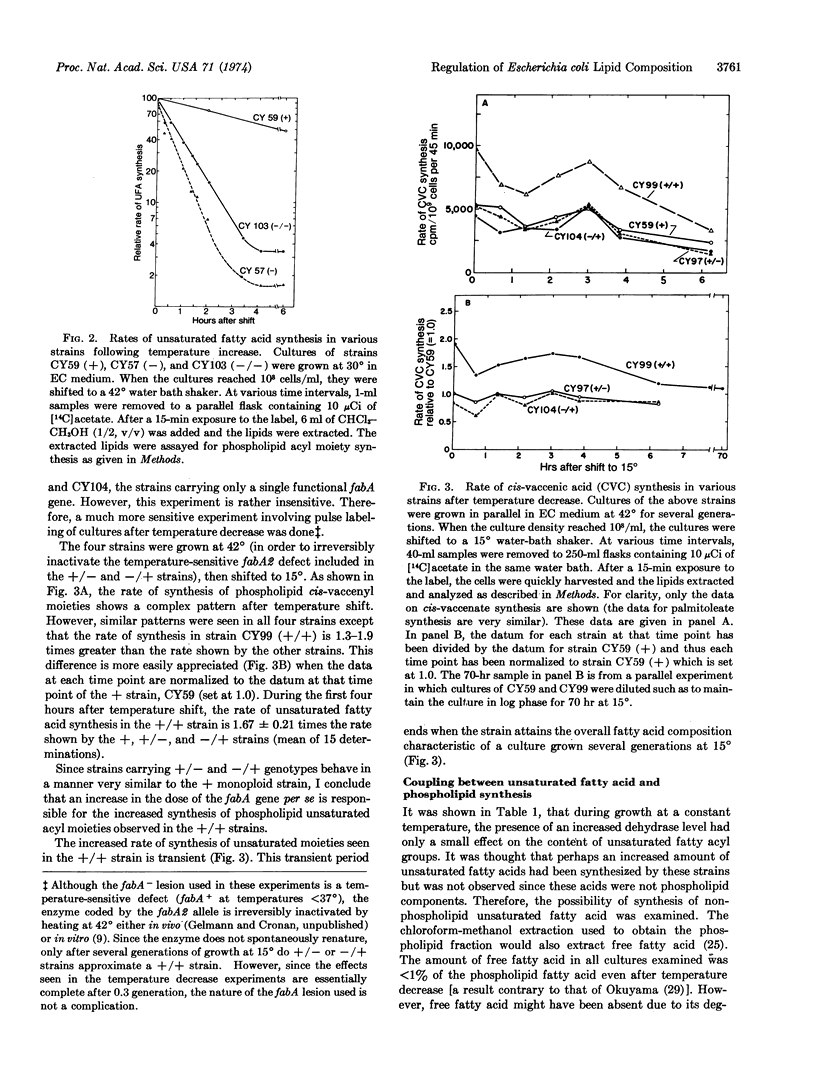
An increase in the dosage of the fabA gene, which codes for the enzyme β-hydroxydecanoyl thioesterase, causes an increase in the unsaturated fatty acyl content of the phospholipids. This increase is small in cells growing at a constant temperature. However, if cells grown at 37 or 42° are shifted to 15°, the rate of unsaturated fatty acyl moiety synthesis approaches twice the rate of a normal strain. These results indicate that the ratio of unsaturated to saturated fatty acyl groups in phospholipids is partially determined by the levels of at least this fatty acid biosynthetic enzyme.
Keywords: gene dosage, enzyme levels
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of the structural gene for L-glycerol 3-phosphate dehydrogenase. J Bacteriol. 1974 May;118(2):598–605. doi: 10.1128/jb.118.2.598-605.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. An estimate of the minimum amount of unsaturated fatty acid required for growth of Escherichia coli. J Biol Chem. 1973 Feb 25;248(4):1188–1195. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Nunn W. D., Batchelor J. G. Studies on the biosynthesis of cyclopropane fatty acids in Escherichia coli. Biochim Biophys Acta. 1974 Apr 26;348(1):63–75. doi: 10.1016/0005-2760(74)90093-9. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Silbert D. F., Wulff D. L. Mapping of the fabA locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1972 Oct;112(1):206–211. doi: 10.1128/jb.112.1.206-211.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Wulff D. L. A role for phospholipid hydrolysis in the lysis of Escherichia coli infected with bacteriophage T4. Virology. 1969 Jun;38(2):241–246. doi: 10.1016/0042-6822(69)90365-1. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Barnes E. M., Jr, Wakil S. J. Control of fatty acid composition in phospholipids of Escherichia coli: response to fatty acid supplements in a fatty acid auxotroph. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1057–1064. doi: 10.1073/pnas.64.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani M., Ioneda T., Wakil S. J. Studies on the control of fatty acid metabolism. 3. Incorporation of fatty acids into phospholipids and regulation of fatty acid synthetase of Escherichia coli. J Biol Chem. 1971 Jan 10;246(1):50–56. [PubMed] [Google Scholar]
- Fan D. P. Deletions in limited homology recombination in Escherichia coli. Genetics. 1969 Feb;61(2):351–361. doi: 10.1093/genetics/61.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann E. P., Cronan J. E., Jr Mutant of Escherichia coli deficient in the synthesis of cis-vaccenic acid. J Bacteriol. 1972 Oct;112(1):381–387. doi: 10.1128/jb.112.1.381-387.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haest C. W., de Gier J., van Es G. A., Verkleij A. J., van Deenen L. L. Fragility of the permeability barrier of Escherichia coli. Biochim Biophys Acta. 1972 Oct 23;288(1):43–53. doi: 10.1016/0005-2736(72)90221-0. [DOI] [PubMed] [Google Scholar]
- Harder M. E., Beacham I. R., Cronan J. E., Jr, Beacham K., Honegger J. L., Silbert D. F. Temperature-sensitive mutants of Escherichia coli requiring saturated and unsaturated fatty acids for growth: isolation and properties. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3105–3109. doi: 10.1073/pnas.69.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Control of fatty acid synthesis in bacteria. J Bacteriol. 1972 Apr;110(1):96–102. doi: 10.1128/jb.110.1.96-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr Unsaturated fatty acid synthesis is not required for induction of lactose transport in Escherichia coli. J Biol Chem. 1974 Feb 10;249(3):724–731. [PubMed] [Google Scholar]
- Okuyama H. Phospholipid metabolism in Escherichia coli after a shift in temperature. Biochim Biophys Acta. 1969 Jan 21;176(1):125–134. [PubMed] [Google Scholar]
- Okuyama H., Wakil S. J. Positional specificities of acyl coenzyme A: glycerophosphate and acyl coenzyme A: monoacylglycerophosphate acyltransferases in Escherichia coli. J Biol Chem. 1973 Jul 25;248(14):5197–5205. [PubMed] [Google Scholar]
- Ray T. K., Cronan J. E., Jr, Mavis R. D., Vagelos P. R. The specific acylation of glycerol 3-phosphate to monoacylglycerol 3-phosphate in Escherichia coli. Evidence for a single enzyme conferring this specificity. J Biol Chem. 1970 Dec 10;245(23):6442–6448. [PubMed] [Google Scholar]
- Silbert D. F. Arrangement of fatty acyl groups in phosphatidylethanolamine from a fatty acid auxotroph of Escherichia coli. Biochemistry. 1970 Sep 1;9(18):3631–3640. doi: 10.1021/bi00820a021. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Cohen M., Harder M. E. The effect of exogenous fatty acids on fatty acid metabolism in Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1699–1707. [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Temperature control of phospholipid biosynthesis in Escherichia coli. J Bacteriol. 1971 May;106(2):449–455. doi: 10.1128/jb.106.2.449-455.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]



