Abstract
All forms of diabetes are characterized by a loss of functional β-cell mass, and strategies for expanding β-cell mass could have significant therapeutic benefit. We have recently identified the transcription factor Nkx6.1 as an essential maintenance factor of the functional β-cell state. In addition, Nkx6.1 has been proposed to control β-cell proliferation, but a role for Nkx6.1 in regulating β-cell mass has not been demonstrated. Here, we show that Nkx6.1 is required for postnatal β-cell mass expansion. Genetic inactivation of Nkx6.1 in newly formed β-cells caused a drastic decrease in early postnatal β-cell proliferation, leading to reduced β-cell mass and glucose intolerance. Interestingly, Nkx6.1 was dispensable for prenatal β-cell proliferation. We found that Nkx6.1 regulates the expression of several β-cell maturation markers as well as expression of the nutrient sensors Glut2 and Glp1r. Manifestation of the β-cell mass defect at the transition to postnatal feeding suggests that Nkx6.1 could regulate β-cell growth by enabling β-cells to respond to nutrient-dependent proliferation signals, such as glucose and Glp1. Identification of β-cell-intrinsic regulators that connect nutrient-sensing and proliferation suggests new therapeutic targets for expanding functional β-cell mass.
Introduction
The establishment of sufficient β-cell mass depends on the rapid expansion of β-cell numbers during early postnatal life (1–5). The extent of this early postnatal β-cell growth is postulated to influence later susceptibility to type 2 diabetes (6). Postnatal β-cell mass expansion is driven by β-cell proliferation (7), which is controlled by the cell cycle regulators Cyclin D1 or Cyclin D2 (encoded by Ccnd1 and Ccnd2, respectively) (2,3). It has been shown that β-cells are highly proliferative in the perinatal period and that this early proliferation is necessary to establish sufficient β-cell mass for maintaining glucose homeostasis (1–5). However, the cell extrinsic and intrinsic factors that drive β-cell proliferation and mass expansion during the perinatal period are still poorly defined.
Glucose has been identified as a systemic factor that stimulates β-cell proliferation (8,9), and recent studies suggest that glucose is a significant driver of early postnatal β-cell proliferation (10). Furthermore, it has been shown that glucose metabolism in β-cells produces signals that increase Cyclin D2 expression and β-cell proliferation (11–13). Independent of glucose, β-cell proliferation is also stimulated by gut-derived hormone GLP-1 (Glp1), which is secreted by intestinal enteroendocrine cells in response to food intake (14,15). Thus there is an established link between feeding, increases in blood glucose levels, and β-cell proliferation. However, β-cells also exhibit significant proliferation during fetal life, when blood glucose concentrations are low and glucose has little effect on β-cell proliferation (16). The distinct mechanisms used in prenatal and postnatal β-cells to regulate proliferation remain unclear.
The β-cell-restricted transcription factor Nkx6.1 is essential for maintaining the functional state of β-cells during adulthood (17). Both in vitro and in vivo experiments have suggested a role for Nkx6.1 in β-cell proliferation (17–19), but whether it is required for β-cell growth in vivo is unknown. To reveal a possible role for Nkx6.1 in β-cell mass expansion, we inactivated Nkx6.1 in newly formed β-cells of the embryo and examined the effects on β-cell proliferation and mass during the prenatal and postnatal period.
Research Design and Methods
RIP-Cre (20), Nkx6.1flox (21), Nkx6.1 null (22), and R26-YFP mice (23) have been described. RIP-Cre;Nkx6.1flox/+;R26-YFP mice served as control mice in all experiments. All experiments were approved by the Institutional Animal Care and Use Committee of the University of California.
Methods for tissue preparation, immunofluorescence staining, and terminal deoxynucleotidyl transferase dUTP nicked end labeling (TUNEL) have been previously described (21). The following primary antibodies were used: guinea pig anti-insulin (Dako), 1:2,000; mouse anti-Nkx6.1 (BCBC #2023), 1:500; rabbit anti-Glut2 (Millipore), 1:1,000; rabbit anti-Glp1r (S. Heller, Novo Nordisk), 1:2,000; rat anti-GFP (C. Kioussi, Oregon State University), 1:1,000; rabbit anti-Ki67 (Laboratory Vision), 1:500; rabbit anti-Ucn3 (M. Huising, University of California, Davis), 1:500; rabbit anti-MafA (Bethyl), 1:200; and rabbit anti-Pdx1 (Abcam), 1:500. Staining with antibodies raised in mice was performed using the M.O.M. Kit (Vector Laboratories). When necessary, nuclei were counterstained with DAPI (Sigma) at 0.1 μg/mL. Primary antibodies were detected with donkey-raised secondary antibodies conjugated to Cy3, Cy5, or Alexa 488 (Jackson ImmunoResearch). β-Cell mass and marker+ area were determined as described (21). Images were captured on a Zeiss Axio Observer Z1 microscope with an ApoTome module and processed with Zeiss AxioVision 4.8 software. All images were processed in accordance with Diabetes journal guidelines.
The quantitative RT-PCR (qRT-PCR) analysis was performed as previously described (17) on total RNA isolated from postnatal day 2 pancreata from individual mice. Primers used are as follows: Nkx6.1 (f-CTTCTGGCCCGGAGTGATG; r-GGGTCTGGTGTGTTTTCTCTTC), Ucn3 (f-GCTGTGCCCCTCGACCT; r-TGGGCATCAGCATCGCT), Adh1 (f- GCAAAGCTGCGGTGCTATG; r-TCACACAAGTCACCCCTTCTC), Angptl7 (f- TGACTGTTCTTCCCTGTACCA; r-CAAGGCCACTCTTACGTCTCT), Dlk1 (f-CCCAGGTGAGCTTCGAGT; r-GGAGAGGGGTACTCTTGTTGAG), Gstm2 (f-ACACCCGCATACAGTTGGC; r-TGCTTGCCCAGAAACTCAGAG), Zyx (f-TCCCACCGCAGGTATCATC; r-GGAGCTAGAAGGGGTCTTCCA), and Gapdh (f-CATGTTCCAGTATGACTCCACTC; r-GGCCTCACCCCATTTGATGT).
Glucose tolerance tests and blood glucose measurements were performed as described (17). For glucose tolerance tests, a 1.5 g/kg body weight intraperitoneal injection of glucose was administered after overnight fasting.
All values are shown as mean ± SEM; P values were calculated using a two-tailed Student t test in Microsoft Excel. P < 0.05 was considered significant.
Results
Nkx6.1 Inactivation in Embryonic β-Cells Causes Hyperglycemia and Reduced β-Cell Mass
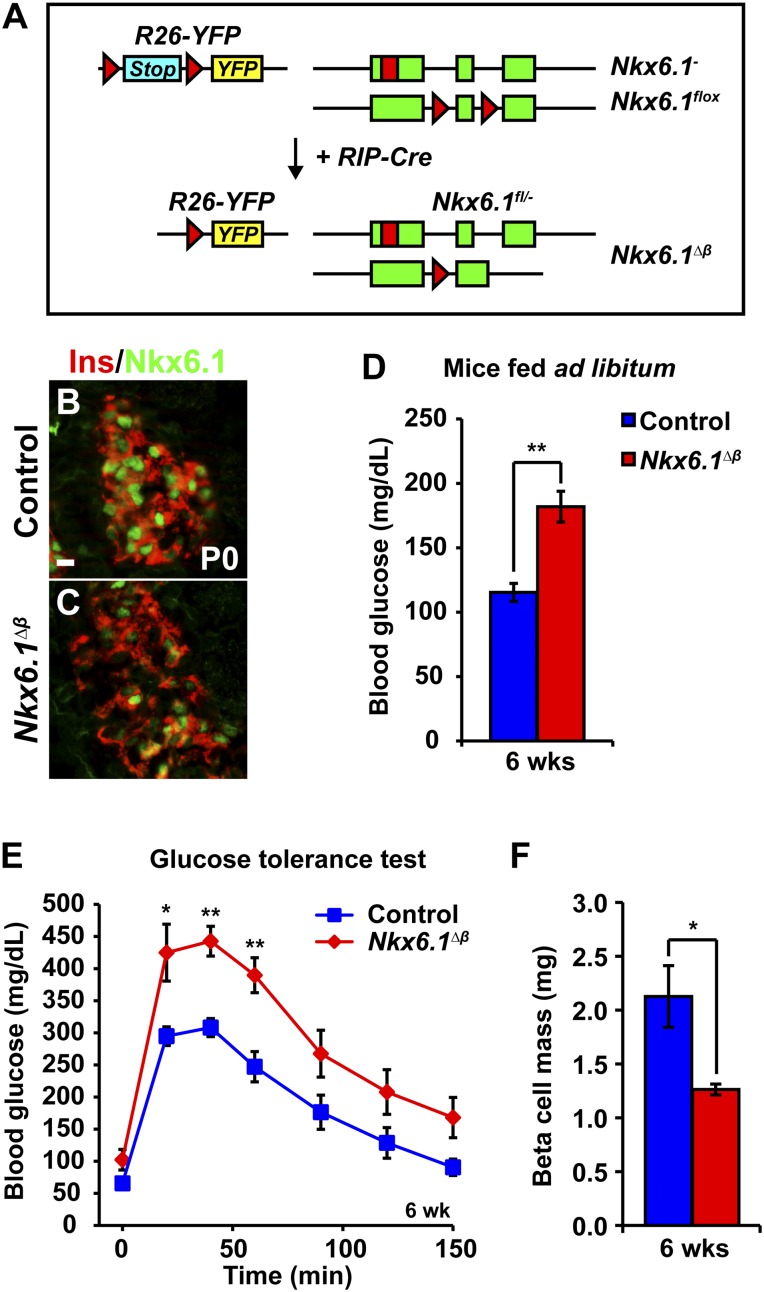
To investigate the role of Nkx6.1 in perinatal β-cell development, we intercrossed mice to generate progeny carrying a Nkx6.1 null allele (Nkx6.1−), a Nkx6.1 conditional loss of function allele (Nkx6.1flox), and the rat insulin2-Cre transgene (RIP-Cre). Additionally, the mice carried a conditional YFP reporter gene targeted to the Rosa-26 locus (R26-YFP), resulting in heritable YFP expression upon RIP-Cre-mediated recombination of a translational stop signal. Thus, in RIP-Cre;Nkx6.1flox/-;R26-YFP (hereafter referred to as Nkx6.1∆β) mice, YFP labels all cells in which Nkx6.1 has been inactivated (Fig. 1A).
Figure 1.
Nkx6.1 deletion in newly formed β-cells leads to glucose intolerance and reduced β-cell mass. A: Schematic of alleles and transgenes used to inactivate Nkx6.1 in fetal β-cells. Rectangles show coding sequences; triangles show loxP sites; red rectangle shows DsRed coding sequence. B and C: Immunofluorescence staining for Nkx6.1 and insulin reveals loss of Nkx6.1 in most β-cells of Nkx6.1∆β mice at P0. D: Blood glucose levels in 6-week-old Nkx6.1∆β mice fed ad libitum compared with control mice (n = 6). E: Intraperitoneal glucose tolerance test shows glucose intolerance in 6-week-old Nkx6.1∆β mice as compared with control mice (n = 6). F: Quantification of β-cell mass reveals decreased β-cell mass in Nkx6.1∆β mice at 6 weeks of age (n = 3). Data shown as mean ± SEM. Scale bars = 20 μm. Ins, insulin; YFP, yellow fluorescent protein. *P < 0.05; **P < 0.01.
Nkx6.1∆β mice were born with the expected Mendelian frequency (data not shown). Consistent with previous reports showing incomplete targeting of β-cells by the RIP-Cre transgene (20), most but not all β-cells were devoid of Nkx6.1 at birth (Fig. 1B and C). At 6 weeks of age, Nkx6.1∆β mice exhibited significantly elevated blood glucose levels (Fig. 1D) and impaired glucose tolerance after intraperitoneal injection of a glucose bolus (Fig. 1E). To investigate whether Nkx6.1 deficiency affects postnatal β-cell growth, we examined β-cell mass in Nkx6.1∆β mice. Compared with littermate controls, 6-week-old Nkx6.1∆β mice exhibited a 40% reduction in β-cell mass (1.26 ± 0.05 mg in Nkx6.1∆β mice vs. 2.13 ± 0.29 mg in controls) (Fig. 1F). Thus Nkx6.1 is necessary to establish appropriate β-cell mass.
Nkx6.1 Is Required for Postnatal, but Not Prenatal, β-Cell Mass Expansion
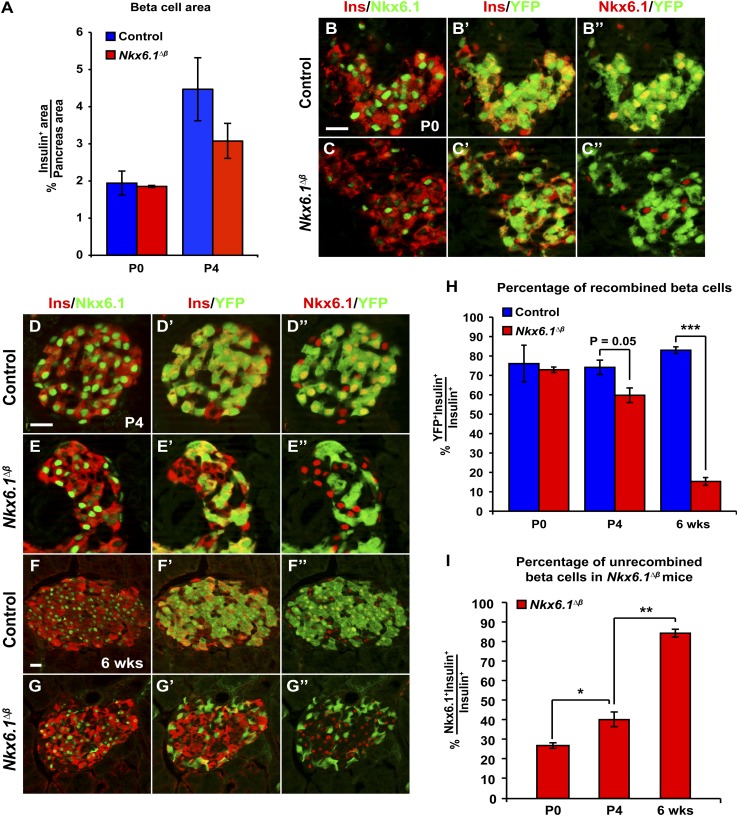
To determine when β-cell mass is first affected in Nkx6.1∆β mice, we measured the relative insulin+ area in Nkx6.1∆β mice immediately after birth. In contrast to 6-week-old mice, β-cell mass in neonatal Nkx6.1∆β mice was indistinguishable from control mice (Fig. 2A), showing that Nkx6.1 is required for postnatal expansion but not for establishing prenatal β-cell mass.
Figure 2.
Nkx6.1 is required for postnatal β-cell mass expansion. A: Quantification of the insulin immunofluorescent area relative to total pancreatic area reveals no difference in β-cell mass between Nkx6.1∆β and control mice at P0 and a slight but not significant decrease at P4 (n = 3). B–G’’: Immunofluorescence staining for insulin, Nkx6.1, and YFP at P0 (B–C’’), P4 (D–E’’), and 6 weeks of age (F–G’’). H: Quantification of insulin+ cells expressing YFP at P0, P4, and 6 weeks shows a progressive decrease of YFP+ recombined β-cells in Nkx6.1∆β mice postnatally (n = 3). I: Quantification of insulin+ cells expressing Nkx6.1 reveals a progressive increase of Nkx6.1-expressing unrecombined β-cells in Nkx6.1∆β mice between P0 and 6 weeks of age (n = 3). Data shown as mean ± SEM. Scale bar = 20 μm. Ins, insulin; YFP, yellow fluorescent protein. *P < 0.05; **P < 0.01; ***P < 0.001.
Because RIP-Cre-mediated recombination of the Nkx6.1flox allele is mosaic and did not delete Nkx6.1 in all β-cells (Fig. 1C), both unrecombined Nkx6.1+ and recombined Nkx6.1-deficient β-cells can contribute to β-cell growth in Nkx6.1∆β mice. To investigate the contribution of recombined β-cells to postnatal β-cell mass expansion, we quantified the percentage of recombined β-cells in Nkx6.1∆β and control mice. In line with our observation that Nkx6.1 is dispensable for prenatal β-cell growth (Fig. 2A), the percentage of recombined YFP+ β-cells was similar in newborn Nkx6.1∆β and control mice (73 ± 1.4% in Nkx6.1∆β mice vs. 76 ± 9.5% in control mice) (Fig. 2B–C’’ and H). Consistent with a slight decrease in overall β-cell mass in Nkx6.1∆β mice at postnatal (P) day 4 (Fig. 2A), a reduction in the percentage of recombined β-cells was discernable in Nkx6.1∆β mice by P4 (Fig. 2D–E’’ and H). At 6 weeks of age, the reduction of YFP+ β-cells was highly significant (15 ± 2.02% of β-cells in Nkx6.1∆β mice vs. 83 ± 1.72% in control mice) (Fig. 2F–H). The decrease of YFP+ β-cells in Nkx6.1∆β mice was accompanied by an age-dependent increase in the percentage of β-cells expressing Nkx6.1 (Fig. 2B–G’’ and I). Closely mirroring the reported 82% recombination efficiency of the RIP-Cre transgene (20), 27 ± 1.41% of β-cells expressed Nkx6.1 in newborn Nkx6.1∆β mice (Fig. 2I). This percentage increased significantly to 40 ± 3.75% at P4 (Fig. 2I). These findings indicate that a selective disadvantage becomes apparent for Nkx6.1-deficient β-cells shortly after birth.
Postnatal, but Not Prenatal, β-Cell Proliferation Depends on Nkx6.1
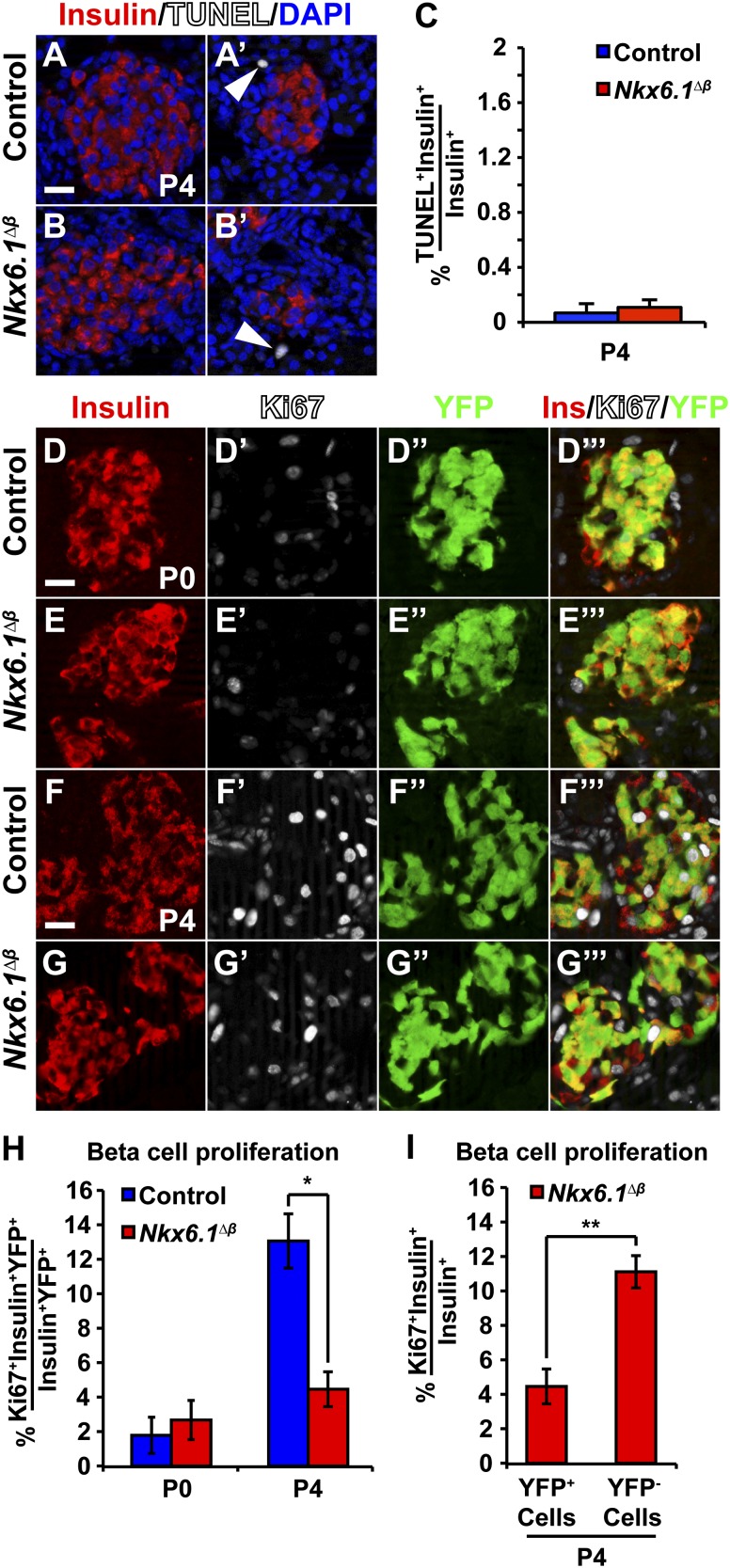
We next investigated whether the postnatal β-cell growth defect in Nkx6.1∆β mice is caused by reduced β-cell proliferation and/or survival. First, we examined the possibility that Nkx6.1 deficiency causes increased β-cell apoptosis by performing TUNEL assays on pancreatic sections. Virtually no TUNEL+ β-cells were detected in either Nkx6.1∆β or control mice at P4 (Fig. 3A–C), indicating that apoptosis does not account for the negative selection of Nkx6.1-deficient β-cells. By contrast, analysis of β-cell proliferation by immunofluorescence staining for Ki67, insulin, and YFP in Nkx6.1∆β mice at P4 revealed reduced numbers of Ki67+ β-cells (Fig. 3F–H). Quantification of Ki67+YFP+ β-cells showed a threefold decrease in β-cell proliferation in 4-day-old Nkx6.1∆β compared with control mice (4.48 ± 1.01% in Nkx6.1∆β mice vs. 13.00 ± 1.58% in control mice) (Fig. 3H). Consistent with our finding that Nkx6.1 inactivation does not affect prenatal β-cell growth (Fig. 2A), the frequency of Ki67+ β-cells did not differ between Nkx6.1∆β and control mice at P0 (2.67 ± 1.14% in Nkx6.1∆β mice vs. 1.78 ± 1.05% in control mice) (Fig. 3D–E’’’ and H). Thus Nkx6.1 is required for β-cell proliferation and expansion during early postnatal life but is dispensable prenatally. Furthermore, the effect of Nkx6.1 deletion on β-cell proliferation is cell autonomous, as revealed by comparing proliferation rates between recombined and unrecombined β-cells in Nkx6.1∆β mice at P4 (4.48 ± 1.01% YFP+insulin+ cells vs. 11.0 ± 0.93% YFP-insulin+ cells expressed Ki67) (Fig. 3I).
Figure 3.
Nkx6.1 is required for postnatal β-cell proliferation. A–C: β-Cells are not apoptotic at P4 in Nkx6.1∆β or control mice based on TUNEL combined with immunofluorescence staining for insulin and DAPI. TUNEL+ cells in the pancreas are shown as a positive control (arrowheads) and TUNEL+insulin+ cells were quantified. D–G’’’: Immunofluorescence staining for insulin, Ki67, and YFP at P0 and P4. H: Quantification of the percentage of insulin+YFP+ cells expressing Ki67 shows decreased β-cell proliferation in Nkx6.1∆β mice at P4, but not at P0 (n = 3). I: Quantification of Ki67-expressing YFP+insulin+ cells and YFP−insulin+ cells in Nkx6.1∆β mice at P4 reveals a selective decrease in proliferation of recombined compared with unrecombined β-cells within the same animal (n = 3). Data shown as mean ± SEM. Scale bar = 20 μm. Ins, insulin; YFP, yellow fluorescent protein. *P < 0.05; **P < 0.01.
Nkx6.1 Deletion Causes a Cell Autonomous Loss of Markers for β-Cell Maturation and Nutrient Sensing
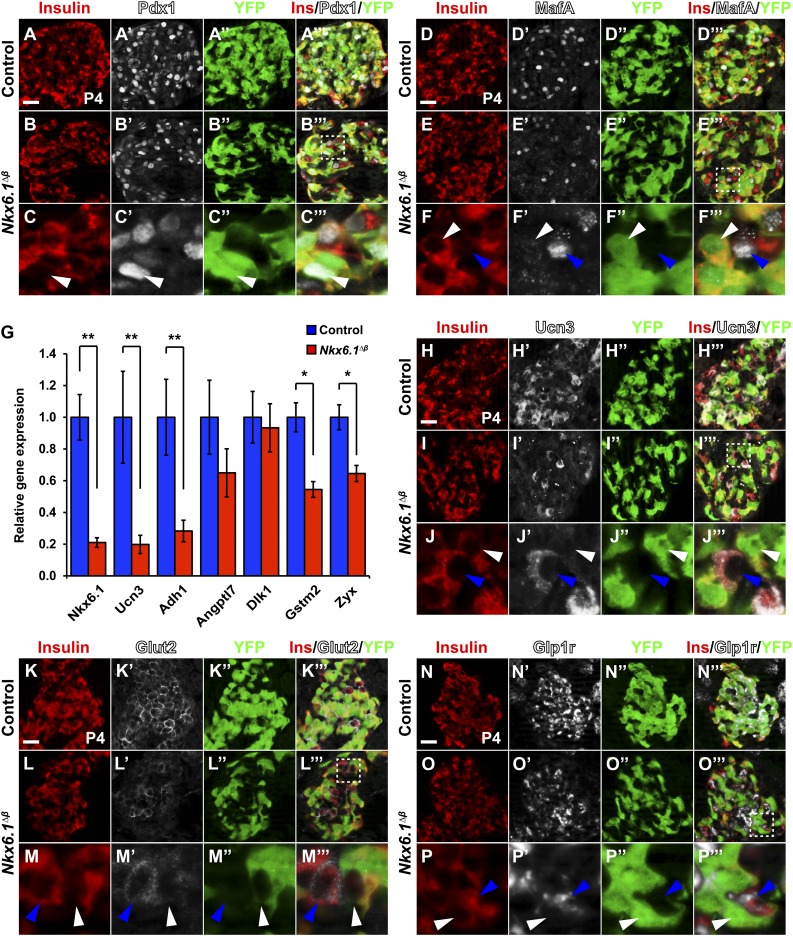
To determine whether loss of Nkx6.1 affects other β-cell markers, we performed immunofluorescence staining for Pdx1 and MafA. While Pdx1 was unaffected, MafA was lost in recombined Nkx6.1-deficient β-cells (Fig. 4A–F’’’). We further assessed whether Nkx6.1 regulates β-cell maturation markers. To this end, we selected genes found to be significantly changed between immature and mature postnatal β-cells (24) and performed qRT-PCR analysis on pancreata from control and Nkx6.1∆β mice at P2, when β-cell mass is similar between Nkx6.1∆β and control mice (Fig. 2A). Of these genes, Ucn3, Adh1, Gstm2, and Zyx were expressed at significantly lower levels in Nkx6.1∆β mice, while Angptl7 and Dlk1 were unchanged (Fig. 4G–J’’’). These results suggest that Nkx6.1 regulates a subset of genes associated with β-cell maturation. Given the postnatal onset of the β-cell proliferation defect in Nkx6.1∆β mice, we next investigated whether Nkx6.1-deficient β-cells are able to receive feeding-induced signals that stimulate β-cell proliferation. We analyzed the expression of Glut2 and the Glp1 receptor (Glp1r), which are known to have a role in the regulation of postnatal β-cell growth (14,16). In accordance with Glut2 being a direct Nkx6.1 target gene (17), Nkx6.1∆β mice exhibited a selective loss of Glut2 expression only in recombined β-cells (Fig. 4K–M’’’). Similarly, recombined β-cells displayed a cell autonomous reduction in Glp1r expression (Fig. 4N–P’’’). The cell autonomous role of Nkx6.1 in regulating β-cell proliferation, Glut2, and Glp1r expression argues against an Nkx6.1-dependent paracrine or systemic factor affecting β-cell proliferation in Nkx6.1∆β mice. These findings demonstrate that Nkx6.1-deficient β-cells lack key sensors for extrinsic stimuli of postnatal β-cell growth.
Figure 4.
Nkx6.1 inactivation leads to a cell autonomous loss of β-cell maturation and nutrient sensing markers. Immunofluorescence staining for insulin, Pdx1, and YFP (A–C’’’) or insulin, MafA, and YFP (D–F’’’) shows Pdx1 but not MafA expression in recombined YFP+insulin+ cells of Nkx6.1∆β mice at P4. Unrecombined YFP−insulin+ cells express Pdx1 and MafA in Nkx6.1∆β mice. G: qRT-PCR analysis of pancreata from Nkx6.1∆β and control mice at P2 for genes associated with β-cell maturation (n = 3). Immunofluorescence staining for insulin, Ucn3, and YFP (H–J’’’); insulin, Glut2, and YFP (K–M’’’); or insulin, Glp1r, and YFP (N–P’’’) shows loss of Ucn3, Glut2, and Glp1r expression in recombined YFP+insulin+ cells but not in unrecombined YFP−insulin+ cells of Nkx6.1∆β mice at P4. For each marker, representative areas are shown in lower panels for Nkx6.1∆β mice, as indicated by a dashed box in the merged middle panel. White arrowheads point to recombined YFP+insulin+ cells and blue arrowheads to unrecombined YFP−insulin+ cells. Data shown as mean ± SEM. Scale bar = 20 μm. Ins, insulin; YFP, yellow fluorescent protein. *P < 0.05; **P < 0.01.
Discussion
The role of Nkx6.1 in β-cell proliferation has been controversial. While in vitro studies have suggested a direct role of Nkx6.1 in stimulating β-cell proliferation through the regulation of Cyclin gene expression (18), in vivo overexpression of Nkx6.1 in β-cells showed no effect on β-cell proliferation or mass (19). Moreover, we have recently reported that β-cell-specific inactivation of Nkx6.1 in adult mice has no overt effect on β-cell mass (17). However, due to the extremely low proliferation rate of β-cells in adult animals (1), the role of Nkx6.1 in β-cell mass expansion could not be rigorously tested in this model. By ablating Nkx6.1 in newly formed β-cells of the embryo, we here show that postnatal β-cell proliferation and mass expansion depends on Nkx6.1 activity. We found that Nkx6.1-deficient β-cells begin to exhibit reduced proliferation between P0 and P4, which manifests in a measurable decrease in the contribution of Nkx6.1-deficient β-cells to β-cell mass as early as P4. We have previously reported that Nkx6.1 deficiency leads to a loss of β-cell identity and, ultimately, their conversion into delta cells (21). It is important to note that this fate conversion occurs later and is not yet observed at P4 (see Fig. 2E–E’’; all YFP+ cells express insulin). Thus the reduced contribution of Nkx6.1-deficient β-cells to β-cell mass is caused by the proliferation defect and cannot be attributed to a β-to-delta–cell fate conversion.
Using chromatin immunoprecipitation sequencing analysis, we have recently shown that Nkx6.1 does not bind to Cyclin gene regulatory regions (17). Therefore, Nkx6.1 is likely an indirect regulator of β-cell proliferation. Consistent with this idea, our current work shows that prenatal β-cell proliferation is unaffected in Nkx6.1∆β mice. Interestingly, we found that the onset of reduced β-cell proliferation in Nkx6.1∆β mice coincides with birth and thus the beginning of food intake, suggesting that Nkx6.1 could enable β-cells to respond to nutrient-dependent inducers of β-cell proliferation. Supporting this notion, Nkx6.1-deleted β-cells fail to express two important nutrient sensors, Glut2 and Glp1r. At the transition from prenatal to postnatal life, glucose becomes an important stimulus of β-cell proliferation (16) and similar to Nkx6.1∆β mice, Glut2-deficient mice exhibit reduced β-cell proliferation during the early postnatal period (25). Since Glp1 regulates β-cell proliferation independent of glucose (15), loss of Glut2 and Glp1r in Nkx6.1∆β mice likely have additive effects on β-cell proliferation. In addition to regulating nutrient sensors, we found that Nkx6.1 also regulates several markers associated with postnatal β-cell maturation (24). It is still largely unclear whether and how these genes affect β-cell maturation, but the regulation of several of these genes by Nkx6.1 suggests a role for Nkx6.1 in β-cell maturation. Collectively, our results demonstrate that Nkx6.1 controls multiple relevant pathways for postnatal β-cell development.
Article Information
Acknowledgments. The authors thank S. Heller (Novo Nordisk) for anti-Glp1r, C. Kioussi (Oregon State University) for anti-GFP, and Mark Huising (University of California, Davis) for anti-Ucn3 antibody. They are grateful to N. Rosenblatt and FenFen Liu for technical assistance.
Funding. This work was supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant R01-DK-068471 to M.S. and the National Institutes of Health training grant T32-GM-008666-15 to J.B.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. B.L.T. and J.B. designed and performed experiments, analyzed data, prepared figures, and wrote the manuscript. M.S. wrote the manuscript. M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 2005;54:2557–2567 [DOI] [PubMed] [Google Scholar]
- 2.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 2004;114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kushner JA, Ciemerych MA, Sicinska E, et al. . Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 2005;25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier JJ, Butler AE, Saisho Y, et al. . Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes 1995;44:249–256 [DOI] [PubMed] [Google Scholar]
- 6.Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 2007;3:758–768 [DOI] [PubMed] [Google Scholar]
- 7.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 8.Alonso LC, Yokoe T, Zhang P, et al. . Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes 1989;38:49–53 [DOI] [PubMed] [Google Scholar]
- 10.Tarussio D, Metref S, Seyer P, et al. . Nervous glucose sensing regulates postnatal β cell proliferation and glucose homeostasis. J Clin Invest 2014;124:413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development 2010;137:3205–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. . Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 13.Salpeter SJ, Klochendler A, Weinberg-Corem N, et al. . Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic β-cells through glycolysis and calcium channels. Endocrinology 2011;152:2589–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–2276 [DOI] [PubMed] [Google Scholar]
- 15.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase Czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes 2001;50:2237–2243 [DOI] [PubMed] [Google Scholar]
- 16.Swenne I. Glucose-stimulated DNA replication of the pancreatic islets during the development of the rat fetus. Effects of nutrients, growth hormone, and triiodothyronine. Diabetes 1985;34:803–807 [DOI] [PubMed] [Google Scholar]
- 17.Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep 2013;4:1262–1275 [DOI] [PMC free article] [PubMed]
- 18.Schisler JC, Fueger PT, Babu DA, et al. . Stimulation of human and rat islet beta-cell proliferation with retention of function by the homeodomain transcription factor Nkx6.1. Mol Cell Biol 2008;28:3465–3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaffer AE, Yang AJ, Thorel F, Herrera PL, Sander M. Transgenic overexpression of the transcription factor Nkx6.1 in β-cells of mice does not increase β-cell proliferation, β-cell mass, or improve glucose clearance. Mol Endocrinol 2011;25:1904–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postic C, Shiota M, Niswender KD, et al. . Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem 1999;274:305–315 [DOI] [PubMed] [Google Scholar]
- 21.Schaffer AE, Taylor BL, Benthuysen JR, et al. . Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic Beta cell identity. PLoS Genet 2013;9:e1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson SB, Schaffer AE, Sander M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development 2007;134:2491–2500 [DOI] [PubMed] [Google Scholar]
- 23.Srinivas S, Watanabe T, Lin CS, et al. . Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum B, Hrvatin SS, Schuetz C, Bonal C, Rezania A, Melton DA. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 2012;30:261–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillam MT, Hümmler E, Schaerer E, et al. . Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2 [published correction appears in Nat Genet 1997;17:503]. Nat Genet 1997;17:327–330 [DOI] [PubMed] [Google Scholar]