Abstract
During the first 7 years of the Diabetes Prevention Program Outcomes Study (DPPOS), diabetes incidence rates, when compared with the Diabetes Prevention Program (DPP), decreased in the placebo (−42%) and metformin (−25%), groups compared with the rates in the intensive lifestyle intervention (+31%) group. Participants in the placebo and metformin groups were offered group intensive lifestyle intervention prior to entering the DPPOS. The following two hypotheses were explored to explain the rate differences: “effective intervention” (changes in weight and other factors due to intensive lifestyle intervention) and “exhaustion of susceptible” (changes in mean genetic and diabetes risk scores). No combination of behavioral risk factors (weight, physical activity, diet, smoking, and antidepressant or statin use) explained the lower DPPOS rates of diabetes progression in the placebo and metformin groups, whereas weight gain was the factor associated with higher rates of progression in the intensive lifestyle intervention group. Different patterns in the average genetic risk score over time were consistent with exhaustion of susceptibles. Results were consistent with exhaustion of susceptibles for the change in incidence rates, but not the availability of intensive lifestyle intervention to all persons before the beginning of the DPPOS. Thus, effective intervention did not explain the lower diabetes rates in the DPPOS among subjects in the placebo and metformin groups compared with those in the DPP.
Introduction
The Diabetes Prevention Program (DPP) (1) showed that intensive lifestyle intervention and metformin administration reduced diabetes incidence compared with placebo-treated control subjects. At the end of the 3 years of active intervention, the DPP was closed and was converted to a long-term follow-up study, the DPP Outcomes Study (DPPOS), to determine whether the reductions in diabetes incidence observed with intensive lifestyle intervention and metformin treatment were maintained (2). Because the intensive lifestyle intervention produced the largest reduction in diabetes incidence, it was offered to all participants during a 6-month “bridge” period before the follow-up (3).
Over a 10-year follow-up period from randomization in the DPP, diabetes risk was reduced by 34% in the original intensive lifestyle intervention group, and by 18% in the metformin group compared with the original placebo group (2). The smaller long-term differences between treatment in progression to diabetes that were observed during the DPPOS phase of the study were due to the following two factors: an increase in the incidence rates among those in the intensive lifestyle intervention group over the 10-year period, and a substantial decrease in rates in the metformin and placebo groups. We considered two hypotheses to explain these results. The “effective intervention” hypothesis posits that substantial numbers of subjects in the metformin and placebo groups achieved weight loss (and related lifestyle changes) due to attending intensive lifestyle intervention classes following the DPP, and reduced their annual incidence rates during DPPOS. The “exhaustion of susceptibles” hypothesis posits that diabetes developed in persons who were most susceptible to diabetes in the metformin and placebo groups during the DPP, and the remaining participants were less susceptible, leading to overall lower incidence rates for these groups during the DPPOS. We present several analyses designed to explore these hypotheses.
Research Design and Methods
The DPPOS objectives were to evaluate the long-term effects of DPP interventions on the development of diabetes and its complications. The protocol and informed consent procedures were approved by all responsible institutional review boards, and all participants provided written informed consent.
Participants
Details of the methods used in the DPP have been presented previously (1,2,4). A total of 3,324 participants enrolled in the DPP between 1996 and 1999 and were randomized to one of the following three treatment arms: intensive lifestyle intervention, metformin, or placebo. Participants were ≥25 years of age and overweight or obese, and had impaired glucose tolerance (IGT) and elevated fasting glucose levels (5). A detailed flowchart of study enrollment has been published (2). All 3,150 surviving DPP participants who had not withdrawn consent were eligible for the DPPOS. Enrollment began in September 2002 and was largely completed within 1 year. By 27 August 2008, the closing date for this report, 88% of participants (2,766 participants) had enrolled and were observed for a median period of 10.0 years (interquartile range 9.0–10.5) after randomization.
Changes in Interventions
At the termination of the DPP in July 2001 (1), there was a 2-week drug “washout period” (6). Following this, all participants were offered the intensive lifestyle intervention modified for groups (3), and 57% of placebo, 58% of metformin, and 40% of intensive lifestyle intervention participants attended some of these sessions. From the end of the DPP until the start of the DPPOS, metformin participants had lost ∼1.5 kg when weight regain began to occur. Placebo participants lost ∼2 kg, whereas intensive lifestyle intervention participants gained ∼1 kg on average (3). This bridge period was conducted until the DPPOS protocol began in September 2002. During the DPPOS, quarterly intensive lifestyle intervention sessions were offered to all participants; intensive lifestyle intervention participants received additional group classes, and metformin participants received an unmasked drug (850 mg twice a day, as tolerated) unless treatment was discontinued for reasons of safety or diabetes development.
Risk Factors
Variables in this analysis included demographic factors; obesity measures (weight in kilograms), height (in meters), waist circumference (in centimeters), BMI (in kilograms per square meter); measures of glycemia (fasting and 2-h glucose levels, and A1C percentage); and insulin sensitivity (1/fasting insulin ratio) and secretion (insulinogenic index, calculated as the ratio of insulin to glucose values between 0 and 30 min) (7). In addition, dietary intake data were collected by the modified Block Food Frequency Questionnaire (8) at regular intervals. Physical activity was self-reported using the Modified Activity Questionnaire (9) as total MET-hours per week. Goals were defined annually for the weight goal (≤7% weight loss), exercise goal (≥150 min/week of moderate physical activity [≥6 MET-h/week, based on the Modified Activity Questionnaire]), and the fat intake goal (total dietary fat <25% of calories). Biochemical tests were analyzed at Northwest Lipid Research Laboratories (Seattle, WA) using methods reported previously (1).
Exploration of the exhaustion of susceptibles hypothesis used the genetic risk score (GRS), which was composed of 34 type 2 diabetes–associated genetic variants, weighting each risk allele by its reported effect size on diabetes risk and summing these values (10). We also calculated a clinical diabetes risk score (DRS) using baseline characteristics sex, age, BMI, sex-specific waist circumference, hypertension medication use, history of gestational diabetes mellitus, smoking, and family history of diabetes (11), as well as analysis of fasting and 2-h glucose levels. We used these scores and glucose levels to explore whether the average risk profiles for the intervention groups (intensive lifestyle intervention, metformin, and placebo groups) became lower over time by calculating the mean of the baseline assigned scores and levels every 6 months for those participants in each group in whom diabetes did not develop. Thus, an individual’s score or level was the same in each time period, but the group average changed as diabetes developed in participants and those participants were no longer included in the mean for the treatment group.
Outcome
The outcome for the DPP/DPPOS was the development of incident diabetes according to American Diabetes Association criteria (fasting glucose ≥126 mg/dL and/or 2-h glucose level ≥200 mg/dL obtained by oral glucose tolerance test), measured annually and confirmed by repeat testing. Participants were observed until diabetes developed, they withdrew from the study, or they were administratively censored as of their last follow-up visit.
Analyses
Time Intervals
Analyses used a common DPPOS start date of 1 September 2002, although most participants were not seen on that exact date. The baseline visit for the DPPOS was the last yearly visit that occurred between 1 August 2001 and 31 August 2002. Follow-up time was divided into the following two periods: 1) 31 July 1996 to 31 July 2001 (i.e., the DPP to the end of the masked intervention phase) and 2) 1 August 2001 through 27 August 2008 (i.e., all visits after the completion of the masked intervention phase, combining the washout, bridge, and DPPOS follow-up periods).
Analytic Methods
Data from participants at DPPOS baseline were described using the mean (±SD) for quantitative variables and frequencies (percentages) for qualitative variables. The effect of weight change over time on diabetes risk was assessed within each treatment group using proportional hazard regression models after adjusting for other factors. The conditional independence of time periods in the DPP and DPPOS for diabetes risk was assumed, as any incident diagnosis of diabetes could occur only once, either in the DPP or DPPOS, to a given participant. The proportional hazards assumption did not hold, therefore the Lin-Wei robust covariance estimates were used in estimating corresponding SEs (12). To assess whether diabetes risk in the DPP was different than that in the DPPOS, hazard ratios (HRs) were computed using Poisson regression models with the log link function using a time-dependent binary covariate for each subject to designate DPP versus DPPOS within each treatment group. SAS version 9.2 (SAS Institute, Cary, NC) was used for all analyses. Two-sided P values are reported with α = 0.05. No adjustments were made for multiple testing.
Results
Overall, 2,766 participants joined the DPPOS (Table 1), of whom 22 had no baseline visit, leaving an effective sample size of 2,744 for analyses. Of these participants, 68% were female, and the average age was 55.2 years. Seventy-two percent of participants did not have diabetes at the start of the DPPOS. A higher proportion of the intensive lifestyle intervention group entered the DPPOS without diabetes (81.2%), with lower fasting insulin levels, better insulin secretion (i.e., higher insulinogenic index), lower BMI and waist circumference, fewer daily calories, and higher physical activity, and they met more of the DPP goals than the other groups, which is consistent with the results of the DPP.
Table 1.
Characteristics of participants at DPPOS entry
| Variable | Overall | DPPOS baseline examination |
||
|---|---|---|---|---|
| Intensive lifestyle intervention | Metformin | Placebo | ||
| N (%) | 2,766 (100) | 910 (100) | 924 (100) | 932 (100) |
| Men, n (%) | 888 (32) | 291 (32) | 307 (33) | 290 (31) |
| Age (years) | 55.2 (10.3) | 55.3 (11.0) | 55.5 (10.1) | 54.8 (10.0) |
| Caucasian (%) | 1,506 (54.4) | 490 (53.8) | 515 (55.7) | 501 (53.8) |
| Not diabetic, n (%) | 1,999 (72.3) | 739 (81.2) | 652 (70.6) | 608 (65.2) |
| Nondiabetic participants | ||||
| Fasting glucose (mmol/L) | 5.72 (0.52) | 5.72 (0.51) | 5.66 (0.53) | 5.79 (0.51) |
| Fasting insulin (pmol/L) | 155.6 (98.8) | 144.2 (86.2) | 158.8 (99.6) | 166.1 (110.4) |
| Insulin glucose ratio (pmol/mmol)* | 140.2 (159.1) | 148.0 (187.6) | 137.6 (102.9) | 133.0 (170.8) |
| BMI (kg/m2) | 32.5 (6.5) | 31.7 (6.6) | 32.7 (6.3) | 33.3 (6.6) |
| Waist circumference (cm) | 101.3 (13.8) | 99.5 (14.2) | 102.0 (13.3) | 102.6 (13.8) |
| Total calories/day (kcal)† | 1,775 (802) | 1,681 (785) | 1,839 (774) | 1,819 (843) |
| Calories from fat (%)† | 31.4 (7.4) | 27.8 (6.7) | 33.7 (6.8) | 33.2 (7.0) |
| Physical activity (METs/week) | 18.2 (18.6) | 20.5 (17.7) | 16.5 (18.4) | 17.2 (19.8) |
| Goals met at DPPOS entry‡ | ||||
| Weight, n (%) | 472 (23.9) | 238 (32.6) | 137 (21.2) | 97 (16.1) |
| Exercise, n (%) | 1,499 (76.6) | 610 (84.3) | 465 (72.7) | 424 (71.4) |
| Fat intake, n (%)† | 382 (19.1) | 256 (34.7) | 56 (8.6) | 70 (11.5) |
| Number of goals met | 1.00 (0.64) | 1.17 (0.65) | 0.94 (0.62) | 0.87 (0.62) |
Data are mean (SD), unless otherwise indicated. Data were missing for 22 enrolled persons who had not undergone a DPPOS baseline examination (see Research Design and Methods). Data were missing for additional participants in varying numbers for some of the variables.
*Calculated as (change in insulin levels after 30 min from fasting)/(change in glucose levels after 30 min from fasting).
†n = 1,995 using the last available visit with dietary data before DPPOS baseline visit.
‡Weight goal is defined as having lost ≥7% of the weight at randomization. Exercise goal is defined as leisure activity of ≥6 MET h/week. 1 MET is 1 kcal/kg/h. Fat intake is defined as the percentage of calories from fat ≤25%.
The impetus for this analysis was that diabetes incidence rates during the DPPOS for all three groups were similar (2). In the DPPOS period (∼4–12 years postrandomization), the intensive lifestyle intervention-to-placebo group HR for diabetes incidence was 1.01 (95% CI 0.84–1.22), intensive lifestyle intervention-to-metformin group HR was 0.93 (0.77–1.12), and the metformin-to-placebo group HR was 0.94 (0.77–1.14), indicating no differences in the rates among the three groups. We also explored rate change patterns by sex, baseline age, BMI group, and initial IGT or IGT plus impaired fasting glucose (IFG) levels, with each group having patterns similar to those of the entire group (see Supplementary Data).
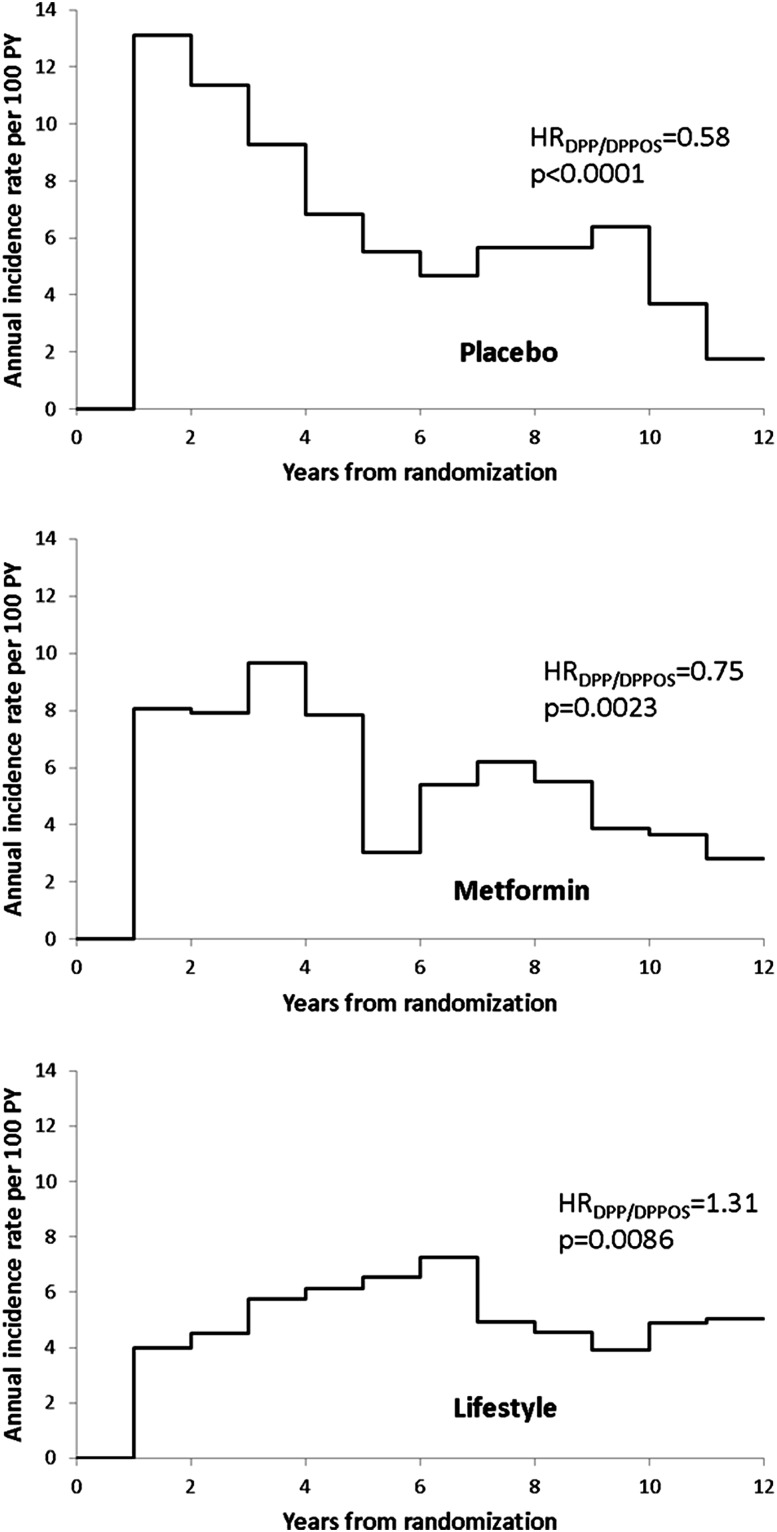
We examined the average annual incidence rates from randomization over time to assess when changes began to occur (Fig. 1). Annual incidence rates in the intensive lifestyle intervention group (Fig. 1, bottom panel) rose slowly through year 4 postrandomization, then declined to steady levels at year 7. Among participants in the metformin group (Fig. 1, middle panel), annual rates were steady until year 5, then fell and remained lower than the rates during the DPP with a continuing downward trend late in the DPPOS. The placebo group (Fig. 1, top panel) showed a very different trend, rising to the highest rate early in the DPP, with a relatively steady decline through the end of the DPP and into the DPPOS, and leveling late in the DPPOS. Figure 1 also shows the HRs for the average annual incidence rates during the DPPOS period compared with the DPP. There was a significant increase in incidence rates (31%) for intensive lifestyle intervention participants in the DPPOS compared with those in the DPP (HR 1.31 [95% CI 1.07–1.61]), whereas both the metformin group (0.75 [0.62–0.90]) and the placebo group (0.58 [0.48–0.69]) had significant decreases in rates after the DPP period (25% and 42%, respectively). The declines in absolute number of cases/100 person-years were largest for the metformin and placebo groups (−2.9 and −5.2 cases/100 person-years, respectively), compared with the increase in the intensive lifestyle intervention group (0.9/100 person-years). The pattern of rate changes in the metformin and placebo groups does not correspond with, or follow a consistent time lag of, the bridge period transition when all participants were offered lifestyle intervention.
Figure 1.
Average annual incidence rates (per 100 person-years [PY]) by time since randomization by initial treatment group.
We explored the effective intervention hypothesis several ways. Since weight change was the primary variable explaining the difference between the treatment groups in the DPP (13), we explored whether the effects of changes in weight in both time periods were similar. HRs for a 1-kg weight change were nearly identical in the DPP and DPPOS within each treatment group (data not shown), indicating that weight loss was acting similarly in both study periods within each treatment group.
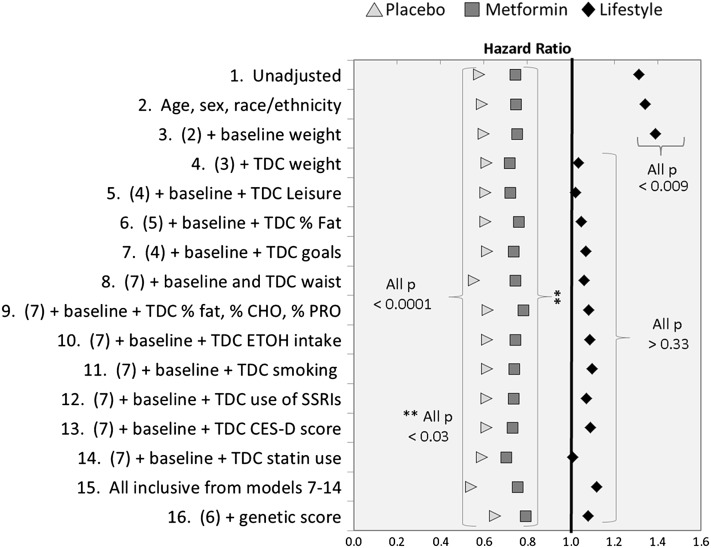
We next asked whether differences in weight change patterns among the groups could partially or fully explain the trends in rates between the DPP and DPPOS. Figure 2 compares the HRs in the DPPOS to those in the DPP, first unadjusted (model 1: intensive lifestyle intervention HR 1.31; metformin HR 0.75; placebo HR 0.58) (as in Fig. 1), and then adjusted for various behavioral variables, added one at a time to prior models, in each treatment group separately. In the intensive lifestyle intervention group, addition of age, sex, race/ethnicity, and baseline weight did not reduce the HR (model 2: 1.34; model 3: 1.39). However, starting with model 4, which added time-dependent weight change, the HR was 1.04, which was no longer different than 1.0 (P = 0.755), and the HR remained at that level with the addition of time-dependent leisure time physical activity and the percentage of calories from fat, suggesting that the resulting weight regain explained the increased intensive lifestyle intervention incidence rates in the DPPOS compared with those in the DPP. However, the same models for metformin and placebo participants showed no such pattern. Each HR remained significantly different from 1.0 (metformin 0.72–0.76; placebo 0.59–0.61) over models 2–6 and very close to the unadjusted values. Because the greater number of study goals that were met, the lower the risk (13,14), we added meeting study goals (model 7), which also did not explain the metformin (HR 0.74, P = 0.002) and placebo (HR 0.62, P < 0.001) differences. Finally, we explored a series of other risk factors that predict diabetes (15) and might have differed by treatment group, including changes in waist circumference (model 8), other aspects of diet (model 9), alcohol consumption (model 10) (16), smoking status (model 11) (17), use of antidepressants (model 12), depression score (model 13) (18), use of statins (model 14) (19,20), and a final model including all these variables (model 15). None of these variables materially changed HRs for either the metformin group (model 15: HR 0.76, P = 0.009) or the placebo group (HR 0.54, P < 0.001). Finally, we added the GRS to the full behavioral model (model 16 = model 6 plus GRS) with no significant change in HR estimate and little change in statistical significance for each treatment group. Thus, weight gain explained the change in diabetes risk between the two periods in the intensive lifestyle intervention group, but accounting for weight changes and other clinical variables did not explain the differences between study periods in the metformin and placebo groups. These observations argue against effective intervention in these groups as an explanation for reduction in the incidence in the metformin and placebo groups during the DPPOS.
Figure 2.
HRs for the DPPOS to DPP time period, by treatment group using sequential adjustment for risk factors. CES-D, Center for Epidemiologic Studies of Depression score; % CHO, percentage of calories from carbohydrate; ETOH, alcohol intake; % fat, percentage of calories from fat; goals, number of five study goals met; waist, waist circumference; % PRO, percentage of calories from protein; smoking, current, former, never cigarette smoker; SSRI, selective serotonin reuptake inhibitors; statin, HMG-CoA reductase inhibitors; TDC, time-dependent covariate; leisure, leisure time physical activity. Model 1 is unadjusted; model 2 is model 1 plus the factors listed.
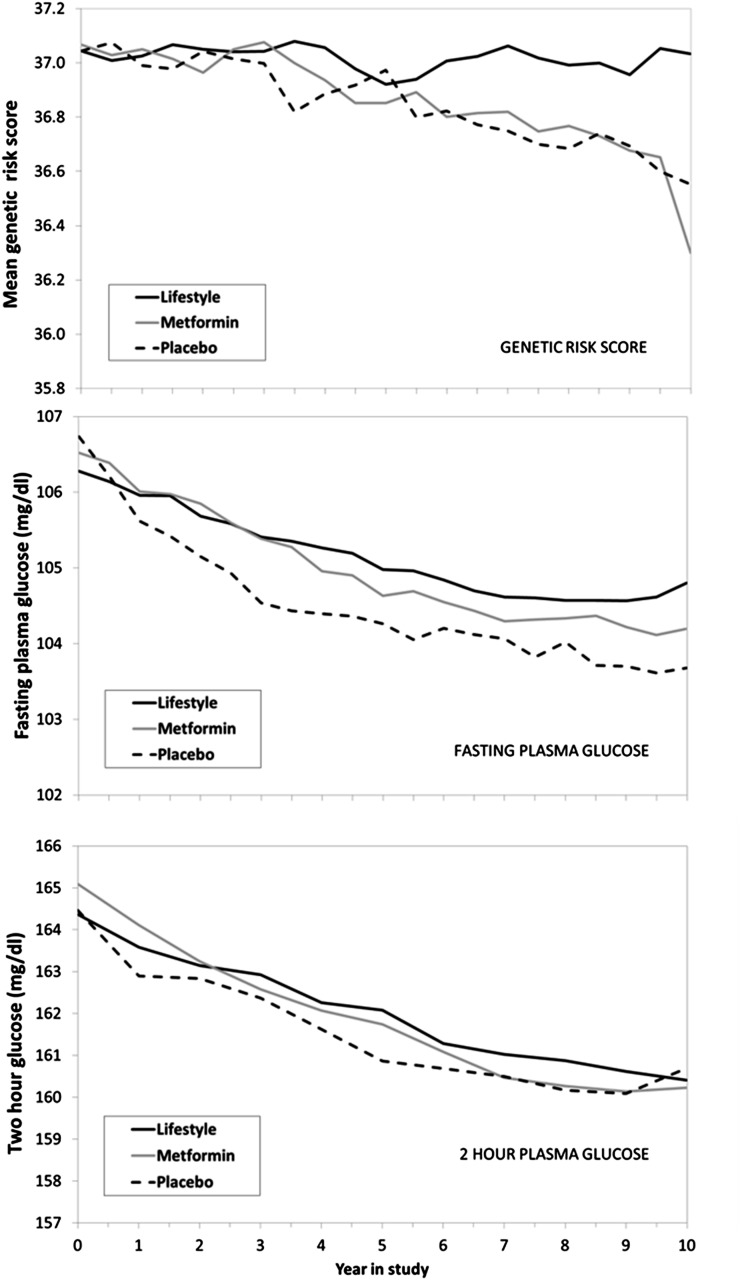
We next explored the exhaustion of susceptibles hypothesis. While conceptually simple in an infectious disease context, it is not straightforward to test, because nearly all measures of susceptibility or change in susceptibility to diabetes over time are confounded by treatment effects. We explored changes in average GRS (10) and DRS (11) for each group over time. Figure 3 (top panel) shows the results of the calculation of the mean GRS among the group of persons who remained without diabetes at every 6-month visit over the course of the DPP and DPPOS. There was a declining trend in the group mean GRS over time, which began to diverge near the start of DPPOS visits. There was little decrease in the mean GRS among participants in the intensive lifestyle intervention group, but both the metformin and placebo groups had lower average scores over time, suggesting that persons remaining nondiabetic had somewhat lower genetic susceptibility. There were small nonsignificant declines in the DRS over time with no difference between treatment groups (data not shown). Since fasting and 2-h glucose levels are strong predictors of diabetes, we calculated the baseline group mean glucose levels at each visit, removing persons who became diabetic over time. The middle (fasting) and bottom (2 h) panels in Fig. 3 show small declines over time, suggesting fewer persons at higher risk, but there is little difference among treatment groups, unlike that seen for GRS.
Figure 3.
Mean baseline GRS (top panel), baseline fasting plasma glucose level (middle panel), and baseline 2-h plasma glucose level (bottom panel) at each visit among groups of persons remaining nondiabetic at each visit.
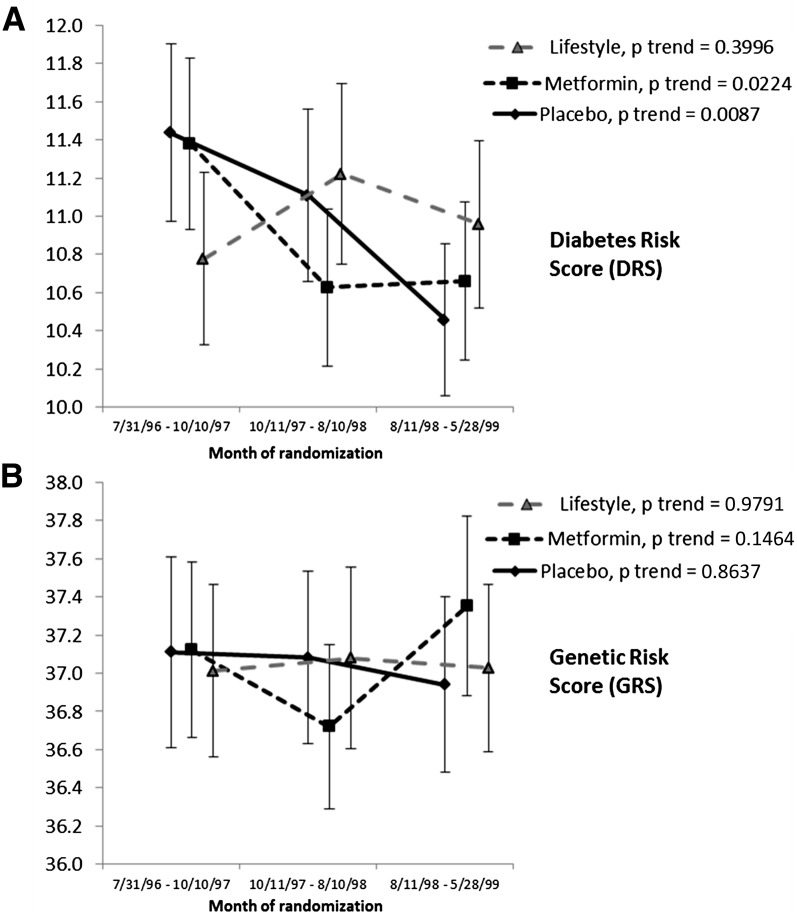
Next, we explored whether participants randomized over the ∼3-year period from July 1996 to May 1998 had different levels of baseline diabetes risk. If participants entering the DPP earlier were at higher risk than those recruited later, they would have entered the DPPOS first, followed by lower-risk subjects, who would have had lower incidence rates later in the follow-up period. Such a pattern would have to be differential by treatment group to aid in understanding incidence patterns. Figure 4 shows both the DRS (panel A) and the GRS (panel B) patterns, stratified into three recruitment periods with approximately equal recruitment numbers in each. Within each period, there were no statistically significant differences among treatment groups; however, there were statistically significant decreasing trends in the estimated DRS in both the metformin and placebo groups, but not in the intensive lifestyle intervention group. While significant, the magnitude of the decline was small. No temporal trend was seen for the GRS during the randomization period.
Figure 4.
A: Mean DRS at randomization by month of randomization, by treatment group (mean, 95% CIs). Each time interval includes 1,072, 1,073, and 1,075 participants, respectively, balanced in the three treatment groups. B: Mean GRS. Each time interval includes 947, 941, and 955 participants, respectively, also well balanced in the three treatment groups.
Discussion
Diabetes incidence rates in the DPPOS time period were similar across the three intervention groups (2), resulting from increasing rates in the intensive lifestyle intervention group, but larger declines in both the metformin and placebo groups. We examined the following two hypotheses to explain these patterns: the effective intervention and the exhaustion of susceptibles. In the former, we postulated that participants in the metformin and placebo groups experienced weight loss sufficient to account for the change in diabetes risk after the group lifestyle classes that were offered during the bridge period between the DPP and the start of the DPPOS (3). Importantly, weight change had a similar association with diabetes risk in each treatment group during both the DPP and DPPOS, providing evidence that weight reduction retained its clinical significance throughout the study period. Models including weight change as a time-dependent variable did explain the increase in rates in the intensive lifestyle intervention group from the DPP to DPPOS. However, weight change, or combinations of other risk factors, did not explain the lower rates seen in either the metformin or placebo groups. These observations argue against the effective intervention hypothesis, contrary to our expectation.
Alternatively, we hypothesized that the decline in incidence rates among participants in the metformin and placebo groups might have been due to the exhaustion of susceptibles. This concept has a long history in the explanation of infectious disease transmission (21) and has been invoked to explain incidence patterns in selected cancers (22). In diabetes epidemiology, the concept has been proposed as a possible explanation for the observed lower diabetes incidence rates in persons >50 years of age among the Pima Indians, together with a possible cohort effect, where older cohorts were less exposed to risk earlier in life (23). In the context of the DPP/DPPOS, a small cohort effect appears to have occurred in the placebo and metformin groups during randomization (Fig. 4), but it did not explain the lower incidence rates in those groups during the DPPOS.
Several of our observations were consistent with the exhaustion hypothesis. First, patterns of annual incidence rates in the untreated placebo group rose rapidly and then began to decline midway through the DPP to even lower rates in the DPPOS, consistent with exhaustion; whereas the slower rise and fall in the metformin group and the absence of a rise in the intensive lifestyle intervention group likely reflect delays in the onset of diabetes, since higher-risk subjects would have been delayed to later time periods through effective interventions, and some would have been prevented for the duration of observation. Second, the mean GRSs (Fig. 3) declined in both the placebo and metformin groups similarly, and both were lower over time than in the intensive lifestyle intervention group. Very small declines in fasting and 2-h glucose levels were also seen, though they were not different between groups. Third, participants randomized early to receive metformin or placebo had somewhat higher mean baseline DRSs that declined significantly over the randomization period, whereas little change was seen in the intensive lifestyle intervention group (Fig. 4). The magnitude of this decline was small, however.
As previously reported (10), the GRS was significantly associated with diabetes risk, but in the highest quartile of GRS, intensive lifestyle intervention was effective in reducing risk. We interpret the lack of decline in the intensive lifestyle intervention GRS to mean that genetically high-risk subjects were retained in this group for a longer period due to an effective intervention, but were lost from the other two treatment groups because they developed diabetes. While consistent with the exhaustion hypothesis, the magnitude of the changes in the mean GRS over time are relatively small, predicting only a 1.1% decrease in risk (10). Similarly, Fig. 2 shows that the addition of the GRS to model 6 (i.e., model 16) does not materially change the HRs, suggesting a limited impact of this score. Nonetheless, these results are consistent with an exhaustion hypothesis. There was small decline in the DRS over time, but there were not differences between groups. We did, however, see a baseline difference in the DRS during the recruitment period (Fig. 4). This would have resulted in fewer higher-risk participants entering the DPPOS in the later time intervals, and would have reduced the treatment group risk profile in a manner similar to that in the exhaustion hypothesis.
We also reviewed the long-term follow-up experience of similar studies of persons with IGT to determine whether a decline in rates over time occurred among participants in the control or placebo groups, which would lend additional support for the exhaustion hypothesis. Studies reviewed included the long-term follow-up of Pima Indians with IGT (24), the Finnish Diabetes Prevention Study (14), the DREAM (25,26) and DREAM-ON follow-up studies (27), the Da Qing Prevention Study (28), and the ADDITION-Denmark study (29). Only in the latter study were lower rates of diabetes seen 1.5–3 years after screening for high-risk IFG levels and IGT (29), with the highest rates among those with combined IFG levels plus IGT, a pattern similar to ours. None of the other reports showed evidence of lower incidence rates of diabetes within 4–6 years and up to 20 years after randomization; however, none reported annual incidence rates as our study and the ADDITION-Denmark study have done. When examining only cumulative incidence, it is often difficult to ascertain the underlying pattern of incidence rates, and we were not aware of such patterns in the placebo group until this analysis was undertaken. Thus, lack of agreement among other studies may be an artifact of data presentation. Only one of six studies directly supports the phenomenon of exhaustion, as we postulated. In addition, most of these studies enrolled subjects with either a single IGT result on an oral glucose tolerance test (24,30) or two IGT results (31), but they did not require a separate fasting glucose elevation at entry, as was done in the DPP (fasting glucose ≥5.3 mmol/L [≥95 mg/dL]). Only in the DREAM trial (25,26) did the majority of participants have both IGT and IFG, or IFG alone. Persons with IGT and elevated fasting glucose levels have subsequent rates of new diabetes higher than those with lower levels of fasting glucose (29,32). It seems unlikely that requiring an elevated fasting glucose level is responsible, since among persons with isolated IFG or IGT in the ADDITION-Denmark study, a pattern of exhaustion was also seen, as it was in our data (see Supplementary Data).
Other possible explanations deserve mention. The incidence rates in the metformin group during the DPPOS period were significantly lower than those during the DPP. Whether long-term use of metformin has effects different from those after shorter periods of use, as seen initially in the DPP, is unknown. However, only 57% of the participants in the nondiabetic metformin group in the DPPOS took ≥80% of the prescribed metformin dose (2), which is lower than that during the DPP (72%) (1). Similarly, it is unlikely that the use of metformin among participants in the placebo group accounted for their lower rates, since only 3% reported taking metformin prescribed outside the study (2). It is also possible that long-term population changes in diabetes risk occurring outside the trial might have affected the DPPOS participants, but this seems unlikely since this effect would need to be different by treatment group to explain the observed differences.
This analysis has some limitations. It was a post hoc exploratory analysis, with multiple analytic comparisons made. Whether there remain important unmeasured risk variables is an open question, though the primary ones used in this analysis are strong and widely predictive across studies. The exhaustion analysis was limited to a few approaches. The GRS analysis and the lower risk for participants randomized later in the metformin and placebo groups were consistent with exhaustion. However, there are few well-established approaches to test this hypothesis, and we are left with limited evidence to support it.
Conclusions
No combination of risk variables explained the decline in rates for the metformin and placebo groups compared with the intensive lifestyle intervention group between the DPP and DPPOS. Thus, it does not appear that effective intervention was the reason for the decline in rates. There was support for the exhaustion of susceptibles hypothesis, since the mean GRS did decline more in the metformin and placebo groups than in the intensive lifestyle intervention group, and there was a significant trend of recruitment of lower-risk participants later in the metformin and placebo groups. Only one of six long-term studies of high-risk persons was consistent with the exhaustion hypothesis. Thus, we identified some internal support for the exhaustion hypothesis, and no support for the effective intervention hypothesis as the reason for the lower rates in the metformin and placebo groups in the DPPOS. Importantly, weight loss remained an effective strategy to reduce diabetes risk over the entire study period, and the long-term reductions in the relative risk of diabetes seen in the DPPOS in the intensive lifestyle intervention group (2) would have been larger, had not the metformin and placebo group rates declined over time. These findings have implications for treatment intervention duration and diabetes prevention trial planning, as the long-term observed effects of the treatments, while remaining highly significant (2), were reduced over time by increases in rates in the intensive lifestyle intervention group and even larger absolute decreases in rates in the metformin and placebo groups.
Supplementary Material
Article Information
Acknowledgments. The authors thank the participants in the Diabetes Prevention Program for their commitment and dedication.
Funding. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding of the clinical and coordinating centers that designed and undertook the study and collected, managed, analyzed, and interpreted the data. The General Clinical Research Center Program and the National Center for Research Resources supported data collection at many of the clinical centers. The study was funded by the NIDDK as a cooperative agreement. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and by the Indian Health Service. Funding was also provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development; the National Institute on Aging; the National Eye Institute; the National Heart, Lung, and Blood Institute; the Office of Women’s Health; the National Institute on Minority Health and Health Disparities; the Centers for Disease Control and Prevention; the Department of Veterans Affairs; and the American Diabetes Association. The sponsor of this study was represented on the Steering Committee and played a part in the study design, how the study was performed, and the publication of the article. The funding agency was not represented on the writing group, although all members of the Steering Committee had input into the contents of the report.
Duality of Interest. Lipha (Merck-Sante) provided medicines, and LifeScan donated materials. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.F.H. helped to conceive and conduct the study; and wrote, reviewed, and critically edited the manuscript. E.H. helped to conceive the study and write the manuscript. E.B.-C., G.A.B., J.C., J.C.F., S.F., R.G., S.E.K., W.C.K., M.B.M., and E.V. helped to conduct the study, and reviewed and critically edited the manuscript. C.A.C. and J.M.L. developed the analysis plan and analyzed the data. All authors in the writing group had access to all of the data. R.F.H. and E.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
The Diabetes Prevention Program (DPP) Writing Group: Richard F. Hamman, MD, DrPH (co-chair); Edward Horton, MD (co-chair); Elizabeth Barrett-Connor, MD; George A. Bray, MD; Costas Christophi, PhD; Jill Crandall, MD; Jose Florez, MD, PhD; Sarah Fowler, PhD; Ron Goldberg, MD; Steven E. Kahn, MB, ChB; William C. Knowler, MD, DrPH; John Lachin, PhD; Mary Beth Murphy, MSN; and Elizabeth Venditti, PhD.
Footnotes
Clinical trial reg. nos. NCT00004992 and NCT00038727, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0333/-/DC1.
The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Fowler SE, Hamman RF, et al.; Diabetes Prevention Program Research Group . 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venditti EM, Bray GA, Carrion-Petersen ML, et al.; Diabetes Prevention Program Research Group . First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes (Lond) 2008;32:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Prevention Program Research Group The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care 2000;23:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program Research Group Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003;26:977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne CD, Wareham NJ, Brown DC, et al. Hypertriglyceridaemia in subjects with normal and abnormal glucose tolerance: relative contributions of insulin secretion, insulin resistance and suppression of plasma non-esterified fatty acids. Diabetologia 1994;37:889–896 [DOI] [PubMed] [Google Scholar]
- 8.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol 1999;9:314–324 [DOI] [PubMed] [Google Scholar]
- 9.Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc 1997;29:5–9 [PubMed] [Google Scholar]
- 10.Hivert MF, Jablonski KA, Perreault L, et al.; DIAGRAM Consortium; Diabetes Prevention Program Research Group . Updated genetic score based on 34 confirmed type 2 diabetes Loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes 2011;60:1340–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alssema M, Vistisen D, Heymans MW, et al.; DETECT-2 collaboration . The Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance (DETECT-2) update of the Finnish diabetes risk score for prediction of incident type 2 diabetes. Diabetologia 2011;54:1004–1012 [DOI] [PubMed] [Google Scholar]
- 12.Lachin JM. Biostatistical Methods: The Assessment of Relative Risks. New York, Wiley-Interscience, 2000 [Google Scholar]
- 13.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindström J, Peltonen M, Eriksson JG, et al.; Finnish Diabetes Prevention Study (DPS) . Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia 2013;56:284–293 [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009;169:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crandall JP, Polsky S, Howard AA, et al.; Diabetes Prevention Program Research Group . Alcohol consumption and diabetes risk in the Diabetes Prevention Program. Am J Clin Nutr 2009;90:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wannamethee SG, Shaper AG, Perry IJ; British Regional Heart Study . Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care 2001;24:1590–1595 [DOI] [PubMed] [Google Scholar]
- 18.Rubin RR, Ma Y, Marrero DG, et al.; Diabetes Prevention Program Research Group . Elevated depression symptoms, antidepressant medicine use, and risk of developing diabetes during the diabetes prevention program. Diabetes Care 2008;31:420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742 [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkelstein W Jr, Samuel M, Padian NS, et al. The San Francisco Men’s Health Study: III. Reduction in human immunodeficiency virus transmission among homosexual/bisexual men, 1982-86. Am J Public Health 1987;77:685–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moger TA, Aalen OO, Halvorsen TO, Storm HH, Tretli S. Frailty modelling of testicular cancer incidence using Scandinavian data. Biostatistics 2004;5:1–14 [DOI] [PubMed] [Google Scholar]
- 23.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol 1978;108:497–505 [DOI] [PubMed] [Google Scholar]
- 24.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med 1988;319:1500–1506 [DOI] [PubMed] [Google Scholar]
- 25.Bosch J, Yusuf S, Gerstein HC, et al.; DREAM Trial Investigators . Effect of ramipril on the incidence of diabetes. N Engl J Med 2006;355:1551–1562 [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC, Yusuf S, Bosch J, et al.; DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators . Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 27.Gerstein HC, Mohan V, Avezum A, et al.; DREAM On (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication Ongoing Follow-up) Investigators . Long-term effect of rosiglitazone and/or ramipril on the incidence of diabetes. Diabetologia 2011;54:487–49521116607 [Google Scholar]
- 28.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen SS, Glümer C, Sandbaek A, Lauritzen T, Borch-Johnsen K. Determinants of progression from impaired fasting glucose and impaired glucose tolerance to diabetes in a high-risk screened population: 3 year follow-up in the ADDITION study, Denmark. Diabetologia 2008;51:249–257 [DOI] [PubMed] [Google Scholar]
- 30.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 31.Tuomilehto J, Lindström J, Eriksson JG, et al.; Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 32.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.