Abstract
In chronic eosinophilic leukemia (CEL), the transforming oncoprotein FIP1L1-PDGFRA is a major target of therapy. In most patients, the tyrosine kinase inhibitor (TKI) imatinib induces complete remission. For patients who are intolerant or resistant, novel TKI have been proposed. We examined the in vitro effects of 14 kinase blockers on growth and function of EOL-1 cells, a FIP1L1-PDGFRA+ eosinophil cell line. Major growth-inhibitory effects were seen with all PDGFR-blocking agents, with IC50 values in the low nM-range: ponatinib: 0.1-0.2 nM, sorafenib: 0.1-0.2 nM, masitinib: 0.2-0.5 nM, nilotinib: 0.2-1 nM, dasatinib: 0.5-2 nM, sunitinib: 1-2 nM, midostaurin: 5-10 nM. These drugs were also found to block activation of PDGFR-downstream signaling molecules, including Akt, S6, and STAT5 in EOL-1 cells. All effective TKI produced apoptosis in EOL-1 cells as determined by microscopy, Annexin-V/PI, and caspase-3-staining. In addition, PDGFR-targeting TKI were found to inhibit cytokine-induced migration of EOL-1 cells. In all bioassays employed, ponatinib was found to be the most potent compound in EOL-1 cells. In addition, ponatinib was found to downregulate expression of the activation-linked surface antigen CD63 on EOL-1 cells, and to suppress growth of primary neoplastic eosinophils. We also examined drug effects on Ba/F3 cells expressing two clinically relevant imatinib-resistant mutant-forms of FIP1L1-PDGFRA, namely T674I and D842V. Strong inhibitory effects on both mutants were only seen with ponatinib. In summary, novel PDGFR-targeting TKI may be alternative agents for the treatment of patients with imatinib-resistant CEL. Although several different PDGFR-targeting agents are effective, the most potent drug appears to be ponatinib.
Introduction
Chronic eosinophilic leukemia (CEL) is a myeloproliferative neoplasm characterized by marked and persistent peripheral blood eosinophilia and molecular and/or cytogenetic evidence of clonal expansion of eosinophils (1-5). In many cases, eosinophil-induced organ damage, often in form of endomyocardial fibrosis and/or thrombosis, is seen (1-5). In a majority of all patients with CEL, the disease-associated CHIC2-deletion and the resulting oncogenic fusion gene, FIP1L1-PDGFRA, are found (1-9).
A number of recent studies have shown that the PDGFR-targeting tyrosine kinase inhibitor (TKI) imatinib blocks in vitro growth of neoplastic eosinophils carrying FIP1L1-PDGFRA (10-15). It has also been described that imatinib can produce complete and long-lasting hematologic remissions in FIP1L1-PDGFRA+ CEL (16-19). However, imatinib is not effective in all patients, which may result from point mutations in the FIP1L1-PDGFRA gene or other mechanisms (20-23). In rare cases, intolerance against imatinib develops. Therefore, current research is seeking novel TKI that act on FIP1L1-PDGFRA+ cells and may overcome resistance. Indeed, several novel TKI, such as nilotinib, dasatinib, sorafenib or PKC412 have been described to block the kinase-activity of FIP1L1-PDGFRA and thus malignant cell growth (12,14,15,24-26). More recently, ponatinib was found to block the growth of Ba/F3 cells carrying various mutant-forms of FIP1L1-PDGFRA (27).
In the current study, we analyzed and compared the effects of 14 kinase-inhibitors on growth, survival, migration and function of neoplastic human eosinophils.
Materials and Methods
Reagents
Bosutinib, masitinib and midostaurin (PKC412) were purchased from LC Laboratories (Woburn, MA, USA), ponatinib from Selleck Chemicals (Houston, TX, USA), piceatannol and pimozide from Sigma Aldrich (San Louis, MO, USA), and dasatinib, sorafenib, sunitinib, tozasertib, vorinostat, everolimus (RAD001), erlotinib, gefitinib, and lapatinib from ChemieTek (Indianapolis, IN, USA). Imatinib, nilotinib, and BEZ235 were kindly provided by Dr.E.Buchdunger and Dr.P.W.Manley (Novartis, Basel, Switzerland). Stock solutions of drugs were prepared by dissolving in DMSO (Merck, Darmstadt, Germany). A specification of targeted drugs is shown in Supplementary Table S1. RPMI 1640 medium and fetal calf serum (FCS) were from PAA Laboratories (Pasching, Austria), 3H-thymidine from Amersham (Buckinghamshire, United Kingdom), and the Annexin V-FITC Kit from eBiosciences (San Diego, CA, USA). Recombinant human (rh) stroma cell-derived factor-1 alpha (SDF-1α) and rh eotaxin were purchased from R&D Systems (Minneapolis, MN, USA), and rh interleukin-5 (IL-5) from BD Bioscience (San José, CA, USA). A specification of monoclonal antibodies (mAb) used in this study is shown in Supplementary Table S2.
Cell lines
The FIP1L1-PDGFRA+ cell line EOL-1 was purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). To confirm the identity of EOL-1 and the expression of FIP1L1-PDGFRA, cells were analyzed by conventional cytogenetic analysis, fluorescence in situ hybridization (FISH) and reverse transcriptase (RT) PCR essentially as described (6,7,11,14). In these experiments, EOL-1 cells were found to express FIP1L1-PDGFRA and the following karyotype: 50,XY,+4,+6,t(7;9)(q36;q22),+8,+19; this karyotype is largely resembling previously published results (10,28). EOL-1 cells were maintained in RPMI 1640 medium supplemented with 20% heat-inactivated FCS and antibiotics at 37°C and 5% CO2. Ba/F3 cells expressing the imatinib-resistant mutants T674I and D842V of FIP1L1-PDGFRA, were generated according to published protocols (29). Ba/F3 cells were cultured in RPMI 1640 medium and 10% FCS.
Isolation and culture of primary human eosinophils
Primary neoplastic eosinophils were obtained from one patient with FIP1L1-PDGFRA+ CEL (75% eosinophils in differential counts) and one with aggressive systemic mastocytosis (ASM-eo) and eosinophilia (25% eosinophils). In the patient with CEL, the presence of the FIP1L1-PDGFRA mutant was confirmed by FISH. As assessed by conventional karyotyping, no additional chromosome abnormalities were detected in neoplastic cells in this patient. Non-neoplastic (reactive) eosinophils were obtained from a patient with reactive hypereosinophilia (HER) (15% eosinophils). Eosinophils were enriched from the bone marrow (BM) using Ficoll (CEL, HER) or from the peripheral blood by Dextran sedimentation (ASM-eo). For proliferation experiments, only cells that showed sufficient uptake of 3H-thymidine over 48 hours, were used. All patients gave written informed consent before BM (iliac crest puncture for routine staging) or peripheral blood samples (routine staging) were obtained. The study was approved by the Local Ethics Committee of the Medical University of Vienna. Purified eosinophils were washed and examined for cell viability and the percentage of eosinophils by Wright-Giemsa staining.
Evaluation of drug responses in Ba/F3 cells carrying PDGFRA mutants
Ba/F3 cells harbouring PDGFRA mutants were seeded at 3×105 cells/ml and cultured in complete medium in the absence or presence of various concentrations of ponatinib, sorafenib, imatinib, dasatinib, masitinib, nilotinib, midostaurin, and sunitinib (1-1,000 nM) at 37°C and 5% CO2. The numbers of viable cells were determined at 0 and 24 hours of treatment. Apoptotic cells were detected by flow cytometry using Annexin-V and 7–AAD staining (Becton Dickinson Immunocytometry Systems, Erembodegem, Belgium). Cells were analyzed on a FACSCanto Cytometer (Becton Dickinson Immunocytometry Systems).
Measurement of 3H-thymidine uptake in neoplastic human eosinophils
To determine growth-inhibitory effects of various drugs, EOL-1 cells (2×104 cells/well) or primary eosinophils (1.5×105 cells/well) were incubated in control medium or various concentrations of targeted drugs (PDGFR blockers: 0.01-0.1 μM, other drugs: up to 10 μM) in 96-well culture plates (TPP, Trasadingen, Switzerland) at 37°C for 48 hours. After incubation, 0.5 μCi 3H-thymidine was added (37°C, 16 hours). Cells were then harvested on filter membranes (Packard Biosciences, Meriden, CT) in a Filtermate 196 harvester (Packard Bioscience). Filters were air-dried, and the bound radioactivity was counted in a ß-counter (Top-count NXT, Packard Bioscience). All experiments were performed in triplicates.
Evaluation of apoptosis in EOL-1 cells
After incubation with drugs (37°C, 48 hours), the percentage of apoptotic cells was quantified on Wright-Giemsa-stained cytospin preparations. Apoptosis was defined according to conventional cytomorphologic criteria (30). For flow cytometric determination of apoptosis, combined Annexin V/propidium iodide-staining and staining for activated caspase-3 were performed as described (14,31,32). EOL-1 cells were exposed to targeted drugs at various concentrations (PDGFR blockers: 0.005-10 nM; other drugs: up to 10 μM) or control medium at 37°C for 48 hours. Thereafter, cells were washed and incubated with Annexin V-FITC (Bender MedSystems, Vienna, Austria) in binding-buffer for 15 minutes. Cells were again washed, and propidium iodide (1 μg/ml) (Bender MedSystems) was added. For caspase-3 staining, cells were fixed in 4% formaldehyde and permeabilized in methanol as reported (31,32). Thereafter, cells were washed and then incubated with a PE-conjugated mAb against active caspase-3 (BD Biosciences) for 30 minutes. After washing, cells were analyzed by flow cytometry on a FACSCalibur (Becton Dickinson, San José, CA, USA).
Analysis of PDGFRA phosphorylation by Western blotting
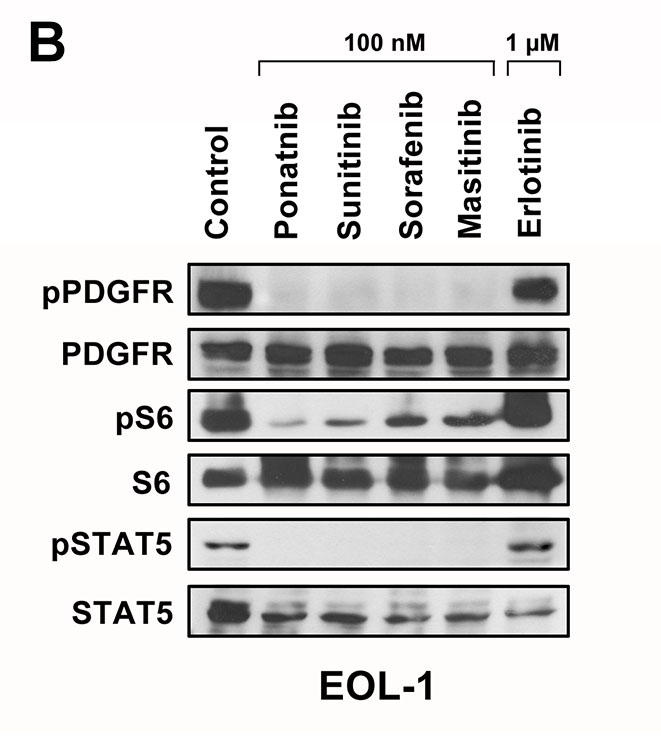
EOL-1 cells were incubated with various targeted drugs (100 nM or 1 μM) or control medium at 37°C for 4 hours. Ba/F3 cells transformed by FIP1L1-PDGFRA variants were incubated in control medium in the absence (Control) or presence of ponatinib or imatinib (each 1 μM) at 37°C for 2 hours. Western blotting was performed as described (14,30) using antibodies against PDGFRA or phosphorylated (p) PDGFRA (Santa Cruz Biotechnology, Santa Cruz, CA), antibodies against S6 or pS6 (Cell Signaling Technology, Danvers, MA) and antibodies against STAT5 or pSTAT5 (tyrosine 694) (BD Bioscience, San José, CA). Antibody-reactivity was made visible by donkey-anti-rabbit IgG or sheep-anti-mouse IgG (both from GE Healthcare, Buckinghamshire, UK) and Lumingen PS-3 detection reagent (Thermo Scientific, Rockford, IL).
Flow cytometric evaluation of surface and cytoplasmic antigens in EOL-1 cells
Expression of cell surface antigens on EOL-1 cells was determined by flow cytometry after culture in control medium or medium supplemented with ponatinib, sorafenib, sunitinib, masitinib and nilotinib (0.1, 0.5, or 10 nM) for 24 hours. After incubation, cells were washed and subjected to flow cytometry using PE-labeled mAb against CD25, CD63, CXCR4 (CD184), FAS (CD95), and HLA-DR. Flow cytometry was performed on a FACSCalibur (Becton Dickinson) as described (14). Staining reactions were controlled by isotype-matched antibodies. For staining of cytoplasmic molecules, EOL-1 cells were incubated in various concentrations of ponatinib, sorafenib, sunitinib, masitinib and erlotinib (0.01, 0.1, and 1 μM) for 4 hours. Cells were then fixed in 4% formaldehyde, permeabilized in methanol (−20°C), and then incubated with mAb M89-61 against phosphorylated Akt (serine 473), mAb N7-548 against pS6, mAb 47 against pSTAT5 (tyrosine 694), or mAb C92-605 against activated caspase 3 (30 minutes) before being analyzed.
Analysis of chemotactic migration of EOL-1 cells
Migration of EOL-1 cells was determined using a modified Boyden chamber-assay with Transwell polycarbonate membranes (5 μm pore size, 0.33 cm2; Corning Incorporated, Corning, NY). The lower chambers were filled with 600 μl RPMI 1640 medium containing 20% heat-inactivated FCS in the absence (Co) or presence of SDF-1α (1-100 ng/mL), IL-5 (0.5-500 ng/ml), or eotaxin (5-1,000 ng/ml). The upper chambers were loaded with EOL-1 cells (1.5 × 105 per well) that had been preincubated with control medium or medium containing 10 nM or 100 nM of ponatinib, sorafenib, masitinib, nilotinib, imatinib, or dasatinib. Cells were allowed to migrate into the lower chambers at 37°C for 4 hours. Then, viable migrated cells in the lower chambers were counted by flow cytometry using a FACS Calibur (Becton Dickinson) and expressed as percent of total cells.
Statistical analysis
To determine the level of significance in differences in cell growth, apoptosis, and migration, the paired Student́s t test was applied. Results were considered to be significantly different, when p<0.05.
Results
Effects of TKI on growth of EOL-1 cells
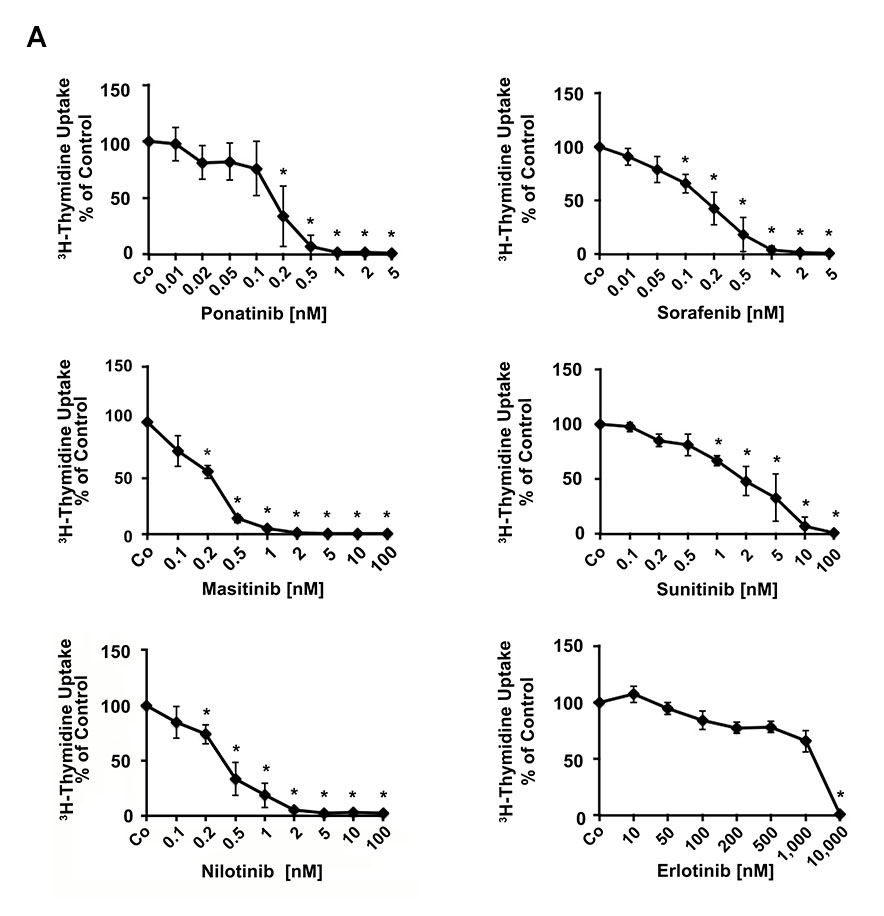
A series of kinase blockers were tested for their effects on proliferation of EOL-1 cells measured by 3H-thymidine uptake. As shown in Table 1 and Figure 1A, the PDGFR-blocking TKI were all found to inhibit growth of EOL-1 cells. Drug effects were seen in the low nanomolar range, with the following rank-order of potency (IC50): ponatinib (0.1-0.2 nM), sorafenib (0.1-0.2 nM), masitinib (0.2-0.5), nilotinib (0.2-1 nM), dasatinib (0.5-2 nM), sunitinib (1-2 nM), and midostaurin (5-10 nM) (Table 1, Figure 1A). The HDAC inhibitor vorinostat also induced growth inhibition, but IC50 values were higher (100-200 nM) compared to PDGFR-blocking drugs (Table 1). The other kinase inhibitors (not affecting PDGFR) such as lapatinib, gefitinib, or erlotinib, showed no effects on growth of EOL-1 cells (Table 1).
Table 1.
Effects of various drugs on proliferation and survival of EOL-1 cells
| Apoptosis |
||||
|---|---|---|---|---|
| compound | 3H-Thymidine Uptake IC50 [nM] | Microscopy ED50 [nM] | Annexin-V/PI ED50 [nM] | Caspase-3 ED50 [nM] |
| Ponatinib | 0.1-0.2 | 0.2-0.5 | 0.01-0.05 | 0.1-0.2 |
| Sorafenib | 0.1-0.2 | 0.05-0.1 | 0.1-0.2 | 0.1 |
| Masitinib | 0.2-0.5 | 0.2-0.5 | 0.2-0.3 | 0.2-0.5 |
| Imatinib | 0.2-0.5 | 0.3-0.5 | 0.2-0.3 | 0.3 |
| Nilotinib | 0.2-1 | 0.2-0.5 | 0.2-0.3 | 0.2-0.5 |
| Everolimus | 0.2-0.5 | n.t. | 0.5-1 | 1-5 |
| Dasatinib | 0.5-2 | 1-5 | 1-5 | 1-5 |
| Sunitinib | 1-2 | 1-5 | 1-2.5 | 1-5 |
| Midostaurin | 5-10 | 10-50 | 5-10 | 10-50 |
| BEZ235 | 20-50 | n.t. | 10-25 | 10-25 |
| Tozasertib | 20-50 | n.t. | 10-25 | 50-100 |
| Vorinostat | 100-200 | n.t. | 250-500 | 250 |
| Bosutinib | 500-1,000 | n.t | 100-250 | 100-500 |
| Lapatinib | 1,000-5,000 | n.t. | 2,500-5,000 | 2,500-5,000 |
| Gefitinib | 1,000-10,000 | n.t. | 100-500 | 1,000-2,500 |
| Erlotinib | 1,000-10,000 | n.t. | 1,000-2,500 | 2,500-5,000 |
| Pimozide | 2,500-5,000 | n.t. | 1,000-2,500 | 2,500-5,000 |
| Piceatannol | 7,500-10,000 | n.t. | 5,000-7,500 | 2,500-5,000 |
EOL-1 cells were incubated with various concentrations of targeted drugs at 37°C for 48 hours. Proliferation was measured by analyzing 3H-thymidine uptake. Apoptosis was determined by light microscopy (Giemsa staining), Annexin-V/PI staining or active caspase-3 staining by flow cytometry. Abbreviations: n.t., not tested. IC50, inhibitory concentration (50%); ED50, effective dose/concentration (50%).
Figure 1. Effects of various kinase blockers on proliferation of neoplastic eosinophils.
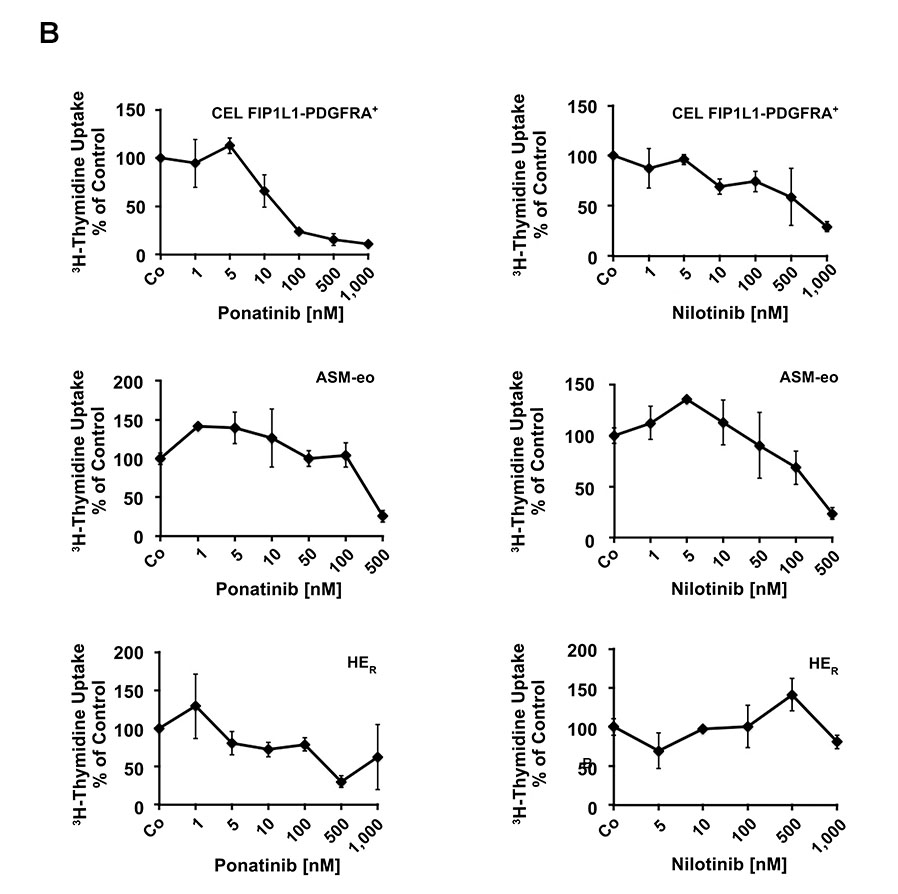
(A) EOL-1 cells were incubated in control medium (Co) or in various concentrations of targeted drugs as indicated at 37°C for 48 hours. After incubation, 3H-thymidine uptake was measured. Results are expressed as percent of control and represent the mean±SD from 3 independent experiments. Asterisk: p<0.05. (B) Primary eosinophils obtained from a patient with FIP1L1-PDGFRA+ CEL (upper panels), one with aggressive systemic mastocytosis with eosinophilia (ASM-eo; middle panels) and one with reactive hypereosinophilia (HER, lower panels), were incubated in control medium (Co) or in various concentrations of targeted drugs as indicated at 37°C for 48 hours. After incubation, 3H-thymidine uptake was measured. Results are expressed as percent of control and represent the mean±SD of triplicates.
Effects of TKI on proliferation of primary neoplastic cells
To confirm growth-inhibitory effects of TKI, we applied the most effective TKI on primary neoplastic eosinophils obtained from a patient with FIP1L1-PDGFRA+ CEL. In addition, drug effects were tested on eosinophils obtained from a patient with ASM and eosinophilia (ASM-eo) and one with HER. In these experiments, we were able to show that ponatinib and nilotinib dose-dependently inhibit the proliferation of primary neoplastic FIP1L1-PDGFRA+ eosinophils (Figure 1B). In the patient with KIT D816V+ ASM-eo and the patient with HER, ponatinib was also found to inhibit the proliferation of eosinophils, but IC50 values were higher compared to that obtained in the patient with CEL (Figure 1B). Nilotinib was also found to exert growth-inhibitory effects on neoplastic eosinophils in the patient with ASM-eo (Figure 1B). However, no substantial effects of nilotinib on reactive eosinophils were seen.
PDGFRA-targeting TKI induce apoptosis in EOL-1 cells
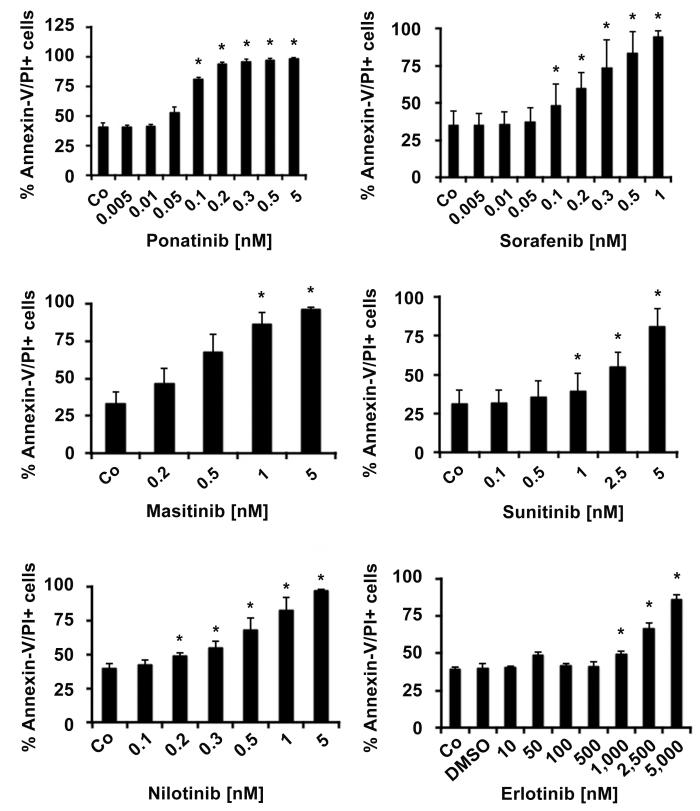
To define the mechanism of TKI-induced growth inhibition in neoplastic eosinophils, we examined apoptosis in EOL-1 cells by light microscopy, Annexin-V/PI staining and caspase 3 staining. In all 3 assays, the effective PDGFRA-blockers, namely ponatinib, sorafenib, masitinib, nilotinib, and sunitinib, induced apoptosis in EOL-1 cells in a dose-dependent manner, with pharmacologic ED50 values (Table 1, Figure 2). The Aurora kinase blocker tozasertib and the HDAC blocker vorinostat were also found to induce apoptosis in EOL-1 cells, but ED50 values were higher than those obtained with the PDGFRA blockers.
Figure 2. PDGFR-targeting drugs induce apoptosis in EOL-1 cells.
EOL-1 cells were cultured in the absence (Co) or presence of various concentrations of targeted drugs as indicated at 37°C for 48 hours. Apoptosis was determined by Annexin-V/PI-staining by flow cytometry. Results are expressed as percent of control and represent the mean±S.D. of 3 or 4 independent experiments. Asterisk: p<0.05. In the lower right panel, the DMSO control is also shown.
Effects of TKI on activation of various signal transduction molecules in EOL-1 cells
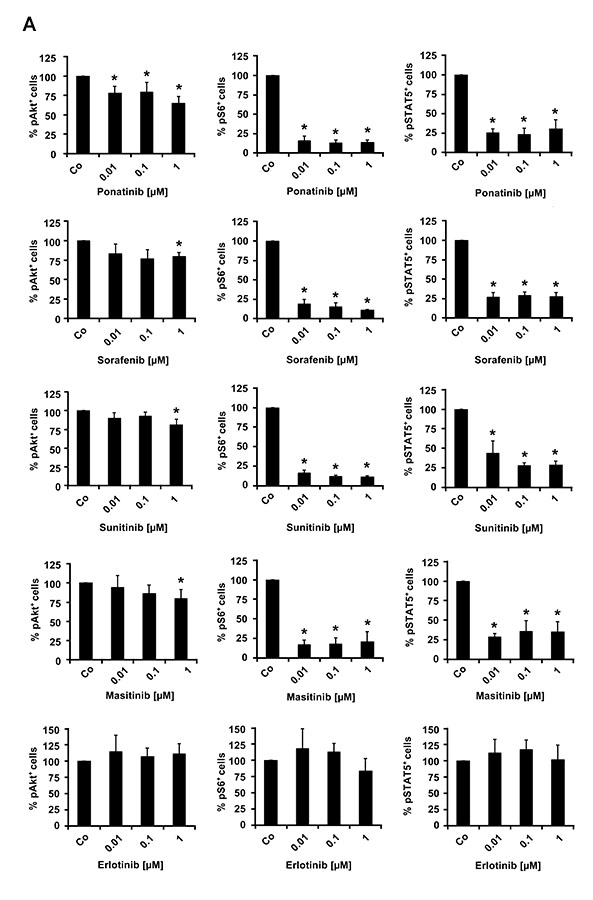
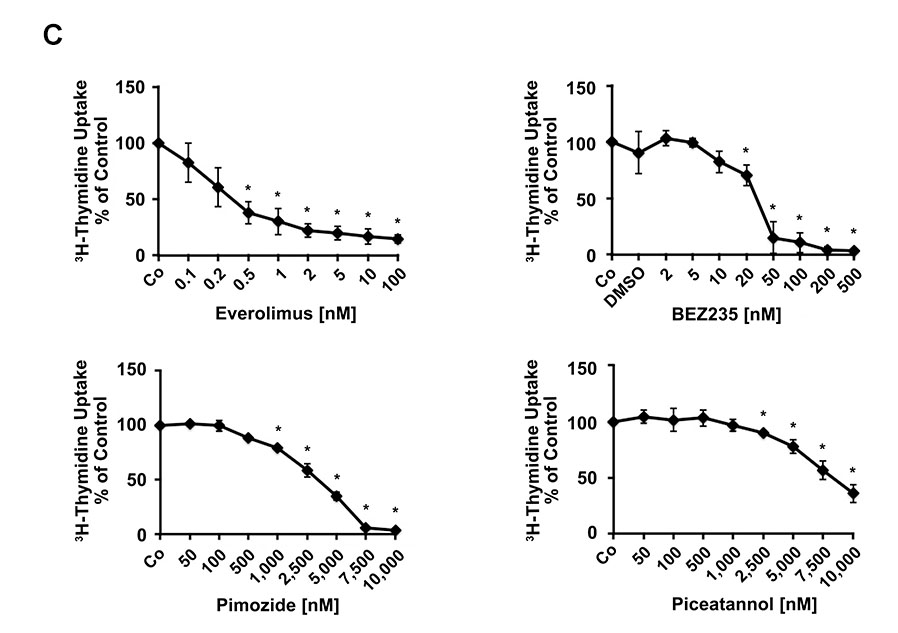
In a next step, we asked whether the effective TKI block one or more PDGFRA-downstream signaling molecules in EOL-1 cells. As assessed by flow cytometry, all effective PDGFRA-targeting TKI were found to inhibit the phosphorylation of PI3 kinase-downstream Akt, mTOR-downstream S6 as well as STAT5 phosphorylation (Figure 3A). Drug effects on PDGFRA phosphorylation and phosphorylation of PDGFRA-downstream signaling molecules (S6 and STAT5) were confirmed by Western blotting (Figure 3B). We also examined the effects of various clinically relevant signal transduction inhibitors, including the mTOR-targeting agent everolimus (RAD001), the PI3 kinase/mTOR blocker BEZ235, and the experimental STAT5-blocking agents pimozide and piceatannol on growth of EOL-1 cells. All these agents produced growth inhibition in EOL-1 cells. However, the IC50 values were higher compared to that obtained with PDGFR-targeting TKI (Table 1, Figure 3C).
Figure 3. Effects of various targeted drugs on PDGFR-downstream signaling molecules in EOL-1 cells.
(A) EOL-1 cells were incubated in control medium in the absence (Co) or presence of ponatinib, sorafenib, masitinib, sunitinib or erlotinib (each 0.01, 0.1 or 1 μM) at 37°C for 4 hours. After incubation, cells were analyzed for expression of pAKT, pS6, and pSTAT5, by flow cytometry as described in the text. Results are expressed as percent of control and represent the mean±SD from at least 3 independent experiments. DMSO (solvent) per se did not show any effect on phosphorylation of tested molecules (not shown). Asterisk, p<0.05. (B) EOL-1 cells were incubated in control medium in the absence (Control) or presence of ponatinib, sunitinib, sorafenib, masitinib or erlotinib (each 0.1 or 1 μM) at 37°C for 4 hours. After incubation, cells were analyzed for expression of pPDGFR, pS6 and pSTAT5 by western blotting as described in the text. (C) EOL-1 cells were incubated in control medium (Co) or in various concentrations of everolimus, BEZ235, pimozide, or piceatannol at 37°C for 48 hours. After incubation, 3H-thymidine uptake was measured. Results are expressed as percent of control and represent the mean±SD from 3 independent experiments. Asterisk: p<0.05.
Effects of various TKI on expression of cell surface antigens in EOL-1 cells
Neoplastic eosinophils are considered to express high levels of CD25 and CD63 as well as other activation-linked cell surface antigens. We examined the effects of various targeted drugs (producing growth inhibition) on expression of these surface antigens in EOL-1 cells. We found that ponatinib, and less effectively sorafenib, downregulate expression of CD25 and CD63 in EOL-1 cells (Supplementary Figure S1). By contrast, no effects of ponatinib or sorafenib on expression of HLA-DR, CXCR4 and CD95 were seen. We were also unable to detect effects of the other TKI tested on surface antigen expression in EOL-1 cells (not shown).
Effects of TKI on cytokine-induced migration of EOL-1 cells
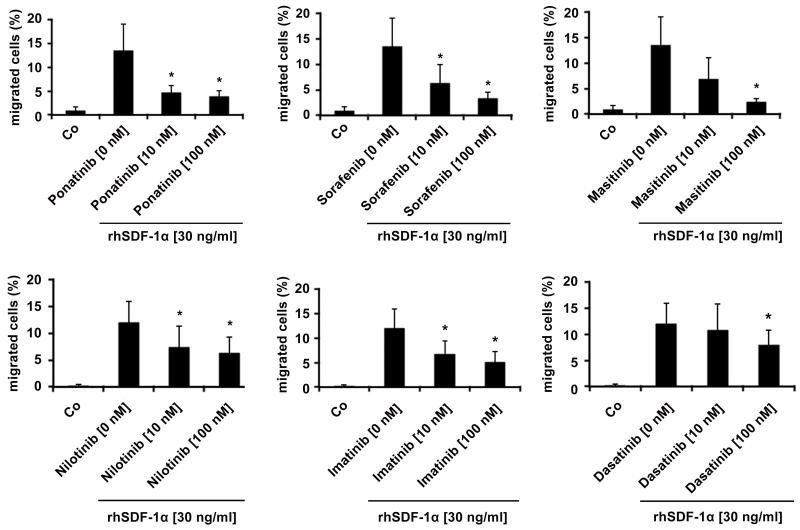
Eosinophil-induced organ damage is usually associated with migration of eosinophils into local tissue sites. Several different cytokines can promote eosinophil migration (33-35). We were therefore interested to learn whether the targeted drugs applied interfere with cytokine-induced migration of neoplastic eosinophils. In a first step, we applied 3 chemotaxins, namely IL-5, eotaxin, and SDF-1α. Unexpectedly, neither IL-5 nor eotaxin was found to induce chemotaxis in EOL-1 cells (not shown). However, SDF-1α was found to induce migration of EOL-1 cells in a dose-dependent manner (Supplementary Figure S2). We next asked whether the PDGFRA-targeting TKI interfere with SDF-1α-induced migration of EOL-1 cells. We found that all six drugs tested, including ponatinib effectively block SDF-1α-induced migration of EOL-1 cells (Figure 4). The most effective drugs were ponatinib, sorafenib, and masitinib. With all three drugs, an almost complete inhibition of EOL-1 cell migration was seen at 100 nM (Figure 4).
Figure 4. Effects of targeted drugs on SDF-1α-induced migration of EOL-1 cells.
EOL-1 cells were pre-incubated in control medium (Co) or in medium containing ponatinib, sorafenib, masitinib, nilotinib, imatinib, or dasatinib (each 10 or 100 nM) at 37°C for 1 hour. Then, migration of cells against SDF-1α (30 ng/ml) was determined in a double-chamber chemotaxis assay (4 hours) as described in the text. Numbers of viable migrated cells are expressed as percent of total (100% input) cells. Results represent the mean±S.D. of 4 independent experiments. Asterisk: p<0.05.
Effects of various kinase blockers on growth of Ba/F3 cells expressing imatinib-resistant mutants of FIP1L1-PDGFRA
Resistance against imatinib in CEL is usually associated with the acquisition of point mutations in the FIP1L1-PDGFRA fusion gene. We asked which of the effective PDGFRA blockers would inhibit growth of neoplastic cells expressing clinically relevant (imatinib-resistant) mutants of FIP1L1-PDGFRA. To address this question, we employed Ba/F3 cells expressing the T674I and the D842V mutants of FIP1L1-PDGFRA. As visible in Table 2 and Supplementary Figure S3, sunitinib was found to inhibit the growth of Ba/F3 cells expressing the T674I mutant of FIP1L1-PDGFRA. By contrast, no substantial effects of masitinib or nilotinib on Ba/F3 cells expressing this mutant were found. Ba/F3 cells expressing the other mutant, D842V, were found to be resistant against sunitinib, nilotinib and masitinib. Confirming previous observations (27), ponatinib was found to inhibit growth of Ba/F3 cells expressing the T674I mutant, and, less effectively, the growth of Ba/F3 cells expressing the D842V mutant (Table 2). We were also able to confirm that ponatinib inhibits the phosphorylation of PDGFRA as well as phosphorylation of key downstream signaling molecules in Ba/F3 cells exhibiting wild type and mutant forms of FIP1L1-PDGFRA (Supplementary Figure S4).
Table 2.
Effects of various targeted drugs on proliferation of mutant-bearing Ba/F3 cells
| compound | Ba/F3 F/P IC50 [nM] | Ba/F3 F/P T674I IC50 [nM] | Ba/F3 F/P D842V IC50 [nM] |
|---|---|---|---|
| Ponatinib | 0.6 | 9 | 154 |
| Sorafenib | 0-5 | 10-50 | >1000 |
| Imatinib | 0-5 | >500 | >1000 |
| Dasatinib | 5-10 | >500 | 1000 |
| Masitinib | 8.3 | >1000 | >1000 |
| Nilotinib | 8.5 | 1736 | 4093 |
| Midostaurin | 10-50 | 10-50 | >500 |
| Sunitinib | 52.7 | 293.9 | >1000 |
Ba/F3 cells expressing wt F/P or F/P mutants were incubated in various concentrations of targeted drugs at 37°C for 24 hours. The numbers of viable cells were counted by flow cytometry. Results are expressed as IC50 values (nM). Abbreviations: F/P, FIP1L1-PDGFRA; IC50, inhibitory concentration (50%).
Discussion
Chronic eosinophilic leukemia is a neoplasm characterized by uncontrolled expansion of eosinophils with consecutive organ damage (1-5). In many patients, eosinophils express the FIP1L1-PDGFRA oncoprotein. This oncogenic mutant contributes essentially to factor-independent growth and accumulation of eosinophils in CEL. The PDGFR kinase blocker imatinib is successfully used to suppress growth of neoplastic eosinophils in patients with FIP1L1-PDGFRA+ CEL (16-19). Notably, most CEL patients treated with imatinib enter continuous complete remission. However, not all patients with CEL show a long-lasting response to imatinib, but relapse after a certain time interval. For these patients, alternative drugs have to be considered. In the present study, we have examined the effects of various clinically relevant TKI on growth and survival of EOL-1 cells. Our data show that several of these TKI, including ponatinib, sorafenib, masitinib, nilotinib, and dasatinib suppress growth and survival of EOL-1 cells in the low nM range. In addition, these drugs were found to inhibit cytokine-induced migration of eosinophils. However, only ponatinib was found to block the growth of Ba/F3 cells expressing the imatinib-resistant FIP1L1-PDGFRA mutants T674I and D842V. These observations may have clinical implications regarding the treatment of patients with drug-resistant CEL.
Recently, several different TKI that can inhibit growth of FIP1L1-PDGFRA+ cells have been identified (12,14,15,24-27). Some of these agents were found to suppress the growth of Ba/F3 cells expressing various imatinib-resistant mutants of FIP1L1-PDGFRA. However, most of these studies were performed in cell lines and only a few of them compared the potency of novel TKI. In the present study, we employed the EOL-1 cell line as a model of human neoplastic eosinophils carrying FIP1L1-PDGFRA. Using this cell line, we were able to compare the potencies of various novel TKI. The results of our study show that various novel TKI, including ponatinib, sorafenib, masitinib, nilotinib, and dasatinib inhibit the growth of EOL-1 cells at rather low concentrations (low nM range). In all bioassay tested, the most potent agent was ponatinib (IC50: 0.1-0.2 nM). The most likely explanation for the different potencies is that these agents not only block the activity of FIP1L1-PDGFRA, but also several other pro-oncogenic kinases. This has already been described for ponatinib and nilotinib as well as for dasatinib (14,24-27). With regard to masitinib, only little is known about additional targets expressed in EOL-1 cells. It also remains unknown which additional targets are most relevant and contribute to enhanced drug potency.
A number of different signalling pathways are activated downstream of the PDGFR. Our data show that all effective TKI that inhibited the proliferation of EOL-1 cells, also blocked the activation of various PDGFR-downstream signaling molecules, including pAkt, pS6, and pSTAT5, in EOL-1 cells. Whether all these effects were mediated through inhibition of PDGFRA or were mediated (also) via other upstream kinase-targets or even via direct inhibition of PDGFRA-downstream signaling molecules, remains at present unknown.
Recent data have shown that growth-inhibitory TKI produce apoptosis in EOL-1 cells (14,24-27). In the present study, we were able to confirm these observations for all effective TKI analyzed. Similar to previous studies, these drugs were found to induce apoptosis in EOL-1 cells in a low nM range. As expected, ponatinib was the most potent inducer of apoptosis in neoplastic eosinophils.
So far, only a very few studies have examined drug effects on primary human neoplastic eosinophils. We here show that the effective TKI, including ponatinib and nilotinib, block spontaneous proliferation of primary neoplastic eosinophils. Primary bone marrow cells derived from a patient with reactive hypereosinophilia were also found to respond to these drugs, but the IC50 values were higher compared to neoplastic eosinophils. These data suggest that ponatinib and other novel TKI are effective agents that can suppress neoplastic cell growth in CEL.
A number of cytokines and chemokines have been described to induce migration of normal and/or neoplastic blood eosinophils (33-35). Among these are IL-5 and eotaxin. However, in neoplastic eosinophils, only SDF-1α may act as a major chemoattractant. Notably, we were unable to show that IL5 or eotaxin induce a major chemotactic effect in EOL-1 cells. In a next step, we examined the effects of the most effective TKI on SDF-1α-induced migration of EOL-1 cells. In these experiments, we found that ponatinib, sorafenib, masitinib, and nilotinib are potent inhibitors of migration of EOL-1 cells against SDF-1α. These data are of special interest as eosinophil-associated organ damage in CEL is considered to be a multi-step process, where eosinophil migration and invasion into local tissue sites is one of the initial important events (36). Blocking these early initiating steps in organ damage may be of clinical significance and may be judged as a preferred drug action.
Another important event in eosinophil-induced organ damage is eosinophil activation with consecutive release of eosinophil-derived cytotoxic mediators/proteins (36). In addition, during eosinophil activation, several surface markers are considered to be expressed at higher levels. These elevated (activation-linked) cell surface antigens are also detectable on circulating eosinophils in CEL patients as well as in EOL-1 cells. In the present study, we found that ponatinib, but not the other TKI tested, inhibit expression of CD25 and CD63 in EOL-1 cells. Whether this de-activating effect may be of functional or even clinical significance remains at present unknown.
In most patients with CEL, imatinib produces long-lasting complete remissions at relatively low doses, e.g. 100 mg/day orally. However, in some of these patients resistance against imatinib may occur (20-23). In several of these patients, point mutations in FIP1L1-PDGFRA can be detected (20-23). Two of the more frequent mutants are T674I and D842V. We found that only ponatinib is able to produce growth inhibition in Ba/F3 cells carrying the T674I or the D842V mutant forms of FIP1L1-PDGFRA. Some of the other TKI, like sorafenib and midostaurin, also blocked the growth of Ba/F3 cells bearing the T674I mutant, but did not affect growth of Ba/F3 cells containing FIP1L1-PDGFRA D842V. These data suggest that ponatinib may be a preferable drug for the treatment of TKI-resistant CEL exhibiting these mutant forms of FIP1L1-PDGFRA. However, further preclinical studies and clinical trials are required to show that ponatinib is indeed an effective and safe agent in patients with CEL. This is of particular importance as ponatinib exhibits a broad kinase target spectrum and has shown beneficial effects in patients with TKI-resistant chronic myeloid leukemia, but also produced a number of adverse events in first clinical trials (37).
In summary, our data show that various TKI can block the growth and survival of neoplastic human eosinophils, including cells carrying FIP1L1-PDGFRA. The most potent and broadly acting agent, that blocks several different functions of eosinophils and clinically relevant mutant forms of FIP1L1-PDGFRA, is ponatinib.
Supplementary Material
Acknowledgements
This work was supported by the Austrian Science Fund (FWF), SFB grants F4704-B20 and F4705-B20, grants from KU Leuven (GOA/11/010 to P.V.), FWO-Vlaanderen (G.0509.10 to P.V.), the Foundation against Cancer (grant 2010-204 to P.V. and E.L.). ELS L is a postdoctoral fellow of FWO-Vlaanderen. Peter Vandenberghe is a senior clinical investigator of FWO-Vlaanderen. We like to thank Margit König and Trude Pass for skilful technical assistance.
Footnotes
Conflict of Interest Disclosure
The authors declare no conflict of interests relevant to this manuscript.
References
- 1.Gotlib J. Molecular classification and pathogenesis of eosinophilic disorders: 2005 update. Acta Haematol. 2005;114:7–25. doi: 10.1159/000085559. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006 Jun;133:468–492. doi: 10.1111/j.1365-2141.2006.06038.x. [DOI] [PubMed] [Google Scholar]
- 3.Bain BJ, Fletcher SH. Chronic eosinophilic leukemias and the myeloproliferative variant of the hypereosinophilic syndrome. Immunol Allergy Clin North Am. 2007;27:377–388. doi: 10.1016/j.iac.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Gotlib J. Chronic eosinophilic leukemia/hypereosinophilic syndrome. Cancer Treat Res. 2008;142:69–106. [PubMed] [Google Scholar]
- 5.Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophil disorders. Blood Rev. 2009;23:157–165. doi: 10.1016/j.blre.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 7.Gotlib J, Cools J, Malone JM, 3rd, et al. The FIP1L1-PDGFRalpha fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: implications for diagnosis, classification, and management. Blood. 2004;103:2879–2891. doi: 10.1182/blood-2003-06-1824. [DOI] [PubMed] [Google Scholar]
- 8.Bain BJ, Pierre R, Imbert M, et al. Chronic eosinophilic leukaemia and the idiopathic hypereosinophilic syndrome. In: Jaffe ES, Harris NL, Stein H, editors. The World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haemopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2001. pp. 29–31. [Google Scholar]
- 9.Bain BJ, Gilliland DG, Vardiman JW, et al. Chronic eosinophilic leukaemia, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2008. pp. 51–53. [Google Scholar]
- 10.Griffin JH, Leung J, Bruner RJ, et al. Discovery of a fusion kinase in EOL-1 cells and idiopathic hypereosinophilic syndrome. Proc Natl Acad Sci (USA) 2003;100:7830–7835. doi: 10.1073/pnas.0932698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cools J, Quentmeier H, Huntly BJ, et al. The EOL-1 cell line as an in vitro model for the study of FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2004;103:2802–2805. doi: 10.1182/blood-2003-07-2479. [DOI] [PubMed] [Google Scholar]
- 12.Verstovsek S, Giles FJ, Quintás-Cardama A, et al. Activity of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, against human FIP1L1-PDGFR-alpha-expressing cells. Leuk Res. 2006;30:1499–1505. doi: 10.1016/j.leukres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Robert C, Apàti A, Chomienne C, et al. All-trans-retinoic acid enhances apoptosis induction by tyrosine kinase inhibitors in the eosinophilic leukemia-derived EoL-1 cell line. Leuk Res. 2008;32:343–346. doi: 10.1016/j.leukres.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner C, Gleixner KV, Peter B, et al. Dasatinib inhibits the growth and survival of neoplastic human eosinophils (EOL-1) through targeting of FIP1L1-PDGFRalpha. Exp Hematol. 2008;36:1244–1253. doi: 10.1016/j.exphem.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Wicklein D, Ramos Leal N, Salamon J, et al. Nilotinib and imatinib are comparably effective in reducing growth of human eosinophil leukemia cells in a newly established xenograft model. PLoS One. 2012;7:e30567. doi: 10.1371/journal.pone.0030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardanani A, Reeder T, Porrata LF, Li CY, Tazelaar HD, Baxter EJ, Witzig TE, Cross NC, Tefferi A. Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. Blood. 2003;101:3391–3397. doi: 10.1182/blood-2002-10-3103. [DOI] [PubMed] [Google Scholar]
- 17.Jovanovic JV, Score J, Waghorn K, et al. Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood. 2007;109:4635–4640. doi: 10.1182/blood-2006-10-050054. [DOI] [PubMed] [Google Scholar]
- 18.Baccarani M, Cilloni D, Rondoni M, et al. The efficacy of imatinib mesylate in patients with FIP1L1-PDGFRalpha-positive hypereosinophilic syndrome. Results of a multicenter prospective study. Haematologica. 2007;92:1173–1179. doi: 10.3324/haematol.11420. [DOI] [PubMed] [Google Scholar]
- 19.Metzgeroth G, Walz C, Erben P, et al. Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: a phase-II study. Br J Haematol. 2008;143:707–715. doi: 10.1111/j.1365-2141.2008.07294.x. [DOI] [PubMed] [Google Scholar]
- 20.Cools J, Stover EH, Wlodarska I, et al. The FIP1L1-PDGFRalpha kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia. Curr Opin Hematol. 2004;11:51–57. doi: 10.1097/00062752-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Lierman E, Michaux L, Beullens E, et al. FIP1L1-PDGFRalpha D842V, a novel panresistant mutant, emerging after treatment of FIP1L1-PDGFRalpha T674I eosinophilic leukemia with single agent sorafenib. Leukemia. 2009;23:845–851. doi: 10.1038/leu.2009.2. [DOI] [PubMed] [Google Scholar]
- 22.Salemi S, Yousefi S, Simon D, et al. A novel FIP1L1-PDGFRA mutant destabilizing the inactive conformation of the kinase domain in chronic eosinophilic leukemia/hypereosinophilic syndrome. Allergy. 2009;64:913–918. doi: 10.1111/j.1398-9995.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- 23.von Bubnoff N, Gorantla SP, Engh RA, et al. The low frequency of clinical resistance to PDGFR inhibitors in myeloid neoplasms with abnormalities of PDGFRA might be related to the limited repertoire of possible PDGFRA kinase domain mutations in vitro. Oncogene. 2011;30:933–943. doi: 10.1038/onc.2010.476. [DOI] [PubMed] [Google Scholar]
- 24.Cools J, Stover EH, Boulton CL, et al. PKC412 overcomes resistance to imatinib in a murine model of FIP1L1-PDGFRα-induced myeloproliferative disease. Cancer Cell. 2003;3:459–469. doi: 10.1016/s1535-6108(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 25.Lierman E, Folens C, Stover EH, et al. Sorafenib is a potent inhibitor of FIP1L1-PDGFRalpha and the imatinib-resistant FIP1L1-PDGFRalpha T674I mutant. Blood. 2006;108:1374–1376. doi: 10.1182/blood-2006-02-004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Bubnoff N, Gorantla SP, Thöne S, et al. The FIP1L1-PDGFRA T674I mutation can be inhibited by the tyrosine kinase inhibitor AMN107 (nilotinib) Blood. 2006;107:4970–4971. doi: 10.1182/blood-2006-01-0285. [DOI] [PubMed] [Google Scholar]
- 27.Lierman E, Smits S, Cools J, et al. Ponatinib is active against imatinib-resistant mutants of FIP1L1-PDGFRA and KIT, and against FGFR1-derived fusion kinases. Leukemia. 2012;26:1693–1695. doi: 10.1038/leu.2012.8. [DOI] [PubMed] [Google Scholar]
- 28.Saito H, Bourinbaiar A, Ginsburg M, et al. Establishment and characterization of a new human eosinophilic leukemia cell line. Blood. 1985;66:1233–1240. [PubMed] [Google Scholar]
- 29.Schwaller J, Frantsve J, Aster J, et al. Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myelo- and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion genes. EMBO J. 1998;17:5321–5333. doi: 10.1093/emboj/17.18.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 31.Blatt K, Herrmann H, Mirkina I, et al. The PI3-kinase/mTOR-targeting drug NVP-BEZ235 inhibits growth and IgE-dependent activation of human mast cells and basophils. PLoS One. 2012;7:e29925. doi: 10.1371/journal.pone.0029925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadzijusufovic E, Peter B, Herrmann H, et al. NI-1: a novel canine mastocytoma model for studying drug resistance and IgER-dependent mast cell activation. Allergy. 2012;67:858–868. doi: 10.1111/j.1398-9995.2012.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JM, Rambaldi A, Biondi A, et al. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989;19:701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberg ME, Ownbey R, Mehlhop PD, et al. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- 35.Hoermann G, Cerny-Reiterer S, Sadovnik I, et al. Oncostatin M is a FIP1L1/PDGFRA-dependent mediator of cytokine production in chronic eosinophilic leukemia. Allergy. 2013 doi: 10.1111/all.12139. in press. [DOI] [PubMed] [Google Scholar]
- 36.Valent P, Gleich GJ, Reiter A, et al. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012;5:157–176. doi: 10.1586/ehm.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.