Abstract
Stratifying the management of type 2 diabetes (T2D) has to take into account marked variability in patient phenotype due to heterogeneity in its pathophysiology, different stages of the disease process, and multiple other patient factors including comorbidities. The focus here is on the very challenging subgroup of patients with T2D who are overweight or obese with insulin resistance (IR) and the most refractory hyperglycemia due to an inability to change lifestyle to reverse positive energy balance. For this subgroup of patients with T2D, we question the dogma that IR is primarily harmful to the body and should be counteracted at any cost. Instead we propose that IR, particularly in this high-risk subgroup, is a defense mechanism that protects critical tissues of the cardiovascular system from nutrient-induced injury. Overriding IR in an effort to lower plasma glucose levels, particularly with intensive insulin therapy, could therefore be harmful. Treatments that nutrient off-load to lower glucose are more likely to be beneficial. The concepts of “IR as an adaptive defense mechanism” and “insulin-induced metabolic stress” may provide explanation for some of the unexpected outcomes of recent major clinical trials in T2D. Potential molecular mechanisms underlying these concepts; their clinical implications for stratification of T2D management, particularly in overweight and obese patients with difficult glycemic control; and future research requirements are discussed.
Introduction
It is now recognized that glycemic management in patients with type 2 diabetes (T2D) should be stratified with respect to choice of glucose-lowering agents and HbA1c targets (1,2). This comes about with increasing realization of the marked heterogeneity in patients with T2D with respect to pathophysiology, stage of disease, and comorbidities (1–4). Importantly, this same heterogeneity in the phenotype of patients recruited to major T2D clinical trials must complicate interpretation of their main outcomes. For example, if a particular approach to intensively lower blood glucose is harmful to only one subgroup of patients, then its potential benefit to all other patient subgroups may be missed. In this Perspective, we consider the subgroup of patients with T2D who are overweight and obese with severe insulin resistance (IR) and difficult-to-control hyperglycemia due to their inability to reverse a positive energy balance through lifestyle measures. We propose that IR protects critical tissues, such as the heart, from nutrient-induced damage in this subgroup and that “approaches” to intensively lower blood glucose that override IR (e.g., high-dose insulin therapy) will cause them harm. We believe that the concept of “insulin-induced metabolic stress” provides a plausible explanation for many of the unexpected outcomes of major T2D clinical trials. The important implications of this concept for ongoing diabetes research, drug development, and clinical care of patients with T2D are discussed.
IR: Offense or Defense
IR is nearly always considered to be “harmful” and at the root of T2D (5). The regulation of insulin sensitivity, however, is an integral component of normal metabolic physiology. Diurnal, seasonal, age-related, pregnancy-associated, and illness-induced fluctuations in food intake and energy expenditure necessitate homeostatic versatility, including the capacity to vary insulin sensitivity so as to optimize partitioning between tissues of a variable nutrient supply. For example, in response to short-term overfeeding, skeletal and cardiac muscle become transiently insulin resistant (6,7), a physiological adaptation that favors the diversion of excess nutrients to adipose tissue for storage. We have proposed, as have others, that this induction of IR, particularly when an excess nutrient supply becomes more chronic, protects important tissues from nutrient-induced dysfunction (8–11).
Thus, to override IR in overnourished patients with T2D with certain glucose-lowering therapies, such as insulin, may equate to overriding a defense mechanism, as the tissues will no longer be protected from excess nutrient entry. In the heart, this could cause metabolic cardiomyopathy with greater risk of heart failure, arrhythmias, and cardiac death, including reduced survival from myocardial infarction.
Concept of Insulin-Induced Metabolic Stress and Its Relevance for the Heart
There is normally a reciprocal relationship between plasma free fatty acids (FFAs) and glucose levels in blood. In the fasted state, blood glucose is low and FFA levels are elevated due to their release from adipose tissue. In the fed state, blood glucose and insulin levels rise and FFA levels fall due to the suppression of lipolysis by insulin. The myocardium, with its high-energy needs, adapts to the predominant nutrient source through complex interactions between glucose and FFA metabolism (12,13). High FFA levels during fasting inhibit the uptake and oxidation of glucose by the myocardium, thus sparing glucose for use by the brain (6,12). In poorly controlled T2D, this reciprocal relationship is lost and circulating glucose and FFA are simultaneously elevated (6). This places the myocardium at increased risk of nutrient overload and myocardial glucolipotoxicity (14,15), a process by which elevated glucose synergizes with FFAs to induce cellular damage (16).
Because of the presence of the high Km glucose transporter GLUT2 that rapidly equilibrates extracellular and intracellular glucose independently of insulin, the β-cell is particularly vulnerable to glucolipotoxicity (10,16). Other tissues, such as heart and skeletal muscle, that express the insulin-regulated glucose transporter GLUT4 have the capacity to protect themselves from glucolipotoxicity by developing IR, which restrains glucose entry into cells and therefore the glucose arm of this potentially damaging process (17). Accordingly, we propose that the myocardium is “safeguarded” against glucolipotoxicity by IR (Fig. 1). Consequently, treating patients with poorly controlled T2D with large amounts of exogenous insulin could override this block against glucose entry, providing all the ingredients for glucolipotoxicity (Fig. 1). In support of this concept, treatment of patients with T2D with 10 days of exogenous insulin increased the myocardial lipid content by 80%, the level of which was positively associated with the initial mean glucose concentration (18). Even in normal subjects, short-term hyperinsulinemia and hyperglycemia caused an increase in myocardial lipid content, indicative of how hyperinsulinemia together with hyperglycemia can contribute to cardiac lipid accumulation (19).
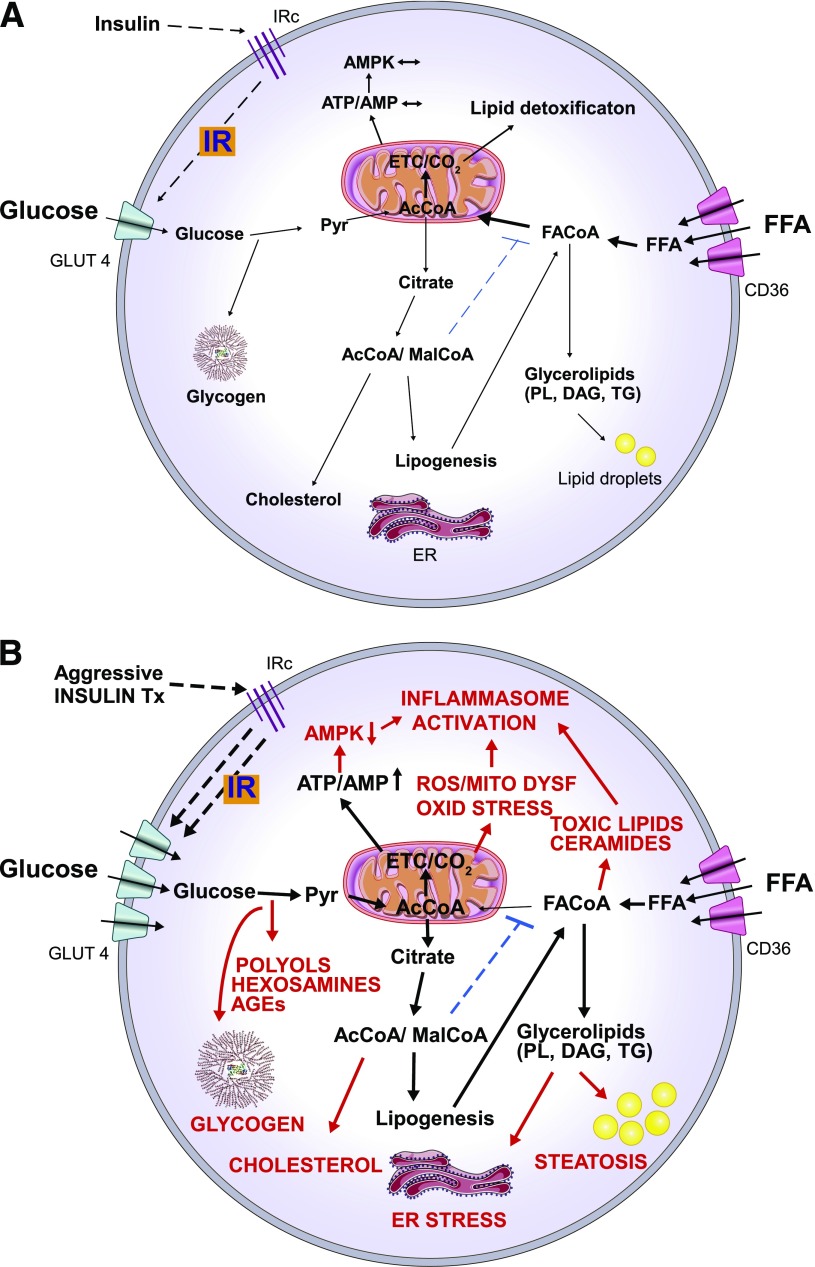
Figure 1.
Hypothetical model illustrating the molecular basis of insulin-induced metabolic stress in patients with poorly controlled T2D in which both blood glucose and FFA levels are persistently elevated. Depicted here is a cell in which (A) IR protects from nutrient overload and metabolic stress by limiting glucose flux into the cell and (B) the IR protection is overridden by a high dose of exogenous insulin therapy, which promotes excess glucose uptake and glucolipotoxicity. Excess glucose supply to the mitochondria results in reducing equivalent overload of the electron transfer chain and enhanced production of ATP and ROS, resulting in oxidative damage. The resulting increased ATP/AMP ratio inhibits AMPK, which has the effect of decreasing FFA oxidation (limiting nutrient detoxification) favoring fat deposition. Enhanced glucose uptake can also result in excessive glycogen deposition and increased activities of the toxic polyol, hexosamine, and AGE formation pathways. Glucose that is metabolized via the anaplerosis pathway can also increase cytosolic acetyl-CoA (AcCoA) and malonyl-CoA (MalCoA). AcCoA and MalCoA are then available for cholesterol and fatty acid synthesis, increasing the lipid load on the cell. MalCoA also inhibits fatty acyl-CoA (FACoA) entry into the mitochondria such that FACoA is more available for synthesis of complex lipids, including glycerolipids (phospholipids, diacylglycerols, and triglycerides) and ceramides. This can result in endoplasmic reticulum stress and the accumulation of lipid droplets (steatosis). Increased ROS production, toxic lipid accumulation, and reduced AMPK activity are factors that also activate the inflammasome contributing to cardiac injury. The overall effect is nutrient overload and metabolic stress causing cell dysfunction or death and cardiac inflammation. CD36, free fatty acid transporter; DAG, diacylglycerols; ER, endoplasmic reticulum; ETC, electron transport chain; GLUT4, facilitative glucose transporter 4; IRc, insulin receptor; MITO DYSF, mitochondrial dysfunction; OXID STRESS, oxidative stress; Pyr, pyruvate; PL, phospholipids; TG, triglycerides; Tx, treatment.
We propose the concept of insulin-mediated metabolic stress for situations in which IR that protects against excess nutrient entry into cells is overridden by high insulin levels (Fig. 1). The combination of high levels of glucose and FFA entry into cells will overload the electron transfer chain with reducing equivalents resulting in mitochondrial dysfunction and increased reactive oxygen species (ROS) production (9,20). The increased glucose entry will also alter the malonyl-CoA/AMPK metabolic network to favor the partitioning of the FFA toward synthesis of complex lipids, including cholesterol and ceramide (21,22), and glucolipotoxicity, contributing to both mitochondrial dysfunction and endoplasmic reticulum stress (22–24) (Fig. 1). The increased FFA levels will also impede glucose oxidation, particularly at the level of pyruvate dehydrogenase, such that glucose flux into pathways above this step, including glycogen synthesis, the polyol and hexosamine pathways, and the production of advanced glycation end product (AGE) precursors, are likely to be increased (14,25). Recently, there has been increased interest in the role of the inflammasome in cardiomyopathy and ischemia/reperfusion injury (26). Relevant to insulin-induced metabolic stress, the inflammasome can be activated by increased glucose metabolism, saturated fatty acids, ceramides, and elevated ROS production (27) (Fig. 1).
With respect to rodent models and the potential for excessive insulin action to affect cardiac function, insulin signaling in the heart has been shown to worsen systolic function in pressure-overloaded rodent hearts (28). Furthermore, cross talk has been shown to occur between insulin receptor and β2-adrenergic receptor signaling in the mouse heart, with insulin having an effect to impair β2-adrenergic receptor–regulated cardiac contractility (29).
Tissue Selectiveness in the Development of IR: the Heart and Skeletal Muscle
The partitioning of nutrient supply to various tissues in response to insulin is affected by the relative insulin sensitivities of those tissues. This is potentially important when using insulin to treat insulin-resistant patients with T2D, as the least insulin-resistant tissues will be more prone to insulin-induced metabolic stress. There is some evidence that IR is easier to override in the myocardium than in skeletal muscle (30,31), which could relate to the different signaling pathways by which IR develops in these two tissues (17,32). The patients with T2D in both these studies appeared to have quite good myocardial insulin responsiveness once high doses of exogenous insulin were used (30,31). Of concern, exogenous insulin tripled myocardial glucose uptake without any compensatory reduction in FFA uptake in one of these studies, consistent with the capacity of a high dose of exogenous insulin to override IR to drive excess nutrient entry into the heart, thus placing the cardiomyocyte at risk for nutrient-induced damage (30).
Of note, insulin use during cardiac ischemia preconditioning in mice resulted in loss of cardioprotection from ischemia/reperfusion injury afforded by the preconditioning (33). Furthermore, this may have related to an increase in the glycogen content of heart prior to the prolonged ischemic event, indicative of an effect of excessive insulin to augment cardiac damage through a nutrient toxicity mechanism (33).
IR and Consequences of Insulin Therapy for the Endothelial Cell and Atherosclerosis
The endothelial cell is the most directly exposed cell of the body to the nutrient and hormonal mix of blood and plays a key role in the pathogenesis of both micro- and macrovascular complications of diabetes (34,35). In considering mechanisms, insulin signal transduction in the endothelial cell occurs via the phosphoinositide 3-kinase/AKT (PI3K/AKT) pathway and the mitogen-activated protein kinase (MAPK) pathway (32) (Fig. 2). Transduction via the PI3/AKT pathway is believed to protect against vascular risk because of its effects to stimulate vasodilatory endothelial nitric oxide production, reduce the expression of adhesion molecules, inhibit proliferation of vascular smooth muscle cells, and prevent adhesion and activation of platelets (36,37). Signaling via the MAPK pathway is believed to have contrary effects through the production of the vasoconstrictor mediator endothelin-1 (34,37) (Fig. 2). Interestingly, nutrient excess has been shown to selectively cause IR in the PI3K/AKT pathway, such that under these circumstances insulin action through the “bad” MAPK pathway predominates (34,38). Thus, successful lowering of blood glucose and FFAs by exogenous insulin should result in improved endothelial functioning due to a reduction in nutrient-induced IR of the “good” PI3K/AKT pathway (35). On the other hand, use of high doses of insulin therapy in subjects who are refractory to its glucose-lowering effect (i.e., the subgroup of patients of most interest in this Perspective) will not improve PI3K/AKT insulin signaling in the endothelium but will have increased harmful signaling through the MAPK pathway, increasing risk of atherosclerotic vascular complications (35) (Fig. 2).
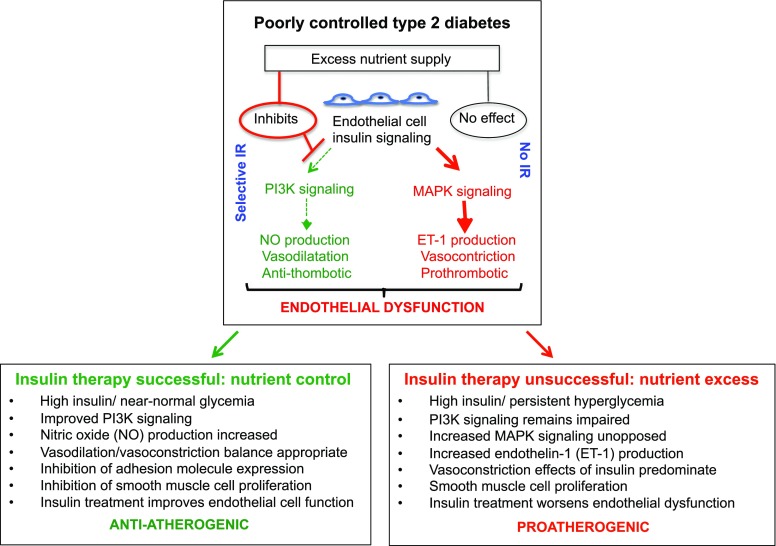
Figure 2.
Differential beneficial or adverse effects of insulin therapy on vascular endothelial cells depending on the level of metabolic control achieved. Insulin signaling in endothelial cells can be via the PI3K (causes vasodilation and is anti-thrombotic) and the MAPK pathway (causes vasoconstriction and is prothrombotic) such that there is a balance of beneficial and harmful effects. In response to an excess nutrient supply, as occurs in metabolically uncontrolled T2D, selective IR in the PI3K pathway will occur such that signaling through the MAPK pathway will be unopposed, increasing the risk of vascular events. Insulin treatment will either improve or worsen vascular health depending on whether it is effective or not at bringing blood nutrient levels under control. If high exogenous insulin therapy is successful at improving blood nutrient levels, then insulin signaling through the PI3K pathway will increase such that insulin therapy will be beneficial to blood vessel health. However, if high dose exogenous insulin therapy fails to control blood nutrients (i.e., in refractory patients), then the IR in the PI3K pathway will not be relieved and insulin signaling will predominate via the harmful MAPK pathway and increase the risk of vascular events.
Insulin-Induced Metabolic Stress in Noncardiovascular Tissues
Overriding IR with high-dose insulin in refractory patients also could have implications for other insulin-responsive tissues, such as the liver and adipose tissue. In the liver, it is clear that hyperinsulinemia is a requirement for nonalcoholic fatty liver disease; however, hyperglycemia is well known to accelerate its progression to nonalcoholic steatohepatitis and cirrhosis (39). There is some evidence that hyperglycemia may accentuate the inflammatory phenotype of visceral adipose tissue in obese subjects (40), such that insulin-induced metabolic stress could also operate in this tissue. Resultant adipose metabolic injury could indirectly harm the heart and vasculature through the increased release of inflammatory mediators (41).
T2D Clinical Studies and the Concept of Insulin-Induced Metabolic Stress
If our premise is correct—that intensive treatment of T2D with insulin is harmful to those overweight and obese patients unable to improve lifestyle to achieve negative energy balance—then there should be support for the premise in clinical trials. Here, we review major clinical trials in patients with T2D of intensive versus conventional glucose control (Table 1), insulin versus other glucose-lowering therapies (Table 2), and lifestyle and other pharmacotherapy cardiovascular outcome trials (Table 3). Essential considerations in reviewing these trials should be the degree to which the use of insulin therapy was intensive and whether the patients were obese and/or gained weight and were in poor glycemic regulation at study entry (more likely in the difficult-to-control patients).
Table 1.
Major trials of intensive vs. conventional glucose lowering in subjects with T2D
| UKPDS-33 (Int-SU or Insulin) | UKPDS-34 (Int-Metformin) | ADVANCE | ACCORD | VADT | |
|---|---|---|---|---|---|
| Subjects (n) | 3,867 | 753a | 11,140 | 10,251 | 1,794 |
| Baseline characteristics | |||||
| Age (years) | 53 | 53 | 66 | 62 | 60 |
| BMI (kg/m2) | 28 | 32 | 28 | 32 | 31 |
| Diabetes duration (years) | 0 | 0 | 8 | 10 | 11.5 |
| Cardiovascular disease history (%) | NR | NR | 32 | 35 | 40 |
| HbA1c (%) | 7.1 | 7.2 | 7.5 | 8.3 | 9.4 |
| Glucose-lowering therapy use | Int vs. Conv | Int vs. Conv | Int vs. Conv | Int vs. Conv | Int vs. Conv |
| Insulin (%) | 38 vs. 16 | NR | 41 vs. 24 | 77 vs. 55 | 87 vs. 77b |
| Metformin (%) | 10 vs. 10 | NR | 74 vs. 67 | 95 vs. 87 | 60 vs. 54b |
| Sulfonylurea (%) | 54 vs. 25 | NR | 92 vs. 59 | 78 vs. 68 | 53 vs. 44b |
| TZD (%) | — | — | 17 vs. 11 | 92 vs. 58 | 37 vs. 28b |
| Results | |||||
| Duration of follow-up (years) | 10.0 | 10.7 | 5.0 | 3.5 | 5.6 |
| HbA1c achieved, Int vs. Conv (%) | 7.0 vs. 7.9 | 7.4 vs. 8.0 | 6.5 vs. 7.3 | 6.4 vs. 7.5 | 6.9 vs. 8.4 |
| Change in weight to study end (kg) | 6 vs. 2.5 | 1 vs. 1 | −0.1 vs. −1.0 | 3.5 vs. 0.4 | 8.2 vs. 4.1 |
| Change in weight, Int vs. Conv (kg/year of follow-up) | 0.6 vs. 0.25 | 0.1 vs. 0.1 | 0.0 vs. −0.2 | 1.1 vs. 0.1c | 1.5 vs. 0.7 |
| Primary cardiovascular outcome (HR or RR [95% CI]) | NR | NR | 0.94 [0.84–1.06] | 0.90 [0.78–1.04] | 0.88 [0.74–1.05] |
| Myocardial infarct (nonfatal) (HR or RR [95% CI]) | 0.79 [0.58–1.09] | 0.69 [0.35–1.64] | 0.98 [0.78–1.23] | 0.76 [0.62–0.92] | NR |
| Myocardial infarct (all) (HR or RR [95% CI]) | 0.84 [0.71–1.00] | 0.61 [0.41–0.89] | NR | NR | 0.82 [0.59–1.14] |
| Myocardial infarct (extended follow-up) (RR [95% CI]) | 0.85 [0.74–0.97]d | 0.67 [0.51–0.89]d | — | — | — |
| Cardiovascular death (HR or RR [95% CI]) | 1.02 [0.66–1.57] | NR | 0.88 [0.74–1.04] | 1.35 [1.04–1.76] | 1.32 [0.81–2.14] |
| All-cause mortality (HR or RR [95% CI]) | 0.94 [0.8–1.1] | 0.64 [0.45–0.91] | 0.93 [0.83–1.06] | 1.22 [1.01–1.46] | 1.07 [0.81–1.42] |
| All-cause mortality (extended follow-up) (RR [95% CI]) | 0.87 [0.79–0.96]d | 0.73 [0.59–0.92]d | — | — | — |
| Comments | Younger, leaner, recent-onset diabetes. Less aggressive glycemic control and moderate weight gain in Int group. | Younger, obese, recent-onset diabetes. Less aggressive glycemic control and low weight gain in Int group. | Older, leaner, long duration of diabetes. High use of SU and low use of insulin and TZD. No weight gain in Int group. | Older, more obese, long duration of diabetes. High use of insulin and TZD and high weight gain in Int group. | Older, obese, long duration of diabetes. High use of insulin and high weight gain in Int group. |
UKPDS (46–48), ADVANCE (50), ACCORD (42), VADT (49). Conv, conventional treatment; RR, risk ratio; Int, intensive treatment; NR, not recorded; SU, sulfonylurea. ACCORD and VADT had intensive glucose lowering involving intensive insulin use and were associated with weight gain greater than 1.0 kg/year. Bold typeface indicates significant changes.
Metformin-based intensively treated vs. conventionally treated subjects only.
Glucose-lowering therapy at last recorded study visit.
Measured at 3 years.
UKPDS 10-year post-trial follow-up.
Table 2.
Major clinical trials of insulin treatment vs. conventional glucose lowering in subjects with T2D (ACCORD study also shown)
| ACCORD | DIGAMI 1 | DIGAMI 2 | BARI 2D | ORIGIN | |
|---|---|---|---|---|---|
| Subjects (n) | 10,251 | 620 | 780a | 1,944b | 12,537c |
| Baseline characteristics | |||||
| Age (years) | 62 | 68 | 68 | 62 | 64 |
| BMI (kg/m2) | 32 | 27 | 28 | 32 | 30 |
| Diabetes duration (years) | 10 | 10 | 8.3 | 10.4 | 0–5.4 |
| Cardiovascular disease history (%) | 35 | 100 | 100 | 100 | 59 |
| HbA1c (%) | 8.1 | 8.1 | 7.2 | 7.7 | 6.4 |
| Glucose-lowering therapy use | Int vs. Conv | Insulin vs. Conv | Insulin vs. Conv | Insulin vs. insulin sensitivity | Insulin vs. Conv |
| Insulin (%) | 77 vs. 55 | 72 vs. 49d | 85 vs. 39f | 61 vs. 29i | 80 vs. 11g |
| Metformin (%) | 95 vs. 87 | NR | 6 vs. 22f | 75 vs. 11i | 47 vs. 60g |
| Sulfonylurea (%) | 78 vs. 68 | NR | 7 vs. 33f | 52 vs. 18i | 25 vs. 47g |
| TZD (%) | 92 vs. 58 | NR | — | 4 vs. 62i | NR |
| Results | |||||
| Duration of follow-up (years) | 3.5 | 1.0 | 2.1 | 5.3 | 6.2 |
| HbA1c achieved, Insulin (or Int) vs. Conv (%) | 6.4 vs. 7.5 | 7.3 vs. 7.6d | 6.8 vs. 6.8g | 7.5 vs. 7.0i | 6.2 vs. 6.5g |
| Change in weight, Insulin (or Int) vs. Conv (kg) | 3.5 vs. 0.4 | NR | 4.7 vs. 0.2 | 2.1 vs. 0.3 | 1.6 vs. −0.5 |
| Change in weight, Insulin (or Int) vs. Conv (kg/year of follow-up) | 1.1 vs. 0.1 | NR | 2.2 vs. 0.1 | 0.7 vs. 0.1 | 0.3 vs. −0.1 |
| Primary cardiovascular (HR or RR [95% CI]) | 0.90 [0.78–1.04] | NR | 1.22 [0.95–1.56] | 1.03 [0.98–1.08]j | 1.02 [0.94–1.11] |
| Myocardial infarct (nonfatal) (HR or RR [95% CI]) | 0.76 [0.62–0.92] | 1.30 [0.83–2.04]d | NR | NR | 1.02 [0.94–1.11] |
| Myocardial infarct (all) (HR or RR [95% CI]) | NR | 0.99 [0.70–1.39]d | 1.36 [0.91–2.03] | — | 1.02 [0.88–1.19] |
| Cardiovascular death (HR or RR [95% CI]) | 1.35 [1.04–1.76] | 0.72 [0.53–0.98]d | NR | NR | 1.00 [0.89–1.13] |
| All-cause mortality (HR or RR [95% CI]) | 1.22 [1.01–1.46] | 0.71 [0.49–0.96]d | 1.26 [0.92–1.72] | 1.00 [0.97–1.03] | 0.98 [0.90–1.08] |
| All-cause mortality (extended follow-up) (HR [95% CI]) | 0.72 [0.55–0.92]e | 1.17 [0.90–1.52]h | |||
| Comments | Obese group. Aggressive glucose lowering with high insulin and TZD use and high weight gain in Int group. | Less obese group. Less intensive glucose lowering in Ins group. Weight gain not recorded. | Less obese group. High weight gain in Ins group. | Obese group. Less intense glucose lowering in Ins group. | Obese group. Short duration of diabetes, lower HbA1c at baseline. Low weight gain in Ins group. |
ACCORD (42), DIGAMI 1 and 2 (55–57), BARI 2D (58), ORIGIN (60). Conv, conventional treatment; Ins, insulin treatment; Int, intensive treatment; RR, risk ratio of insulin (or intensive therapy) vs. comparator therapy; NR, not recorded. ACCORD and DIGAMI 2 studies had intensive glucose lowering involving intensive insulin use and were associated with weight gain greater than 1.0 kg/year. Bold typeface indicates significant changes.
Comparison of group 1 (acute insulin–glucose infusion for 24 h followed by insulin-based long-term glucose control) vs. group 3 (routine metabolic management).
Insulin provision vs. insulin sensitization groups.
Insulin glargine vs. standard care.
At d12-month and
3.4-year time points.
At initial discharge from hospital.
At study end.
DIGAMI 2 follow-up.
At 3 years of follow-up.
Primary cardiovascular in revascularization subgroup (HR 1.24, P = 0.059).
Table 3.
Other major cardiovascular outcome studies in T2D (ACCORD study also shown)
| ACCORD | Look AHEAD | EXAMINE | SAVOR-TIMI 53 | |
|---|---|---|---|---|
| Subjects (n) | 10,251 | 5,145 | 5,380 | 16,492 |
| Baseline characteristics | ||||
| Age (years) | 62 | 59 | 61 | 65 |
| BMI (kg/m2) | 32 | 36 | 29 | 31 |
| Diabetes duration (years) | 10 | 5 | 7 | 10 |
| Cardiovascular disease history (%) | 35 | 14 | 100 | 79 |
| HbA1c (%) | 8.1 | 7.2 | 8.0 | 8.0 |
| Glucose-lowering therapy use | Int vs. Conv | Int lifestyle vs. Conv | Alogliptin vs. placebo | Saxagliptin vs. placebo |
| Insulin (%) | 77 vs. 55 | 33 vs. 40b | 29 vs. 30e | 43 vs. 46g |
| Metformin (%) | 95 vs. 87 | 67 vs. 67b | 65 vs. 67e | 70 vs. 70g |
| Sulfonylurea (%) | 78 vs. 68 | NR | 47 vs. 46e | 39 vs. 40g |
| TZD (%) | 92 vs. 58 | NR | 2.4 vs. 2.5e | 4.7 vs. 4.7g |
| Results | ||||
| Duration of follow-up (years) | 3.5 | 9.6 | 1.5 | 2.1 |
| HbA1c achieved (%) | 6.4 vs. 7.5 | Diff −0.22 Int vs. Convc | 7.7 vs. 8.1f | 7.5 vs. 7.8g |
| Change in weight (kg) | 3.5 vs. 0.4 | Diff −4 Int vs. Convc | 1.1 vs. 1.0f | 0.6 vs. 1.0g |
| Change in weight, Int or DPP-4 inhibitor vs. Conv or placebo (kg/year of follow-up) | 1.1 vs. 0.1 | −0.7 vs. −0.4 | 0.7 vs. 0.7 | 0.3 vs. 0.5g |
| Primary cardiovascular (HR [95% CI]) | 0.90 [0.78–1.04] | 0.95 [0.83–1.09] | 0.96 [≤ 1.16] | 1.00 [0.89–1.12] |
| Myocardial infarct (nonfatal) (HR [95% CI]) | 0.76 [0.62–0.92] | 0.86 [0.69–1.06] | 1.08 [0.88–1.33] | NR |
| Myocardial infarct (fatal) (Event rate or HR [95% CI]) | 0.4% Int vs. 0.3% Conv | 0.44 [0.15–1.26] | NR | NR |
| Myocardial infarct (all) (Event rate or HR [95% CI]) | 4.0% Int vs. 4.7% Conv | 0.84 [0.68–1.04] | NR | 0.95 [0.8–1.12] |
| Heart failure (HR [95% CI]) | 1.18 [0.93–1.49]a | 0.80 [0.61–1.04]d | NR | 1.27 [1.07–1.51] |
| Cardiovascular death (HR [95% CI]) | 1.35 [1.04–1.76] | 0.88 [0.62–1.29] | 0.79 [0.60–1.04] | 1.03 [0.87–1.22] |
| All-cause mortality (HR [95% CI]) | 1.22 [1.01–1.46] | 0.85 [0.69–1.04] | 0.88 [0.71–1.09] | 1.11 [0.96–1.27] |
| Comments | Obese group. Aggressive glucose lowering with high insulin and TZD use and high weight gain in Int group. | Obese group. Weight loss greater with lifestyle intervention. Not a study of intensive glucose lowering. | High risk subjects. Not a study of intensive glucose lowering, but alogliptin group had lower HbA1c. | High-risk subjects. Not a study of intensive glucose lowering, but saxagliptin group had lower HbA1c. Moderate use of insulin. |
ACCORD (42), Look AHEAD (64), EXAMINE (73), SAVOR-TIMI 53 (72). Conv, conventional treatment; Int, intensive treatment (glucose lowering in ACCORD; lifestyle in Look AHEAD); NR, not recorded. Bold typeface indicates significant changes.
Fatal and nonfatal heart failure.
At end of study.
Over course of study.
Hospitalization for heart failure.
At baseline.
At end of study.
At 2 years.
Studies of Intensive Versus Standard Glycemic Control in T2D
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study (median duration of diabetes of 10 years, mean BMI of 32 kg/m2, and mean HbA1c of 8.3% at trial entry) unexpectedly showed that aggressive intensification of glycemic control (achieved HbA1c of 6.4%) compared with standard treatment (achieved HbA1c of 7.5%) in patients with T2D increased all-cause mortality (hazard ratio [HR] 1.22 [95% CI 1.01–1.46]), including death from cardiovascular causes (1.35 [1.04–1.76]) (Table 1) (42). The ACCORD patients had moderately high rates of preexisting cardiovascular disease events (35%) and congestive cardiac failure (5%) at baseline (42). A high percentage of the intensively treated patients were managed on insulin (77%) and/or a thiazolidinedione (TZD) (92%), with weight gain of >10 kg from baseline occurring in 27.8% of the intensive therapy group compared with 14.1% in the standard therapy group (Table 1) (42). Post hoc analyses of ACCORD did not support the hypothesis that increased hypoglycemia in the intensively treated subjects caused the excess deaths, but this could not be excluded (43). These analyses revealed, however, that a higher HbA1c at baseline and a failure to improve average HbA1c throughout the study despite intensive therapy were linked to increased mortality (44,45).
Intensive therapy in the UK Prospective Diabetes Study (UKPDS) (newly diagnosed diabetes, mean BMI of 28 kg/m2, and mean HbA1c of 7.1% at trial entry) was less aggressive (achieved HbA1c of 7.0% compared with 6.4% in ACCORD), had lower insulin use (38%) and less weight gain (0.6 kg/year compared with 1.1 kg/year in ACCORD), and was not associated with an increase in cardiovascular death (46). In the extended follow-up of the UKPDS subjects, the initial intensive therapy for 10 years had a legacy effect in maintaining lower rates of myocardial infarction and all-cause mortality (Table 1) (47). In a relatively small cohort of overweight patients in the UKPDS, intensive therapy with metformin compared with conventional therapy, in which change in weight was negligible in both groups, metformin was associated with a lower risk of all-cause mortality (Table 1) (48). In a secondary analysis of UKPDS overweight patients, intensive therapy with metformin (negligible weight gain) had better outcomes than intensive therapy with either sulfonylurea or insulin (modest weight gain) (48).
The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial (mean duration of diabetes of 8 years, mean BMI of 28 kg/m2, and mean HbA1c of 7.5% at trial entry) and the Veterans Affairs Diabetes Trial (VADT) (mean duration of diabetes of 11.5 years, mean BMI of 31 kg/m2, and mean HbA1c of 9.4% at trial entry) were, like ACCORD, more recent studies of intensive versus less intensive glycemic control in patients with T2D (Table 1) (42,49,50). The glucose-lowering approach for the intensive group in ADVANCE achieved the glycemic target more gradually than in ACCORD, with much less insulin (41%) and TZD (17%) use and without weight gain (Table 1) (42,50). Intensive therapy did not alter macrovascular events or all-cause mortality in ADVANCE (50). Intensive lowering of HbA1c in VADT was also less aggressive but, unlike ADVANCE, with high rates of insulin use in both the intensive (87%) and standard treatment groups (77%) and substantial weight gain (1.5 kg/year) in the intensive arm (49,50). Of note, a similar trend to ACCORD of enhanced cardiovascular death was found in the intensive arm of VADT (HR 1.32 [95% CI 0.81–2.14]) (Table 1) (49).
Steno-2 was a long-term multifactorial intervention study in 160 high-risk patients with T2D with microalbuminuria (median duration of diabetes of 6 years, mean BMI of 29–31 kg/m2, and mean HbA1c of 8.4–8.8% at trial entry) (51). For the 80 patients (63 male, 17 female) randomized to the intervention, more intensive treatment of blood glucose, lipids, and blood pressure for a median of 7.8 years with combination of drug therapy and behavior modification was highly successful in reducing cardiovascular disease events (HR 0.47 [95% CI 0.24–0.73]). A post-trial follow-up, at a median of 13.3 years from study initiation, demonstrated a reduction in all-cause mortality (0.54 [0.32–0.89]) (52). It is not possible to determine which components of the approach contributed to the improved outcome. Of relevance here, the glucose-lowering component in the intervention group was not that intensive, as the HbA1c at the end of the intervention period had only come down from 8.4 to 7.9% and weight gain was negligible in men (BMI increase of 0.7 kg/m2 in 7.8 years) and moderate in women (BMI increase of 2.3 kg/m2) (52).
A meta-analysis of UKPDS, ADVANCE, ACCORD, and VADT showed that intensive versus standard glycemic approaches were associated with a small reduction in major cardiovascular events, mainly due to a 15% reduction in myocardial infarction (46,53), whereas all-cause and cardiovascular-related mortalities were not affected (53). This meta-analysis highlights the seemingly paradoxical finding in ACCORD of greater cardiovascular mortality but a lower rate of nonfatal myocardial infarction (HR 0.78 [95% CI 0.62–0.92]) in the intensively treated group (42). The increased deaths could have been a consequence of worsening atherosclerosis in a subset of patients. For example, the paradox could be explained if intensively treated patients achieving better glycemic control have reduced myocardial infarcts and those patients with ongoing poor control have more fatal events. If not a consequence of worsening atherosclerosis, then alternative mechanisms need to be considered. One alternative mechanism, as discussed earlier in this Perspective, could be a detrimental effect of intensive glycemic treatment causing insulin-induced metabolic stress in the heart in some individuals (a metabolic cardiomyopathy) increasing the risk of cardiac failure and arrhythmias. Furthermore, if hyperinsulinemia in the face of continuing hyperglycemia impairs ischemic preconditioning, an effect supported by rodent experimental findings (33), then this could result in a greater ratio of fatal-to-nonfatal myocardial infarcts in the category of intensively treated patients with poorly controlled T2D.
Underpinning this discussion is the importance of heterogeneity of subjects recruited to major cardiovascular disease outcomes clinical trials. This was evident in a subset analysis of VADT (54). Intensive glucose lowering in VADT patients who had low coronary artery calcium (CAC) scores at baseline significantly reduced the rates of cardiovascular events compared with no effect in those with a high baseline CAC (54).
Randomized Controlled Trials of Insulin Therapy in T2D
There are few randomized controlled trials of exogenous insulin use in T2D. The Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction 1 (DIGAMI 1) study randomized patients with T2D (mean duration of diabetes of 10 years, BMI of 27 kg/m2, and HbA1c of 8.1% at trial entry) admitted with an acute myocardial infarction to an insulin glucose infusion, followed by insulin therapy for >3 months compared with standard treatment (55,56). Insulin therapy reduced rates of cardiovascular mortality and all-cause mortality at 1 and 3.4 years (Table 2) (55,56). The DIGAMI 2 study (mean duration of diabetes of 8.3 years, BMI of 28 kg/m2, and HbA1c of 7.2% at trial entry) did not confirm the findings of DIGAMI 1, rather showing the reverse trend in all-cause mortality with an insulin compared with standard therapy protocol (HR 1.26 [95% CI 0.92–1.72]) (Table 2) (57). Of note, the HbA1c achieved in DIGAMI 2 with insulin was lower than in DIGAMI 1 (6.8% compared with 7.3%), suggesting a more aggressive approach to glucose lowering, and the insulin-treated group had substantial weight gain (2.2 kg/year) (not reported in DIGAMI 1) (55–57). The Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D) study demonstrated that in patients with T2D with coronary artery disease (mean duration of diabetes of 10.4 years, BMI of 32 kg/m2, and HbA1c of 7.7% at trial entry), enhanced insulin provision with insulin and/or sulfonylurea over insulin sensitization with metformin and/or TZDs was associated with worse glycemic control (achieved HbA1c of 7.5% compared with 7.0%) (i.e., not intensive like ACCORD), more severe hypoglycemic events, more weight gain (0.7 compared with 0.1 kg/year) (less than ACCORD), and lower HDL cholesterol levels but not increased cardiovascular events or death (58). However at 5 years of follow-up, there was a nonsignificant trend for major cardiovascular events to be greater in revascularized subjects in the insulin provision compared with the insulin sensitization arm (P = 0.059) (59). The Outcome Reduction With Initial Glargine Intervention (ORIGIN) trial investigated early use of the long-acting insulin analog glargine in subjects with increased cardiovascular risk factors and impaired glucose tolerance or early T2D (mean BMI of 30 kg/m2 and median HbA1c of 6.4% at trial entry) (60). After an average 6.2 years of follow-up, the rates of cardiovascular outcomes did not differ between the insulin glargine and standard care groups. Insulin therapy did increase the risk of serious hypoglycemia and was associated with some weight gain (0.3 kg/year), albeit much less than in ACCORD (60). The important difference between ORIGIN and ACCORD, however, was that patients with T2D with difficult-to-control glycemia, the ones that did poorly in ACCORD, were not recruited in the ORIGIN trial (42,60). The baseline median HbA1c in ORIGIN was very low (6.4%) compared with a mean of 8.3% in ACCORD (Table 2) (42,60).
Evident in the two series of studies (glycemic control and insulin therapy) is marked heterogeneity that makes direct comparison difficult (Tables 1 and 2). However, whenever intensive use of insulin was associated with weight gain of greater than 1.0 kg/year (ACCORD, VADT, DIGAMI 2), cardiovascular and all-cause mortality increased similarly, only reaching statistical significance in ACCORD given the greater sample size (Tables 1 and 2).
Observational Studies of Insulin Compared With Other Glucose-Lowering Therapies
Population-based studies in patients with T2D reported recently from Canada, the U.K., and Sweden have shown, after correction for multiple factors, increased risk of mortality in patients with T2D treated with insulin (61–63). A follow-up analysis of DIGAMI 2, using the total cohort as an epidemiological database, also showed that insulin therapy from the time of hospital discharge was associated with a significant increased risk of the composite of death, reinfarction, or stroke (HR 1.78 [95% CI 1.14–2.40]) (57). These studies, while observational needing cautious interpretation, do raise concerns that should be addressed.
Intensive Lifestyle Intervention and Cardiovascular Outcomes
An alternative approach to overriding IR in the management of T2D is to reduce it by an intervention of intensive lifestyle aimed at weight loss. If our premise is correct, then reduction in nutrient load should reduce metabolic stress on tissues and improve cardiovascular outcomes. The Look AHEAD (Action for Health in Diabetes) study was a trial of a lifestyle intervention aimed at promoting weight loss through decreased calorie intake and increased physical activity compared with standard therapy in patients with T2D (median duration of diabetes of 5 years, mean BMI of 36 kg/m2, and HbA1c of 7.2% at trial entry) (Table 3) (64). The intervention, which had a median follow-up period of 9.6 years, successfully reduced weight by an average of 4 kg more than occurred in the standard care group, and although it was not a trial of intensive glucose lowering, it lowered the average HbA1c by 0.22% compared with the comparator group (64). The lifestyle intervention failed to reduce the composite primary outcome of death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for angina (HR 0.95 [95% CI 0.83–1.09]) (Table 3) (64). However, death from any cause, which was the outcome that resulted in early termination of the glycemic control trial of ACCORD, was reduced by a nonsignificant 15% by the lifestyle intervention compared with control treatment (0.85 [0.69–1.04]) (64). Furthermore, intensive lifestyle intervention showed trends of a 16% reduction in myocardial infarction (P = 0.11), 56% reduction in fatal myocardial infarction (P = 0.13), and 20% reduction in heart failure (P = 0.10). There were no trends for change in rates of hospitalization for angina (P = 0.79) or stroke (P = 0.78) (Table 3) (64).
Also of note in the Look AHEAD study was the trend for patients with a history of cardiovascular disease at baseline who received the lifestyle intervention to do worse in terms of the composite primary outcome (64). This observation is similar to that in the subset of VADT patients who had CAC measurements in whom the intervention did not have a beneficial effect when there were large amounts of CAC while reducing cardiovascular outcomes when there was little to no CAC present (54). Both these sets of data suggest that any intensive method of glycemia or weight management in T2D is likely not to benefit those patients with preexisting cardiovascular disease. What is not discernible from either of these reports is the actual therapies these higher-risk patients received and how many in VADT had difficulty in achieving glycemic control and how many in the Look AHEAD study failed to lose weight (54,64).
Overall, we believe the Look AHEAD results are more in support of than against our premise. Clearly, the lifestyle intervention did not have a detrimental effect on all-cause mortality as occurred with the intensive glucose-lowering approach used in ACCORD. It is also important to point out that the intensive lifestyle intervention in the Look AHEAD study was associated with an array of noncardiovascular event health benefits, including improved glucose control, blood pressure, and overall lipid control, all with the use of fewer medications. Further, participants in the intensive lifestyle arm reported improved quality of life, mobility, and sleep as examples of additional benefits.
Clinical Implications of the Concepts of IR as an Adaptive Defense and Insulin-Induced Metabolic Stress
The implications of these series of studies and our premise relating to insulin-induced metabolic stress apply primarily to patients with T2D with the most difficult-to-control glycemia as a consequence of their inability to control an excess energy balance. These patients are nearly all overweight or obese and either medically unable or not motivated to change their lifestyle (Fig. 3). This subset of patients, for many of whom glycemic management targets have recently been relaxed (1,2), may actually have the most to gain from “appropriate” aggressive glucose lowering, as uncontrolled excess nutrient supply to particularly vulnerable cardiovascular tissues is likely to be harmful. The particular approach to improving the metabolic control, however, is likely to be pivotal to whether the outcomes are of benefit or harm. Glycemia lowering that works by overcoming IR and forcing even more nutrients into already overloaded tissues may paradoxically step up the metabolic injury of critical tissues via insulin-induced metabolic stress. Of note, moderately intensive insulin therapy in overweight or obese patients with poorly controlled T2D led to a dramatic increase in myocardial triglyceride content in two of eight subjects (65). If our premise is substantiated, then alternative approaches to glucose lowering that involve nutrient off-loading to the myocardium and endothelium should hold the most promise to improve cardiovascular outcomes (Fig. 3).
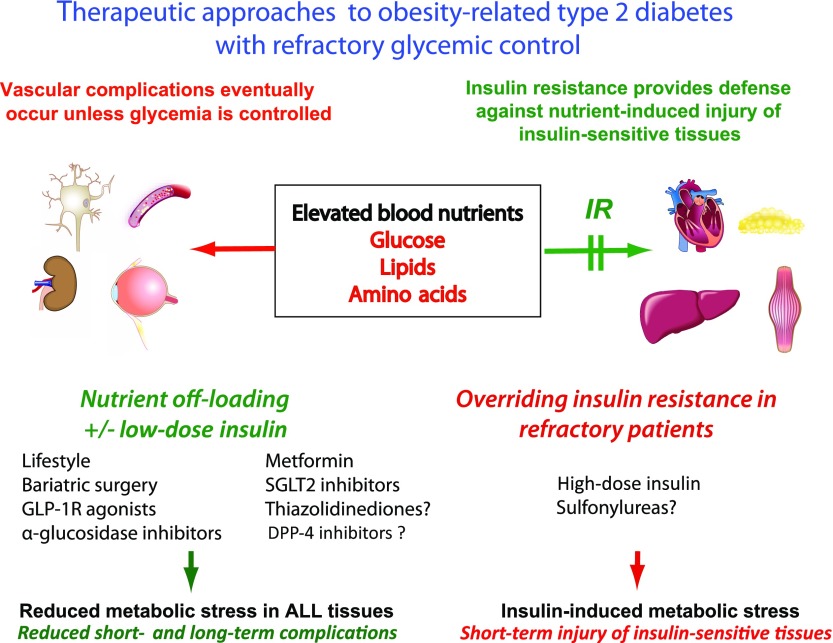
Figure 3.
The potential benefit of nutrient off-loading approaches compared with the overriding of IR to lower blood glucose in subjects with poorly controlled T2D. Insulin-responsive tissues, such as the heart, skeletal muscle, adipose tissue, and the liver, are able to protect themselves from nutrient-induced damage by developing IR. Other tissues, such as nerves, eye, kidney, and the vasculature, are less protected by IR. The clinician has the choice to 1) treat the hyperglycemia with enough insulin to override IR and reduce nutrient toxicity in tissues at longer-term risk of microvascular complications but with the risk of increasing insulin-induced metabolic stress in the insulin-responsive tissues or to 2) use alternative nutrient off-loading approaches to glucose lowering to benefit all tissues. Insulin-induced metabolic stress is more likely to occur with high-dose insulin therapy in patients who are refractory to improved glycemic regulation. Sulfonylureas could potentially have similar effects to high-dose insulin as they increase insulin levels without effect to nutrient off-load. Bariatric surgery, GLP-1 receptor (GLP-1R) agonists, α-glucosidase inhibitors, and SGLT2 inhibitors have well-known mechanisms by which they nutrient off-load. Metformin likely detoxifies nutrients because it is a mild inhibitor of mitochondrial function that is thought to activate AMPK in liver and other tissues. TZDs, via promoting nutrient partitioning to adipose tissue, may nutrient off-load other more critical tissues. DPP-4 inhibitors do not appear to have nutrient off-loading effects.
Key here is that “overriding IR” is not the same as “reducing or improving IR by nutrient off-loading.” Essentially, IR protects against metabolic stress caused by exposure to an excessive nutrient load. If therapies reduce the nutrient load (i.e., off-load nutrients), then there will be reduced requirement for the protection of IR (Fig. 3). On the other hand, overriding IR without reducing nutrient load will overcome the protection and aggravate metabolic stress (Fig. 3).
IR and the Safety of Insulin Therapy
There will remain many patients with T2D who require insulin replacement therapy and others in which it could be harmful (Fig. 4). For patients late in the natural history of the disease, with severe deficiency in endogenous insulin secretion, insulin replacement becomes necessary as the only effective approach for lowering blood glucose. Also for patients who are of normal weight, including many patients with latent autoimmune diabetes of adults (3), and are able to achieve healthy energy balance, insulin therapy is likely to be a safe option. A short course of insulin may also be of use to achieve rapid glycemic control and improve β-cell function in patients with new-onset T2D (66). However, we propose that caution needs to be exercised in using very high-dose insulin regimens in overweight or obese individuals if they are unable to achieve improvements in lifestyle to reverse positive energy balance (Figs. 3 and 4). If used in such patients, insulin should not be used with very aggressive glycemic targets and/or it should be used in combination with other agents that have nutrient off-loading mechanisms of action.
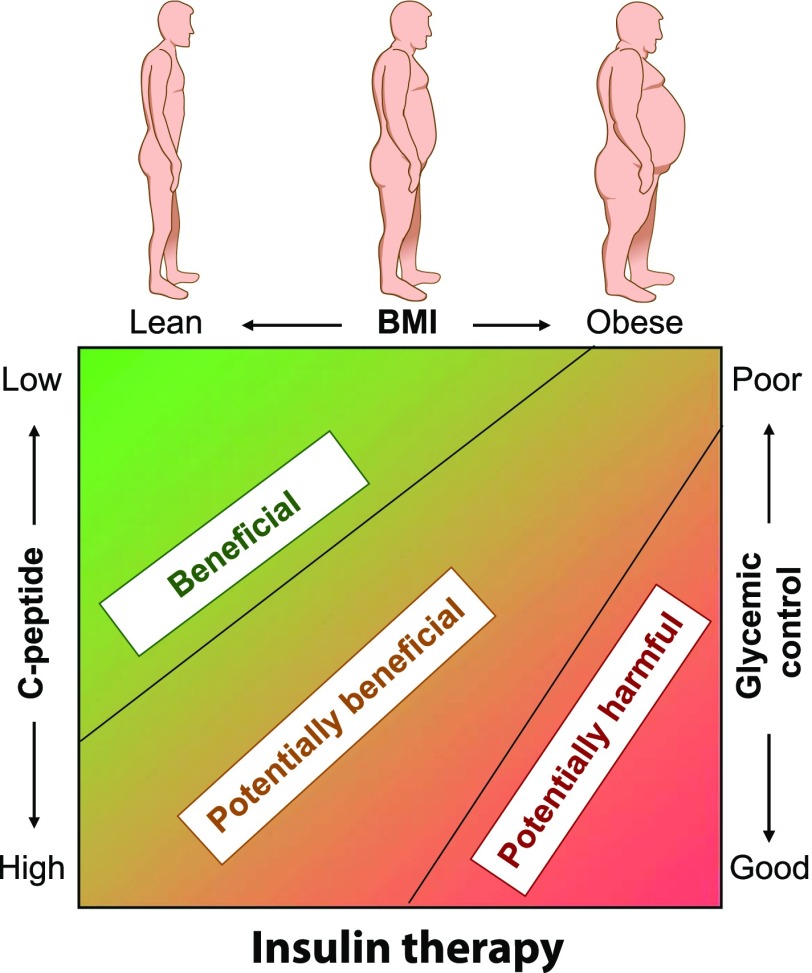
Figure 4.
The appropriateness of insulin therapy in patients with T2D. The need for insulin therapy depends on whether it is being used as replacement in insulin-deficient patients (less likely to cause harm) or to override IR (more likely to cause harm). Insulin-deficient patients are more likely to be lean and in neutral or negative energy balance, with low C-peptide levels and poor glycemic control. Insulin-induced harm is more likely to occur in overweight and obese subjects with IR and high C-peptide levels and an inability to achieve negative energy balance through lifestyle change. In these patients, lifestyle and pharmacological therapies aimed at reversing the excess nutrient imbalance are more advisable than insulin therapy.
Other Glucose-Lowering Therapies in Difficult-to-Control Obesity-Related T2D
Excess nutrient off-loading to critical tissues should be beneficial. Lifestyle measures of increased physical activity and reduced calorie intake should always be advised, but these measures usually have failed in overweight or obese patients with T2D with refractory poor glycemic control. Bariatric surgery is currently the most effective means to achieve negative energy balance and is typically associated with dramatic improvements in glycemic control. Its role in these most difficult patients needs further assessment. Use of newer glucose-lowering agents that favor a negative energy balance, such as the GLP-1 receptor agonists that reduce food intake and the sodium-glucose cotransporter 2 (SGLT2) inhibitors that promote glycosuria, is expected to result in reduction of metabolic stress, as should the more established α-glucosidase inhibitors that slow the rate of carbohydrate absorption (Fig. 3) (67). We await clinical trials assessing the longer-term safety and effects on cardiovascular disease outcomes of such glucose-lowering agents.
The safety of insulin sensitizers in these high-risk subjects with T2D needs careful consideration, as their effects may be beneficial or harmful depending on their mechanism of action, patient factors such as metabolic control, and interactions with other agents such as insulin. TZD drugs are the most effective insulin sensitizers, and from what we know of their mechanism of action, they should protect tissues such as the heart and skeletal muscle from nutrient-induced toxicity. They increase partitioning of excess fuel to “inert” triglyceride stores in adipose tissue and should also promote intracellular nutrient detoxification by activating AMP kinase (AMPK) and consequently fuel oxidation (65,68,69). If successful in controlling glycemia, these effects should reduce metabolic injury to cardiovascular tissues (70). However, the majority of subjects in ACCORD were on TZDs and those that had poor outcomes had poorly controlled glycemia and were also highly likely to be on insulin (45). It might be that the TZDs in poorly controlled patients can synergize with insulin to increase glucose entry into cardiomyocytes to accentuate insulin-induced metabolic stress in the heart (metabolic cardiomyopathy). Clearly, the combination of TZDs and insulin needs to be used with caution in patients with a positive energy balance. Metformin, although often referred to as being an insulin sensitizer, has its main glucose-lowering effect via reducing hepatic glucose production (1). It should thus help to reduce the glycemic nutrient load on peripheral tissues and be beneficial. Unclear, however, is the significance of the unexpected increase in all-cause mortality in UKPDS when metformin was added to sulfonylurea in subjects poorly controlled on sulfonylurea therapy alone (48). The development of new insulin sensitizer medications that do not have a mechanism of action that involves nutrient off-loading may not be advisable. Furthermore, tissue selectivity of agents that improve insulin action via promoting nutrient clearance via their oxidative metabolism may be important for safety. For example, an agent that specifically “browns the fat,” increasing its oxidative capacity (71), is likely to be safer than an agent that works in critical tissues, such as the heart and skeletal muscle, to dispose of excess energy (Fig. 3).
The first two cardiovascular outcome trials of the incretin-enhancing dipeptidyl peptidase-4 (DPP-4) inhibitors, alogliptin in the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndrome (EXAMINE) and saxagliptin in Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53), were recently reported (Table 3) (72,73). Neither showed improvement or worsening in the primary cardiovascular disease outcomes. However, concern was raised by the unexpected outcome of an increase of hospitalization for heart failure with the use of saxagliptin in SAVOR-TIMI 53 (3.5% vs. 2.8%; HR 1.27 [95% CI 1.07–1.51]) (72). Of note, the use of insulin therapy was relatively high in the participants in this study (43.8% and 46.4% in saxagliptin-treated and placebo-treated subjects, respectively), while TZD use was minimal (Table 3) (72). The reason for the increased heart failure with saxagliptin is unknown; however, the possibility that the DPP-4 inhibitors promote a greater degree of hyperinsulinemia favoring cardiac glucolipotoxicity, particularly in subjects also receiving exogenous insulin, should be given consideration.
Perspective and Future Directions
The concept of insulin-induced metabolic stress needs to be further explored through studies of insulin therapy in obese T2D patients with varying levels of metabolic control. Of particular interest will be the effects of insulin on nutrient uptake and storage, as well as tissue function, in the myocardium, skeletal muscle, endothelium, adipose tissue, and liver of the more difficult-to-control patients. Attention needs also be focused on how insulin interacts with other glucose-lowering agents as well as how insulin-induced metabolic stress in skeletal muscle might induce metabolic myopathy and influence a patient’s ability to exercise. An improved understanding of this pathophysiological process may also be valuable to clinicians for counseling and motivating patients with T2D on the critical importance of a healthy lifestyle. Finally, carefully designed randomized clinical trials of pathophysiologically well-defined subgroups of patients with T2D with comparable frequencies of cardiovascular complications at study entry aiming for long-term outcome data are of utmost importance for assessing the safety of intensive insulin therapy, including how best to use it, in the highly heterogeneous disorders of T2D.
Article Information
Acknowledgments. The authors thank many of our colleagues for their time and interest in discussing the concepts written into the manuscript and their provision of constructive feedback.
Funding. Work was supported in part by grants from the National Institutes of Health (R01-DK019514, [N.B.R. and M.P.], P01-HL068758 [N.B.R.], and P30-DK017047 [S.E.K.]), the Canadian Institutes of Health Research (M.P.), the National Health and Medical Research Council (project grant 1028108 [C.J.N.]), and the Department of Veterans Affairs (S.E.K.).
Duality of Interest. C.J.N. has received speaking fees from AstraZeneca, Novartis, and Takeda and has been a member of an advisory board for Sanofi Aventis. S.E.K. has been a member of advisory boards for Boehringer Ingelheim, Elcelyx, Genentech, GlaxoSmithKline, Intarcia, Janssen, Merck, Novo Nordisk, Receptos, and Takeda. O.P. holds shares in Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.J.N. and M.P. wrote the manuscript. N.B.R., S.E.K., and O.P. contributed to the discussion and reviewed and edited the manuscript. C.J.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al.; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomi T, Santoro N, Caprio S, Cai M, Weng J, Groop L. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014;383:1084–1094 [DOI] [PubMed] [Google Scholar]
- 4.Gale EA. Is type 2 diabetes a category error? Lancet 2013;381:1956–1957 [DOI] [PubMed] [Google Scholar]
- 5.Ruderman NB, Shulman GI. The Metabolic Syndrome. In Endocrinology, 6th ed. De Groot LJ, Jamieson JL, Eds. Philadelphia, Elsevier, Saunders, 2010, p. 828–839 [Google Scholar]
- 6.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraegen EW, Saha AK, Preston E, et al. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab 2006;290:E471–E479 [DOI] [PubMed] [Google Scholar]
- 8.Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab 2003;14:398–403 [DOI] [PubMed] [Google Scholar]
- 9.Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 2009;106:17787–17792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolan CJ, Prentki M. The islet beta-cell: fuel responsive and vulnerable. Trends Endocrinol Metab 2008;19:285–291 [DOI] [PubMed] [Google Scholar]
- 11.Nolan CJ, Ruderman NB, Prentki M. Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol 2013;1:9–10 [DOI] [PubMed] [Google Scholar]
- 12.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 13.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 2005;85:1093–1129 [DOI] [PubMed] [Google Scholar]
- 14.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res 2008;79:269–278 [DOI] [PubMed] [Google Scholar]
- 15.Labbé SM, Grenier-Larouche T, Noll C, et al. Increased myocardial uptake of dietary fatty acids linked to cardiac dysfunction in glucose-intolerant humans. Diabetes 2012;61:2701–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes 1996;45:273–283 [DOI] [PubMed] [Google Scholar]
- 17.Wright JJ, Kim J, Buchanan J, et al. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 2009;82:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jankovic D, Winhofer Y, Promintzer-Schifferl M, et al. Effects of insulin therapy on myocardial lipid content and cardiac geometry in patients with type-2 diabetes mellitus. PLoS One 2012;7:e50077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winhofer Y, Krssák M, Jankovic D, et al. Short-term hyperinsulinemia and hyperglycemia increase myocardial lipid content in normal subjects. Diabetes 2012;61:1210–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 2012;15:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 2004;3:340–351 [DOI] [PubMed] [Google Scholar]
- 22.El-Assaad W, Joly E, Barbeau A, et al. Glucolipotoxicity alters lipid partitioning and causes mitochondrial dysfunction, cholesterol, and ceramide deposition and reactive oxygen species production in INS832/13 ss-cells. Endocrinology 2010;151:3061–3073 [DOI] [PubMed] [Google Scholar]
- 23.Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia 2009;52:2369–2373 [DOI] [PubMed] [Google Scholar]
- 24.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 26.Singh LP. The NLRP3 inflammasome and diabetic cardiomyopathy. Cardiovasc Drugs Ther 2014;28:5–6 [DOI] [PubMed] [Google Scholar]
- 27.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab 2012;15:10–18 [DOI] [PubMed] [Google Scholar]
- 28.Shimizu I, Minamino T, Toko H, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest 2010;120:1506–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu Q, Xu B, Liu Y, et al. Insulin inhibits cardiac contractility by inducing a Gi-biased β2-adrenergic signaling in hearts. Diabetes 2014;63:2676–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jagasia D, Whiting JM, Concato J, Pfau S, McNulty PH. Effect of non-insulin-dependent diabetes mellitus on myocardial insulin responsiveness in patients with ischemic heart disease. Circulation 2001;103:1734–1739 [DOI] [PubMed] [Google Scholar]
- 31.Utriainen T, Takala T, Luotolahti M, et al. Insulin resistance characterizes glucose uptake in skeletal muscle but not in the heart in NIDDM. Diabetologia 1998;41:555–559 [DOI] [PubMed] [Google Scholar]
- 32.Cook SA, Varela-Carver A, Mongillo M, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J 2010;31:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fullmer TM, Pei S, Zhu Y, et al. Insulin suppresses ischemic preconditioning-mediated cardioprotection through Akt-dependent mechanisms. J Mol Cell Cardiol 2013;64:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groop PH, Forsblom C, Thomas MC. Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 2005;1:100–110 [DOI] [PubMed] [Google Scholar]
- 35.Cao W, Ning J, Yang X, Liu Z. Excess exposure to insulin is the primary cause of insulin resistance and its associated atherosclerosis. Curr Mol Pharmacol 2011;4:154–166 [DOI] [PubMed] [Google Scholar]
- 36.Vicent D, Ilany J, Kondo T, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest 2003;111:1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potenza MA, Addabbo F, Montagnani M. Vascular actions of insulin with implications for endothelial dysfunction. Am J Physiol Endocrinol Metab 2009;297:E568–E577 [DOI] [PubMed] [Google Scholar]
- 38.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes 2002;51:159–167 [DOI] [PubMed] [Google Scholar]
- 39.Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic Fatty liver disease: a pathogenic duo. Endocr Rev 2013;34:84–129 [DOI] [PubMed] [Google Scholar]
- 40.Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring) 2010;18:884–889 [DOI] [PubMed] [Google Scholar]
- 41.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 42.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calles-Escandón J, Lovato LC, Simons-Morton DG, et al. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riddle MC, Ambrosius WT, Brillon DJ, et al.; Action to Control Cardiovascular Risk in Diabetes Investigators . Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 47.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 48.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 49.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 50.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 51.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393 [DOI] [PubMed] [Google Scholar]
- 52.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–591 [DOI] [PubMed] [Google Scholar]
- 53.Turnbull FM, Abraira C, Anderson RJ, et al.; Control Group . Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 54.Reaven PD, Moritz TE, Schwenke DC, et al.; Veterans Affairs Diabetes Trial . Intensive glucose-lowering therapy reduces cardiovascular disease events in Veterans Affairs Diabetes Trial participants with lower calcified coronary atherosclerosis. Diabetes 2009;58:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation 1999;99:2626–2632 [DOI] [PubMed] [Google Scholar]
- 56.Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol 1995;26:57–65 [DOI] [PubMed] [Google Scholar]
- 57.Mellbin LG, Malmberg K, Norhammar A, Wedel H, Rydén L; DIGAMI 2 Investigators . Prognostic implications of glucose-lowering treatment in patients with acute myocardial infarction and diabetes: experiences from an extended follow-up of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2 Study. Diabetologia 2011;54:1308–1317 [DOI] [PubMed] [Google Scholar]
- 58.Malmberg K, Rydén L, Wedel H, et al.; DIGAMI 2 Investigators . Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 2005;26:650–661 [DOI] [PubMed] [Google Scholar]
- 59.Frye RL, August P, Brooks MM, et al.; BARI 2D Study Group . A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerstein HC, Bosch J, Dagenais GR, et al.; ORIGIN Trial Investigators . Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–328 [DOI] [PubMed] [Google Scholar]
- 61.Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab 2013;98:668–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gamble JM, Simpson SH, Eurich DT, Majumdar SR, Johnson JA. Insulin use and increased risk of mortality in type 2 diabetes: a cohort study. Diabetes Obes Metab 2010;12:47–53 [DOI] [PubMed] [Google Scholar]
- 63.Saleh N, Petursson P, Lagerqvist B, et al. Long-term mortality in patients with type 2 diabetes undergoing coronary angiography: the impact of glucose-lowering treatment. Diabetologia 2012;55:2109–2117 [DOI] [PubMed] [Google Scholar]
- 64.Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zib I, Jacob AN, Lingvay I, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med 2007;55:230–236 [DOI] [PubMed] [Google Scholar]
- 66.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 67.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.LeBrasseur NK, Kelly M, Tsao TS, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab 2006;291:E175–E181 [DOI] [PubMed] [Google Scholar]
- 69.Ye JM, Dzamko N, Cleasby ME, et al. Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia 2004;47:1306–1313 [DOI] [PubMed] [Google Scholar]
- 70.van der Meer RW, Rijzewijk LJ, de Jong HW, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 2009;119:2069–2077 [DOI] [PubMed] [Google Scholar]
- 71.Carobbio S, Rosen B, Vidal-Puig A. Adipogenesis: new insights into brown adipose tissue differentiation. J Mol Endocrinol 2013;51:T75–T85 [DOI] [PubMed] [Google Scholar]
- 72.Scirica BM, Bhatt DL, Braunwald E, et al.; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 73.White WB, Cannon CP, Heller SR, et al.; EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]