Abstract
Purpose of review
An important cause of maternal morbidity and direct maternal death is obstetric haemorrhage at caesarean section. Concerns regarding allogeneic blood safety, limited blood supplies and rising health costs have collectively generated enthusiasm for the utility of methods intended to reduce the use of allogeneic blood transfusion in cases of haemorrhage at caesarean section. This can be achieved by intraoperative cell salvage (IOCS). The aim of this review is to summarize and examine the evidence for the efficacy of IOCS during caesarean section, in women at risk of haemorrhage, in reducing the need for allogeneic blood transfusion.
Recent findings
The majority of the evidence currently available is from case reports and case series. Although this evidence appears to support the use of IOCS in obstetrics, strong clinical evidence or economic effectiveness from clinical trials are essential to support the routine practice of IOCS in obstetrics.
Summary
Current evidence is limited to reported case series and two small controlled studies. Overall, IOCS may reduce the need for allogeneic blood transfusions during caesarean section. Future large randomized trials are required to assess effectiveness, cost effectiveness and safety. The results of the current ongoing SALVO (A randomised controlled trial of intra-operative cell salvage during caesarean section in women at risk of haemorrhage) trial will shed light on these aspects.
Keywords: allogeneic blood transfusion, caesarean section, intraoperative cell salvage, obstetrics
INTRODUCTION
Obstetric haemorrhage is a leading cause of maternal mortality and morbidity in the United Kingdom [1,2] and worldwide [3,4]. The reports of the United Kingdom Confidential Enquiry into Maternal Deaths have consistently recognized haemorrhage as an important cause of direct maternal death. Haemorrhage was found to be the sixth highest cause of direct maternal death (0.39 deaths per 100 000 maternities) during the latest 2006–2008 triennium. The majority of these deaths were due to postpartum haemorrhage and associated with caesarean section [5,6].
In 2011–2012, approximately 163 858 caesarean sections (over 20% of deliveries) were performed in the United Kingdom [7,8]. The obstetric setting accounts for around 4–5% of the total United Kingdom’s blood supply of approximately 1.8 million units of packed red cells per year, with each unit currently costing approximately £125 [9]. Globally, the availability of blood is limited and expensive [10]. There are potential risks associated with allogeneic blood transfusion, such as acute and delayed haemolytic reactions, febrile, urticarial, and anaphylactic reactions, administration errors [11] and risk of transfusion-transmitted infection, including prion disease [12,13].
Intraoperative cell salvage (IOCS) is a blood conservation technique that may reduce the need for allogeneic blood transfusion, therefore lowering risk and conserving the scarce blood supply [10]. During IOCS, the patient’s own blood that is lost during a surgical operation is collected, filtered, washed and centrifuged to produce autologous RBC, which can be reinfused to the patient [14]. The efficiency of red cell recovery by IOCS has been shown to be improved by washing swabs [15]. Since the 1970s, IOCS has been widely utilized in numerous surgical specialities (orthopaedics, cardiac, urologic, vascular, intracranial and gynaecological surgery) to reduce the transfusion of allogeneic blood and its associated risks [16].
The two main reasons for the delayed introduction of IOCS in obstetrics, compared to other specialities, stem from the theoretical risk and ongoing dispute centred on the risk of contamination of salvaged blood with amniotic fluid and the risk of maternal-foetal anti-Rhesus factor D [Rh(D)] alloimmunization. This article aims to review the present evidence concerning the quality and safety of this procedure being introduced in the obstetric clinical setting.
IDENTIFICATION OF THE LITERATURE
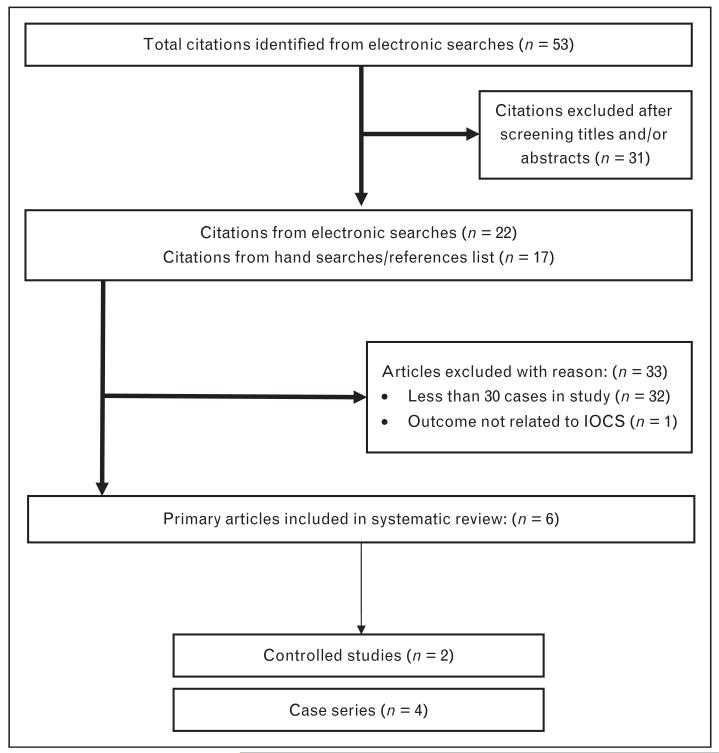
All the published, observational and controlled studies, assessing the efficiency of IOCS during caesarean section, were identified by performing a comprehensive literature search. For a sensitive search to identify the maximum number of relevant citations, a combination of key terms was used in the following databases without language restrictions: Medline, Embase and Cochrane Library. All the known primary and review article reference lists were then examined for further relevant citations (Fig. 1).
FIGURE 1.
Diagram of study selection. IOCS, intraoperative cell salvage.
Following completion of the electronic literature search, the citation lists (titles, medical subject headings and abstracts, wherever available) were independently reviewed by two of the authors (S.K.D. and K.S.K.). To identify which studies evaluated the efficiency of IOCS during caesarean section, the citations were categorized as relevant or not relevant. Subsequently, the citations considered relevant by any of the authors were reviewed in full by taking into consideration their complete manuscripts. To be considered eligible for inclusion in our systematic review, studies fulfilled the criteria below. Two authors (S.K.D. and K.S.K.) independently assessed the study eligibility.
SELECTION CRITERIA
To determine study eligibility, the following selection criteria were used:
-
(1)
Population: women undergoing caesarean section.
-
(2)
Number of cases per study: 30 or more.
-
(3)
Date: published after 1993.
-
(4)
Intervention: treatment with IOCS technique with or without comparison to standard medical treatment.
-
(5)
Outcome: rates of blood transfusion, wherever available the complication rates and health economics.
DATA EXTRACTION AND ASSESSMENT OF STUDY QUALITY
Information regarding the features of the study design, the characteristics of the population, the methods of performing the intervention (IOCS) and the assessment of the outcomes were extracted from each article in duplicate (S.K.D. and K.S.K.).
LITERATURE SEARCH RESULTS
At first, the total number of citations identified was 53. Of these, 22 citations were regarded as relevant. Furthermore, 17 citations were identified following the review of the reference lists of the 22 relevant citations. After evaluating the complete manuscripts of these articles, six studies were finally included in the overview. To be included in the overview, the article must have been published after 1993 and included 30 or more cases. Thirty articles were chosen as a cut point so that studies selected had sufficient numbers in the denominator for a precise enough result to reach reliable conclusions. Figure 1 demonstrates a flow diagram of how studies were selected.
METHODOLOGIC QUALITY ASSESSMENT
Table 1 shows the six studies [13,17-21] that were included in the overview. Of these, two were controlled studies and four were case series.
Table 1.
Characteristics of studies included in the systematic review of the effects of intraoperative cell salvage in caesarean sections
| Study Author, Year | Publication type | Total number of patients reinfused with salvaged blood | Retransfusion rate of salvaged blood (%) | Clinical setting | Clinical outcome |
|---|---|---|---|---|---|
| Jackson et al. 1993 [17] | Retrospective series | 64 | 53 | CS | Uneventful |
| Rainaldi et al. 1998 [20] | Prospective controlled trial | 34 | 100 | CS | Reduced length of hospital stay and allogeneic blood transfusion. Higher postoperative haemoglobin |
| Rebarber et al. 1998 [21] | Retrospective cohort | 139 | 74 | CS | Heparin toxicity (n = 1) |
| Sullivan et al. 2011 [13] | Retrospective series | 36 | CS | ||
| Ralph et al. 2011 [18] | Prospective series | 70 | CS | No adverse clinical signs. One positive anti-S was detected | |
| Brearton et al. 2012 [19] | Retrospective series | 137 | CS |
CS, caesarean section.
CURRENT EVIDENCE: IS INTRAOPERATIVE CELL SALVAGE A WELL TOLERATED PROCEDURE IN OBSTETRICS?
A systematic review on this topic was performed in 2009, and this is an updated review encompassing several more recent case reports and case series [16]. There have been many studies carried out on obstetric patients who have undertaken IOCS. However, to date, after conducting a thorough literature search, only two controlled studies, both published in 1998, have been identified [13,17-21]. Firstly, there was a multicentre cohort study, which looked at the safety of cell salvaged blood collection and auto-transfusion during caesarean section. This study demonstrated no statistically significant differences between the two different groups due to lack of statistical power [21]. A further small, randomized controlled clinical trial looked at the elective use of cell salvage at caesarean section. The study has shown a large decrease in the number of patients needing allogeneic transfusion in those that received salvaged blood opposed to those who did not. Haemoglobin levels were reported to be significantly higher for all 4 days postoperatively in the IOCS group. In addition, patients who received IOCS had a significantly shorter stay in hospital [20]. Since these two papers in 1998, we have been restricted to case series and case reports. Beyond these two controlled studies, this overview identified further four studies that had over 30 cases each.
There have been no reported direct serious adverse events related to the use of IOCS in obstetric patients. In 2011, Ralph et al. [18] demonstrated that there were no haemodynamic changes or any other adverse clinical signs observed during all 70 reinfusions. Out of the 70 patients, one positive anti-S antibody was identified [18]. The implications of various red cell antibodies in pregnancy and their management are covered in the recent Green top guideline [22■]. Recently, there have been three cases in the serious hazards of transfusion report of hypotension following rapid reinfusions via a leucodepletion filter and when using the anticoagulant acid-citrate-dextrose [18,23]. Further evidence about the benefits of using leucodepletion filters is required.
AMNIOTIC FLUID CONTAMINATION
The theoretical risk that contamination of salvaged blood with amniotic fluid may precipitate the syndrome of amniotic fluid embolism (AFE) when salvaged blood is reinfused remains one of the greatest theoretical threats [24]. The potential concerns of AFE in IOCS have not yet been realized [2,5,25-27]. AFE has a low incidence that varies between 1.3 and 12.5 per 100 000 pregnancies overall [28]. Some research has shown that the IOCS technique when combined with the leucocyte depletion filter significantly reduces levels of amniotic fluid contaminants, and therefore the theoretical risk of AFE [25,26,29]. There has only been one obstetric death occurring in a patient who received cell salvage, and the cause of death in that seriously ill patient has not been established [24,28,30].
RED BLOOD CELL CONTAMINATION
The risk of maternal-foetal anti-Rh(D) alloimmunization also raises concern as the cell saver cannot differentiate foetal from maternal red cells. RhD-negative mothers with an RhD-positive fetus should have Kleihauer testing 1 h after the procedure to determine the extent of foeto-maternal haemorrhage and a suitable dose of anti-D immunoglobulin should be administered to prevent RhD alloimmunization [10,31■].
The median foetal red cell contamination was 0.8 ml (range 0.2–12.9 ml) in one study on 70 women who were reinfused salvaged blood via a leucodepletion filter [18]. The concentration of foetal RBC in the maternal circulation increases during pregnancy. Therefore, the potential risk of sensitization can occur throughout pregnancy and at delivery. Approximately 1% of women in the third trimester have transplacental haemorrhage more than 2.5 ml and 0.3% have greater than 15 ml. A recent report has shown comparable amounts of contamination in women about to give birth with a median foetal RBC of 0.48 ml (range 0–4.6 ml) before delivery and a maximum of 9 ml after delivery [18]. This shows that reinfusing a foetal RBC volume of 0.2–12.9 ml in IOCS is similar to that found normally in the maternal circulation after delivery [26,29]. The reported range of foetal RBCs volume collected by cell salvage in previous studies is 0.2–19 ml. At present, the volume of contamination necessary to provoke an antibody response to red cell antigens is unknown [18]. The significance of the risk of alloimmunization by foetal RBCs after a reinfusion of cell-salvaged blood remains uncertain. To evaluate the incidence of antibody formation, large numbers of women need to be followed up to test for antibody formation 3–6 months after reinfusion. A central database could be set up to gather information and consider the risk of alloimmunization [18].
DOES CELL SALVAGE REDUCE COSTS AND THE USE OF ALLOGENEIC BLOOD IN OBSTETRICS?
Brearton et al. [19] evaluated the financial considerations of cell salvage over a 5-year period (2006–2011). During the study period, cell salvaged blood was reinfused to 137 patients. Overall, 47 143 ml of blood was returned. This is equivalent to 189 units of packed red cells. The total cost of cell salvage in 2011 was £9245 for 83 units of blood. This showed a saving of £1130 as allogeneic blood (£125 per unit) would have cost £10 375. The total running cost (collecting and processing) of cell salvaged blood was approximately £100 per patient. There were no intrapostoperative or postoperative complications associated with the patients who had their blood reinfused by using IOCS in this study. In all cases, there were no recorded episodes of hypotension associated with the use of Pall LeukoGuard (Pall Corporation, Pall Life Sciences, Portsmouth, UK) leucocyte depletion filters. The study concluded that the use of cell salvage in obstetrics is an appropriate expenditure to decrease the use of allogeneic blood [19].
The costs, manpower planning and training are other alleged barriers to put into practice the use of cell salvage in obstetrics [32]. Concerns about training and expertise can be overcome by setting up the cell saver into routine practice [18]. Setting up cell salvage preoperatively in patients at high risk of haemorrhage is practicable because of the familiarity and knowledge achieved, which in turn can help in emergency caesarean sections. Guidelines also emphasize this aspect of using cell salvage repeatedly to gain the confidence to use cell salvage quickly and easily [27].
SUPPORT AND INDICATIONS
Despite insufficient evidence from robust, controlled trials, there has recently been an increase in the use of IOCS in obstetrics at caesarean section. To date, the use of IOCS has caused no serious complications leading to poor maternal outcome [24]. In the United Kingdom, IOCS in obstetrics has been recommended in specific circumstances by several official bodies, including the Centre of Maternal and Child Enquires, the National Institute of Clinical Excellence, the Royal College of Obstetricians and Gynaecologists, the Association of Anaesthetists of Great Britain and Ireland and the Obstetrics Anaesthetists’ Association [6,27,33,34].
CONCLUSION
The majority of the evidence currently available is from case reports and case series. Although current evidence supports the use of IOCS in obstetrics, strong clinical evidence from large multicentre clinical trials is essential to support the routine practice of IOCS in obstetrics [16,24]. Future randomized trial should reliably measure the difference, evaluate cost effectiveness and address concerns of adverse effects. We hope that the current ongoing SALVO trial (A randomised controlled trial of intra-operative cell salvage during caesarean section in women at risk of haemorrhage; ISRCTN66118656) would be able to answer these questions.
KEY POINTS.
IOCS is well established in many types of surgeries in which it reduces the use of allogeneic (donor) blood transfusion.
During IOCS, patients’ own blood loss at caesarean section is collected, filtered, centrifuged and reinfused. Swab washing increases efficiency of IOCS.
There are a few studies on use of IOCS in obstetrics. There is a need for robust, multicentre clinical trials to evaluate clinical benefits and cost effectiveness of routine use of IOCS at caesarean section.
To date, the use of IOCS has not been associated with any serious complications leading to poor maternal outcome.
Acknowledgements
We thank Genny Franklin for conducting literature searches and Aoife Ahern for coordinating the compilation of this manuscript.
SALVO study group consists of Jane Daniels, Samantha Parker, Steve Robson, Tracy Roberts, Mairi Harkness, Fang Gao-Smith, Richard Hooper, Ian Wrench, Aarti Ullal, Paul Ayuk, Tommy Mousa, Matthew Hogg, Dominika Dabrowska, Chris Marsh, Vinod Patal, Nicola Osborn, Daryl Thorp-Jones, Vicki Clark, Arlene Wise, Sue Catling, Susan Williams, Jules Allt, James Geoghegan, David Portch, Bini Ajay, Lesley Woods, George Bugg, Sangeeta Pathak, Balaji Packianathaswamy, Parijat Bhattacharjee, Jag Samra, Sanjay Rao and Isobel Gardner
Funding: The SALVO Study is funded by the NIHR Health Technology Assessment Programme (Ref: 10/57/32).
Ethics approval: The SALVO Study has ethical approval from North West Research Ethics Committee – Haydock (Ref 12/NW/0513).
Footnotes
Conflicts of interest
Khalid S. Khan is a Chief Investigator of the SALVO study.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Lewis G, The Confidential Enquiry into Maternal and Child Health (CEMACH) Why Mothers die 2000-2002. The sixth report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. CEMACH; London: 2004. [Google Scholar]
- 2.Lewis G, The Confidential Enquiry into Maternal and Child Health (CEMACH) Saving mothers’ lives: reviewing maternal deaths to make motherhood safer 2003–2005. The seventh report of Confidential Enquiries into Maternal Deaths in the United Kingdom. CEMACH; London: 2007. [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organisation . The world health report 2005: make every mother and child count. WHO Press; Geneva, Switzerland: 2005. [Google Scholar]
- 5.United Nations The Millennium Development Goals Report. 2012 http://www.un.org/millenniumgoals/pdf/MDG%20Report%202012.pdf.
- 6.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118:1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 7.NHS Maternity Statistics - England, 2011-2012. Health and Social Care Information Centre; 2012. http://www.hscic.gov.uk/article/2021/Website-Search?productid=10061&q=NHS+Maternity+Statistics+&sort=Relevance&size=10&page=1&area=both#top. [Google Scholar]
- 8.Royal College of Obstetricians and Gynaecologists The National Sentinel Caesarean Section Audit Report. 2001 http://www.rcog.org.uk/files/rcog-corp/uploaded-files/nscs_audit.pdfPublished.
- 9.Strategic Plan 2012-17. NHS Blood and Transplant; 2012. http://www.nhsbt.nhs.uk/strategicplan/pdf/nhsbt_strategic_plan.pdf. [Google Scholar]
- 10.Catling S. Blood conservation techniques in obstetrics: a UK perspective. Int J Obstet Anesth. 2007;16:241–249. doi: 10.1016/j.ijoa.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Serious Hazards of Transfusion (SHOT) Steering Group The 2010 annual SHOT report. http://www.shotuk.org/wp-content/uploads/2011/10/SHOT-2010-Report1.pdf.
- 12.Hunter N, Foster J, Chong A, et al. Transmission of prion diseases by blood transfusion. J Gen Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan JV, Crouch ME, Stocken G, Lindow SW. Blood cell salvage during cesarean delivery. Int J Gynaecol Obstet. 2011;115:161–163. doi: 10.1016/j.ijgo.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Khan K. A randomised controlled trial of intra-operative cell salvage during caesarean section in women at risk of haemorrhage (Project record) Health Technology Assessment Database. 2012;(1) doi: 10.3310/hta22020. http://onlinelibrary.wiley.com/o/cochrane/clhta/articles/HTA-32012000323/frame.html. [DOI] [PMC free article] [PubMed]
- 15.Haynes SL, Bennett JR, Torella F, McCollum CN. Does washing swabs increase the efficiency of red cell recovery by cell salvage in aortic surgery? Vox Sang. 2005;88:244–248. doi: 10.1111/j.1423-0410.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 16.Geoghegan J, Daniels JP, Moore PA, et al. Cell salvage at caesarean section: the need for an evidence-based approach. BJOG. 2009;116:743–747. doi: 10.1111/j.1471-0528.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- 17.Jackson SH, Lonser RE. Safety and effectiveness of intracesarean blood salvage. Transfusion. 1993;33:181. doi: 10.1046/j.1537-2995.1993.33293158054.x. [DOI] [PubMed] [Google Scholar]
- 18.Ralph CJ, Sullivan I, Faulds J. Intraoperative cell salvaged blood as part of a blood conservation strategy in Caesarean section: is fetal red cell contamination important? Br J Anaesth. 2011;107:404–408. doi: 10.1093/bja/aer168. [DOI] [PubMed] [Google Scholar]
- 19.Brearton C, Bhalla A, Mallaiah S, Barclay P. The economic benefits of cell salvage in obstetric haemorrhage. Int J Obstet Anesth. 2012;21:329–333. doi: 10.1016/j.ijoa.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Rainaldi MP, Tazzari PL, Scagliarini G, et al. Blood salvage during caesarean section. Br J Anaesth. 1998;80:195–198. doi: 10.1093/bja/80.2.195. [DOI] [PubMed] [Google Scholar]
- 21.Rebarber A, Lonser R, Jackson S, et al. The safety of intraoperative autologous blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol. 1998;179:715–720. doi: 10.1016/s0002-9378(98)70070-5. [DOI] [PubMed] [Google Scholar]
- 22.Royal College of Obstetricians and Gynaecologists (RCOG) The management of women with red cell antibodies during pregnancy. Royal College of Obstetricians and Gynaecologists (RCOG); London: 2014. (Green top guideline No. 65). [■ The implications of various red cell antibodies in pregnancy and their management are covered in this recent Green top guideline.] [Google Scholar]
- 23.Liumbruno GM, Liumbruno C, Rafanelli D. Autologous blood in obstetrics: where are we going now? Blood Transfus. 2012;10:125–147. doi: 10.2450/2011.0010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allam J, Cox M, Yentis SM. Cell salvage in obstetrics. Int J Obstet Anesth. 2008;17:37–45. doi: 10.1016/j.ijoa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan I, Faulds J, Ralph C. Contamination of salvaged maternal blood by amniotic fluid and fetal red cells during elective caesarean section. Br J Anaesth. 2008;101:225–229. doi: 10.1093/bja/aen135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters JH, Biscotti C, Potter PS, Phillipson E. Amniotic fluid removal during cell salvage in the cesarean section patient. Anesthesiology. 2000;92:1531–1536. doi: 10.1097/00000542-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Clinical Excellence IPG144 Intraoperative blood cell salvage in obstetrics-guidance. http://www.nice.org.uk/nicemedia/live/11038/30690/30690.pdf2005.
- 28.Liumbruno GM, Meschini A, Liumbruno C, Rafanelli D. The introduction of intra-operative cell salvage in obstetric clinical practice: a review of the available evidence. Eur J Obstet Gynecol Reprod Biol. 2011;159:19–25. doi: 10.1016/j.ejogrb.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Catling SJ, Williams S, Fielding AM. Cell salvage in obstetrics: an evaluation of the ability of cell salvage combined with leucocyte depletion filtration to remove amniotic fluid from operative blood loss at caesarean section. Int J Obstet Anesth. 1999;8:79–84. doi: 10.1016/s0959-289x(99)80002-8. [DOI] [PubMed] [Google Scholar]
- 30.Oei SG, Wingen CBM, Kerkkamp HEM. Cell salvage: how safe in obstetrics? Int J Obstet Anesth. 2000;9:143. [Google Scholar]
- 31.Qureshi H, Massey E, Kirwan D, et al. BCSH guidelines for the use of anti-D immunoglobulin for the prevention of haemolytic disease of fetus and newborn. Transfus Med. 2013;24:8–20. doi: 10.1111/tme.12091. [■ This guideline provides updated practical guidance on the use of anti-D immunoglobulin for rhesus prophylaxis. It takes into account the National Institute of Clinical Excellence 2008 guidance for routine anti-D prophylaxis.] [DOI] [PubMed] [Google Scholar]
- 32.Teig M, Harkness M, Catling S, Clark V. Survey of cell-salvage use in obstetrics in the UK. Int J Obstet Anesth. 2004;16:S30. [Google Scholar]
- 33.The Association of Anaesthetists of Great Britain and Ireland Obstetric Anaesthetists’ Association OAA /AAGBI guidelines for obstetric anaesthetic services revised edition 2005. http://www.aagbi.org/sites/default/files/obstetric05.pdf2005.
- 34.RCOG . Blood transfusion in obstetrics. Royal College of Obstetricians and Gynaecologists; 2007. (Green-top guideline no. 47). http://www.rcog.org.uk/files/rcog-corp/uploaded-files/GT47BloodTransfusions1207amended.pdf. [Google Scholar]