Abstract
Purpose.
Previously, aliphatic β-nitroalcohols (BNAs) have been studied as a means to chemically induce tissue cross-linking (TXL) of cornea and sclera. There are a number of related and possibly more potent agents, known as formaldehyde releasers (FARs), that are in commercial use as preservatives in cosmetics and other personal care products. The present study was undertaken in order to screen such compounds for potential clinical utility as therapeutic TXL agents.
Methods.
A chemical registry of 62 FARs was created from a literature review and included characteristics relevant to TXL such as molecular weight, carcinogenicity/mutagenicity, toxicity, hydrophobicity, and commercial availability. From this registry, five compounds [diazolidinyl urea (DAU), imidazolidinyl urea (IMU), sodium hydroxymethylglycinate (SMG), DMDM hydantoin (DMDM), 5-Ethyl-3,7-dioxa-1-azabicyclo [3.3.0] octane (OCT)] were selected for efficacy screening using two independent systems, an ex vivo rabbit corneal cross-linking simulation setup and incubation of cut scleral tissue pieces. Treatments were conducted at pH 7.4 or 8.5 for 30 minutes. Efficacy was evaluated using thermal denaturation temperature (Tm), and cell toxicity was studied using the trypan blue exclusion method.
Results.
Cross-linking effects in the five selected FARs were pH and concentration dependent. Overall, the Tm shifts were in agreement with both cornea and sclera. By comparison with BNAs previously reported upon, the FARs identified in this study were significantly more potent but with similar or better cytotoxicity.
Conclusions.
The FARs, a class of compounds well known to the cosmetic industry, may have utility as therapeutic TXL agents. The compounds studied thus far show promise and will be further tested.
Keywords: cornea, sclera, tissue cross-linking, keratoconus, progressive myopia
Formaldehyde-releasing agents are in widespread use as preservatives in commercially available cosmetics and shampoos. Proposing an alternative use for these compounds as therapeutic tissue cross-linking agents greatly expands the possibilities for treating keratoconus and progressive myopia.
Introduction
The growing clinical success of ultraviolet-A irradiation (UVA)–riboflavin photochemical corneal cross-linking (CXL) to halt the progression of keratoconus1–8 and post-LASIK keratectasia8,9 suggests that increasing mechanical tissue strength in vivo can be beneficial. CXL increases the stiffness of corneal tissue as shown in animal studies using postmortem mechanical strip testing.10 A majority of patients ultimately gain improvements in topography and gain lines of vision.5–9 Application of CXL has been extended to treat corneal edema,11 corneal melting,12 and corneal infections.13
As clinical trials involving CXL progress in the United States, suggestions have been made to extend its use to the sclera as a treatment for progressive myopia,14 since biomechanical weakening occurs during progressive globe elongation.2 Scleral cross-linking with UVA-riboflavin technology has been reported15 but may be difficult to carry out in the posterior region of the sclera without the use of surgical means. Also, of concern is the potential of damaging the neural retina during UVA irradiation.15 The use of injectable pharmacologic agents that could cross-link the sclera as an alternative to photochemical activation is being explored and includes glyceraldehyde,16 glutaraldehyde,14 genipin,17 and nitroalcohols (Hoang QV, et al. IOVS 2013;54:ARVO E-Abstract 5169).18
The present study serves as an extension of our previous work using nitroalcohols,19,20 wherein we test the corneal and scleral cross-linking efficacy of several related though potentially more potent chemicals from a group known as formaldehyde-releasing agents (FARs). These compounds are used as preservatives in a wide array of popular cosmetic and personal care products21,22 such as skin care products, body wash, fingernail polish, and shampoo, including the former formula for Johnson & Johnson's “No More Tears” baby shampoo.23 Formaldehyde-releasing agents have also been employed in the textile industry as cross-linking agents to impart antiwrinkle properties to clothing.24 Considering their use in everyday items that come into direct contact with the human body, we wanted to examine the efficacy and cell toxicity of FARs as tissue cross-linking (TXL) agents as a first step in their potential development for clinical use. To that end, in vitro and ex vivo cross-linking experiments were conducted using corneal and scleral tissues as collagenous tissue substrates. Effectiveness of cross-linking was evaluated using shifts in the thermal denaturation temperature (Tm) of the collagen found in corneal and scleral tissue as measured by differential scanning calorimetry (DSC), where Tm is the melting temperature of a folded protein.25 Past studies have employed Tm as a measure of the thermal stability of biological tissue.16,26–29 Therefore, we used differences in Tm between chemically treated and control tissue to estimate extent of cross-linking and compared these values to those induced by CXL (i.e., riboflavin photochemical cross-linking), which served as a positive control for corneal samples. Toxicity was assessed using trypan blue exclusion staining.30 Our results suggest that some FARs are potent chemical agents for TXL with moderate cell toxicity thresholds.
Materials and Methods
Chemical Registry
A chemical registry of FARs commonly found in cosmetics and other personal care products (PCPs) was compiled from a review of the literature.21,22 Information used to assemble this registry included characteristics relevant to TXL such as molecular weight, European Union maximum allowed concentration (i.e., “max allowed”), carcinogenicity/mutagenicity, toxicity, hydrophobicity (octanol/water partition coefficient or logP), efficacy of formaldehyde release, and commercial availability of the chemicals. From the FARs identified, five compounds with favorable profiles were selected for cross-linking efficacy and toxicity evaluation. These compounds include diazolidinyl urea (DAU), imidazolidinyl urea (IMU), DMDM hydantoin (DMDM), sodium hydroxymethylglycinate (SMG), and 5-Ethyl-3,7-dioxa-1-azabicyclo [3.3.0] octane (OCT), which were specifically chosen because of the vastness of their use in cosmetics and PCPs as well as their ability to donate formaldehyde in solution under equilibrium conditions.31,32 The cross-linking efficacy and toxicity of two additional FARs, bronopol (BP) and 2-hydroxymethyl-2-nitro-1,3-propanediol (HNPD), which are β-nitroalcohols (BNAs) that we have previously worked with, were included for comparative purposes.
Chemicals
Diazolidinyl urea (N-Hydroxymethyl-N-(1,3-di(hydroxymethyl)-2,5-dioxoimidazolidin-4-yl)-N′-hydroxy-methylurea [DAU]), imidazolidinyl urea (N,N′-methylenebis[N-[3-(hydroxymethyl)-2,5-dioxo-4-imidazolidinyl]]-urea [IMU]), sodium hydroxymethylglycinate (SMG), 5-Ethyl-3,7-dioxa-1-azabicyclo [3.3.0] octane (7a-Ethyldihydro-1H,3H,5H-oxazolo[3,4-c]oxazole [OCT]), 2-bromo-2-nitro-1,3-propanediol or BP, hydroxypropyl methyl cellulose (HPMC, 15 centipoise), dextran (high molecular weight = 425,000–575,000 Da), sodium bicarbonate, and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA). DMDM hydantoin (1,3-bis(hydroxymethyl)-5,5-dimethyl-2,4-imidazolidinedione [DMDM]) was obtained from Oakwood Products, Inc. (West Columbia, SC, USA). 2-Hydroxymethyl-2-nitro-1-3-propanediol (HNPD) was obtained from TCI Chemicals, Inc. (New York, NY, USA). Riboflavin-5-phosphate was obtained from MP Biomedicals (Santa Ana, CA, USA). Dulbecco's phosphate-buffered saline (DPBS) solution (MgCl2 and CaCl2 free) was obtained from Life Technologies (Carlsbad, CA, USA). All chemical solutions and buffers were prepared fresh using Millipore water (double distilled, deionized water, ρ = 18.2 MΩ·cm at 25°C; EMD Millipore, Billerica, MA, USA) on the day of cross-linking.
Chemical and Riboflavin-Mediated Photochemical Cross-Linking (CXL) of the Cornea
Intact cadaveric rabbit heads with clear corneas were obtained fresh (within an hour of euthanasia) in adherence with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Formaldehyde releaser solutions at concentrations equivalent to half the maximum allowed value (1/2max) were administered in a manner designed to simulate therapeutic cross-linking in patients. For all of the corneal experiments (with the notable exception of CXL), the corneal epithelium was left intact. An 8-mm Hessburg-Barron corneal reservoir (JEDMED, St. Louis, MO, USA) was affixed to the corneal surface using the supplied vacuum syringe. A single drop of proparacaine (0.5%) was applied to the corneal surface prior to reservoir application. A buffer solution containing 0.1 M NaHCO3 at either pH 8.5 or 7.4 was used. The pH of the sample and buffer mixture was titrated to the desired pH just prior to application to the eye using an appropriately concentrated HCl solution. Treatments were conducted over a 30-minute period at 25°C with refreshing of the solution every 5 minutes. The control contralateral eye was treated identically with vehicle. Immediately after treatment, a central 6-mm corneal button was trephined from the treated region of each eye, was blotted on both sides using a paper towel to remove excess solution, and was analyzed using DSC (see below). A minimum of two independent determinations were carried out for each condition described using a fresh cadaver head each time.
As a comparison, the same ex vivo system was used to conduct photochemical cross-linking of rabbit cornea as previously described by Wollensak et al.,1 with some changes. To that end, a central 8-mm portion of the corneal epithelium was debrided using a blunt-end scalpel. De-epithelialized corneal tissue was presoaked in 0.1% riboflavin-5-phosphate solution in 1.1% HPMC for 5 minutes. Thereafter, the cornea was exposed to UV light (λmax = 370 nm) at an irradiance of 3 mW/cm2 with an 8-mm aperture for 30 minutes using the Optos XLink Corneal Collagen Cross-Linking System (Optos, Dunfermline, UK). Riboflavin solution was refreshed every 3 minutes for the course of the treatment. The control contralateral eye was treated identically without irradiation.
Scleral Tissue Cross-Linking
The following is a modified version of the procedure that we have previously described for testing TXL efficacy of various BNAs.18 Enucleated porcine globes were purchased from Visiontech, Inc. (Sunnyvale, TX, USA) and were stored at −80°C until time of experimentation (1–2 months). Equatorial scleral strips approximately 6 × 40 mm in size were obtained from multiple eyes. These strips were submerged in DPBS solution containing 1 mM EDTA to inactivate native collagenases and to prevent tissue dehydration during sample preparation. Each strip was further cut into smaller 4- × 3-mm pieces. The scleral pieces were individually transferred to a 24-well plate and were incubated in 1 mL cross-linking solution in 0.1 M NaHCO3 buffer at either pH 8.5 or 7.4 for 30 minutes at 25°C without refreshing the solution. Four concentrations of FAR solution were tested at each pH: (1) max allowed concentration, (2) 1/2max allowed concentration, (3) 1/10 max allowed concentration, and (4) 25 mM. Tissue samples cross-linked with the BNAs BP and HNPD at concentrations of 5 mM (max allowed for BP), 10 mM, and 25 mM were used as positive controls. Negative controls were treated identically with vehicle. Post treatment, all solutions were aspirated, and samples were washed twice using DPBS to remove remnant cross-linking solution before being analyzed by DSC. A minimum of three independent determinations were carried out for each condition using scleral pieces originating from different porcine globes.
Differential Scanning Calorimetry (DSC) and Cross-Link Analysis
Thermal denaturation temperature (Tm) of all samples was measured using a Perkin-Elmer DSC 6000 Autosampler (Waltham, MA, USA). Tissue samples were carefully blotted in a standardized, repetitive manner to remove excess solution/DPBS and transferred to preweighed 50-μL aluminum pans. The pans were immediately hermetically sealed using a DSC pan sealing press, which is used to prevent tissue dehydration due to evaporative losses. The DSC scans were run using Pyris software (version 11.0; Perkin-Elmer, Waltham, MA, USA) from 40°C to 75°C at a rate of 1°C/min, and denaturation curves representing differential heat flow over time were recorded. Differential scanning calorimetry heat flow endotherm data were analyzed using the Pyris data analysis peak search function using a calculation limit of ±0.3°C from the apparent thermal denaturation peak.
Statistical Analysis
Student's t-tests were used to evaluate the significance of observed differences in Tm between cross-linked and control groups. Due to the nature of the ex vivo cadaveric system used for corneal cross-linking, where each cadaver provided the treated eye and contralateral control, corneal samples were subjected to paired t-tests. Conversely, scleral samples were subjected to nonpaired t-tests assuming equal variance of data. Significance of all statistical tests was based on an alpha value of 0.05 (P ≤ 0.05). All ΔTm values are reported in the form of mean value followed by standard error.
FAR Cytotoxicity Threshold
Formaldehyde-releasing agent cell cytotoxicity assays were performed as we have previously described.33 Briefly, healthy human skin fibroblasts (HSFs) from American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in dermal cell basal media (ATCC) using a serum-free fibroblast growth kit provided by the company (ascorbic acid, EGF/TGF-β1, glutamine, hydrocortisone, insulin, and fibroblast growth supplements). Cells were grown in 5% CO2 and 95% ambient air at 37°C until confluent. Once confluent, the cells were detached and seeded into 24-well plates at a density of 5 × 104 cells/well and were once again allowed to reach confluence. Next, cells were treated with FAR solutions over a range of concentrations (0.001–5 mM) for 24 hours. Following cross-linking exposure, all cell media, including FAR solution, were aspirated, and each well was rinsed once with DPBS. Fresh media were then reintroduced and the cells were allowed to recover for 48 hours. Subsequent to cell recovery, cell toxicity was assessed using a modified version of the trypan blue staining protocol. To that end, all culture media were aspirated and each well was rinsed with DPBS. Next, 0.4% trypan blue solution (Gibco, Grand Island, NY, USA) was added to each well for 3 minutes at 25°C. The staining solution was then aspirated, and cells were washed twice with DPBS. Finally, extent of trypan blue staining and morphology of cells were visualized using an inverted microscope (Cat. no. 12-560-45; Fisher Scientific, Pittsburgh, PA, USA).
Results
Identification of FARs
From a broad review of the literature, we were able to identify a total of 62 formaldehyde-releasing agents that can potentially be used for corneal and scleral TXL. These include FARs commonly found in cosmetics and PCPs as well as those that are used in the textile industry. Table 1 depicts the structures, chemical formulae, toxicity, and other pertinent information for the seven FARs that were chosen for evaluation in this study. This is an excerpt from the larger chemical registry that was compiled. None of the chemicals that were tested in this study are known carcinogens (various Material Safety Data Sheets). They range in size up to <400 Da, with IMU being the largest at 388 Da and SMG the smallest at 104 Da (MWt = 127 − 23 [Na] = 104 Da). In most but not all cases, mutagenicity data are available, and these chemicals have been found to be nonmutagenic using Ames, micronucleus, and other standard assays. Furthermore, they exhibit low organismal toxicity as indicated by high (>1000 mg/kg) rat oral LD50 values. The exception is BP, which has a relatively low LD50 oral, rat = 180 mg/kg (Table 1).
Table 1.
Characteristics of Select FARs Pertaining to Tissue Cross-Linking In Vivo
|
Chemical |
Structure |
Predicted Octanol/Water Partition Coefficient, LogP |
% Max Allowed Concentration (mM Conversion) |
Mutagenicity |
Toxicity: Method, Species, Dose, Exposure Time |
| Diazolidinyl urea [DAU; CAS No.: 78491-02-8; MWt: 278.22 g/mol; formula: C8H14N4O7] | −5.398 ± 0.86656 | 0.521 (17.97 mM) | Nonmutagenic*48 | LD50 oral, rat, 2600 mg/kg; LD50 dermal, rabbit, >2000 mg/kg63 | |
| Imidazolidinyl urea [IMU; CAS No.: 39236-46-9; MWt: 388.29 g/mol; formula: C11H16N8O8] | −4.930 ± 0.95957 | 0.621 (15.45 mM) | - | LD50 oral, rat, 11,300 mg/kg64 | |
| Sodium hydroxymethylglycinate [SMG; CAS No.: 70161-44-3; MWt: 127.07 g/mol; formula: C3H6NO3.Na] | −1.19758 | 0.521 (39.06 mM) | Nonmutagenic†54 | LD50 oral, rat, 2100 mg/kg, LD50 dermal, rabbit, >2000 mg/kg54 | |
| DMDM hydantoin [DMDM; CAS No.: 6440-58-0; MWt: 188.18 g/mol; formula: C7H12N2O4] | −1.078 ± 0.65459 | 0.621 (31.88 mM) | Nonmutagenic‡50 | LD50 oral, rat, 3720 mg/kg; LD50 oral, rat, >2000 mg/kg50 | |
| 5-Ethyl-1-aza-3,7 dioxabicyclo[3.3.0]octane [OCT; CAS No.: 7747-35-5; MWt: 143.18 g/mol; formula: C7H13NO2] | 0.274 ± 0.49660 | 0.321 (20.95 mM) | - | LD50 oral, rat, >3600 mg/kg; LD50 dermal, rabbit, 1948 mg/kg53 | |
| Bronopol [BP; CAS No.: 52-51-7; MWt: 199.99 g/mol; formula: C3H6BrNO4] | 1.150 ± 0.63161 | 0.121 (5 mM) | Nonmutagenic§51 | LD50 oral, rat, 180 mg/kg51 | |
| 2-hydroxymethyl-2-nitro-1,3-propanediol [HNPD; CAS No.: 126-11-4; MWt: 151.12 g/mol; formula: C4H9NO5] | −0.115 ± 0.77062 | - | Nonmutagenic||52 | LD50 oral, rat, 1917 mg/kg; LD50 oral, mouse, 10,550 mg/kg62 |
Nonmutagenic: Ames, micronucleus assay.
Nonmutagenic: Ames, 100% sodium hydroxymethylglycinate; mouse micronucleus; rat hepatocyte/DNA repair assay; in vivo, in vitro rat hepatocyte UDS assay.
Nonmutagenic: Ames, salmonella, 55% DMDM, 0.001 to 5 μL/plate; salmonella/mammalian-microsome preincubation mutagenicity assay, salmonella, 2.0 μL/plate; mutagenic, L5178 TK ± mouse lymphoma assay, 0.01 to 1.0 μg/mL; L5178 TK ± mouse lymphoma assay, 0.006 to 0.2 μL/mL; chromosome aberrations assay, Chinese hamster ovary cells, 0.3 μL/mL.
Nonmutagenic: Ames, salmonella, with and without metabolic activation, dose not specified.
Nonmutagenic: Ames, salmonella with and without metabolic activation, 1000 μg/plate; chromosomal aberration.
Efficacy of Corneal Cross-Linking
The ability of five FARs (DAU, IMU, SMG, DMDM, OCT) to cross-link intact cadaveric rabbit cornea, a substrate for collagenous tissue, was assessed using an ex vivo TXL simulation setup. Cross-linking effects were measured using DSC, an assay method based on changes in thermal denaturation temperature (Tm). Our results indicate that two out of the seven FARs studied, DAU and SMG, are effective collagen cross-linking agents for the cornea with the epithelium left intact (in the ex vivo simulation setup) at 1/2max allowed concentrations. Diazolidinyl urea was effective at pH 8.5, and SMG was effective at both pH 8.5 and 7.4. This was evidenced by shifts in the thermal denaturation temperature of corneal tissue (Fig. 1). Sodium hydroxymethylglycinate pH 8.5 showed the greatest upward shift in Tm (ΔTm = 3.573 ± 0.578°C, P < 0.05) followed by DAU at pH 8.5 (ΔTm = 3.210 ± 0.742°C, P < 0.05). Sodium hydroxymethylglycinate also showed effective cross-linking at pH 7.4 (ΔTm = 2.281 ± 0.697°C, P < 0.05). Some inconsistencies in the shifts in Tm induced by SMG at pH 7.4, however, were noted, and a sample size of n = 8 was required to reach statistical significance. A negative shift in Tm on the order of ~0.5°C was observed for DAU at pH 7.4 and for IMU at both pH 8.5 and 7.4 (Fig. 1), but the shift was significant only for IMU at pH 8.5 (ΔTm = −0.69 ± 0.697°C, P < 0.05). The lack of effect under these conditions may reflect issues related to epithelial permeability since both DAU and IMU are significantly larger than SMG. Furthermore, an increase in Tm was observed for DMDM at both pH 8.5 and 7.4 (ΔTm = 2.04 ± 0.225°C and 2.13 ± 0.273°C, respectively) and for OCT at pH 8.5 (ΔTm = 1.10 ± 0.246°C). However, these observed increases in Tm were not statistically significant using paired controls, which included the contralateral eye for each sample. Rabbit cornea cross-linked using UVA-riboflavin (CXL) showed an increase in Tm comparable to values previously reported. In the present study, the ΔTm following CXL was 1.73 ± 0.487°C. We previously reported a ΔTi = 1.9°C,19 which was similar to that reported by Spoerl et al.34 at 2.5°C. Ti values indicate the onset of thermal shrinkage (Ts) temperature, and change in Ti (ΔTi) is another parameter widely used to measure extent of TXL. As previously reported, the CXL effect is relatively mild from a “thermal transition shifting” standpoint if one considers the potential magnitude of shifts in Tm that may be induced using chemical agents. Lastly, it is worth noting that corneal tissue remained clear to visual inspection using either chemical or photochemical cross-linking treatment.
Figure 1.
Corneal TXL potency of five FARs using 1/2max allowed concentrations (epi-on) versus CXL (epi-off). Cadaveric rabbit corneas with intact epithelia were cross-linked using the FARs DAU, IMU, SMG, DMDM, and OCT at the indicated concentrations in 0.1 M NaHCO3 buffer for 30 minutes. Control samples were treated identically with vehicle. A 0.1% riboflavin-5-phosphate solution in 1.1% hydroxypropyl methyl cellulose (HPMC, 15 centipoise) was used for CXL with the corneal epithelium removed. ΔTm indicates average shifts in the denaturation temperature of corneal tissue after TXL compared to the controls as measured by DSC. In this case, each experimental determination was paired with the contralateral cornea from the same cadaver head. Dark blue bars depict shifts at pH 8.5, whereas light blue bars depict shifts at pH 7.4. Error bars represent standard error. Asterisks indicate significant changes in Tm following TXL based on paired t-tests on data from at least two independent trials (P ≤ 0.05).
Efficacy of Scleral Cross-Linking
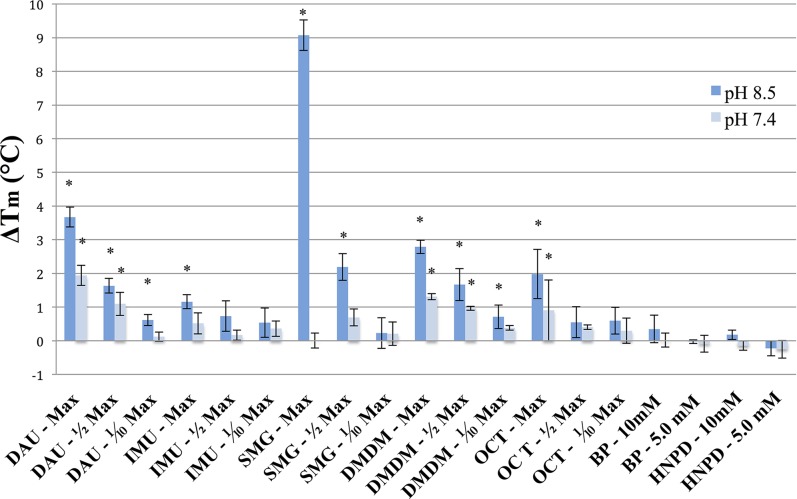
The results for scleral TXL are generally comparable to the results for corneal samples, although different methods of application were used (i.e., refreshing solution every 5 minutes for corneal experiments and not refreshing for scleral experiments). In this case, two additional FARs, BP and HNPD, were also tested. We found that SMG, DAU, and DMDM induced statistically significant cross-linking effects at pH 8.5 and 7.4 (with the exception of SMG at pH 7.4) at concentrations as low as 1/2max allowed. In addition, both a concentration- and pH-dependent effect was observed for the FARs (Fig. 2). A notable exception to the concentration-dependent effect is seen in the thermal denaturation data for SMG at pH 7.4 and 39.06 mM, where little change in Tm is observed (ΔTm = 0.007 ± 0.222°C, P = 0.493), although a dramatic upward shift is seen for the same concentration using a pH of 8.5 (ΔTm = 9.073 ± 0.450°C, P < 0.05). The reason for this difference is unclear since in general, upward shifts in Tm occur for most FARs, albeit consistently greater for pH 8.5 over 7.4. It should be noted that SMG is highly basic in unbuffered solution, requiring the addition of significant amounts of acid in order to achieve the targeted pH of 7.4. Thus, we speculate that the procedure for titrating the buffered SMG solution to pH 7.4 may have impacted the efficacy of TXL in this case. This phenomenon might also explain the inconsistencies in TXL efficacy experienced when intact cornea was cross-linked using SMG at pH 7.4, as mentioned above.
Figure 2.
Concentration and pH dependence of FAR-induced scleral TXL Porcine scleral tissue was cross-linked using three different concentrations of FAR solution in 0.1 M NaHCO3 buffer for 30 minutes. Control samples were treated identically with vehicle. ΔTm indicates average shifts in the denaturation temperature of scleral tissue after TXL compared to the control as measured by DSC. Dark blue bars depict shifts at pH 8.5, and light blue bars depict shifts at pH 7.4. Error bars represent standard error. Asterisks indicate significant changes in Tm following TXL based on nonpaired t-tests on data from three independent trials (P ≤ 0.05).
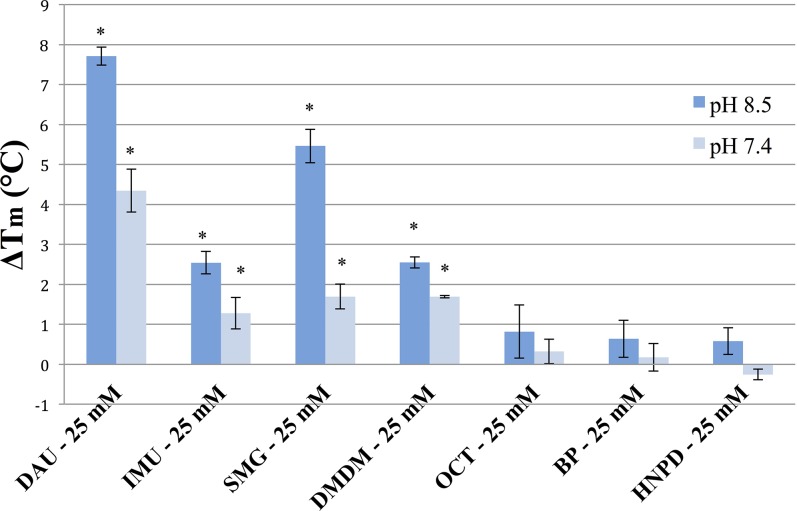
We also tested FARs at a concentration of 25 mM in order to directly compare the cross-linking “potency” of each FAR to the others. We chose a relatively high concentration for this comparison because we wanted to elicit a noticeable cross-linking effect using the BNAs HNPD and BP within 30 minutes. Diazolidinyl urea showed the greatest shifts in thermal denaturation temperature at 25 mM for both pH 8.5 and 7.4 (ΔTm = 7.713 ± 0.226°C and 4.347 ± 0.538°C, respectively, P < 0.05), followed by SMG (ΔTm = 5.463 ± 0.419°C and 1.697 ± 0.311°C, respectively, P < 0.05), DMDM (ΔTm = 2.550 ± 0.142°C and 1.693 ± 0.033°C, P < 0.05), and IMU (ΔTm = 2.543 ± 0.280°C and 1.280 ± 0.392°C, respectively, P < 0.05). OCT, BP, and HNPD exhibited shifts on the order of ~0.5°C for both pHs (with the exception of HNPD at pH 7.4, which had a negative ΔTm), but these shifts were not statistically significant (Fig. 2).
Evaluation of FAR Cytotoxicity
Planar cell culture experiments using HSFs were conducted to determine the toxicity thresholds of the FARs under study. The toxicity threshold was taken to be the highest concentration in mM at which all cells were alive following a 24-hour exposure to the FAR and a 48-hour recovery period. With the exception of BP, the toxicity threshold was found to lie between 0.1 and 1 mM for all FARs (Table 2). Bronopol was the most toxic to HSFs, with a toxicity threshold between 0.01 and 0.001 mM. These values for BP and HNPD were in agreement with those recently reported using the same toxicity testing apparatus.33
Table 2.
FAR Toxicity Thresholds for Human Skin Fibroblasts
|
Concentration, mM |
DAU |
IMU |
SMG |
DMDM |
OCT |
HNPD |
BP |
| 5 | Dead | Dead | Dead | Dead | Dead | Dead | Dead |
| 1 | Dead | Dead | Dead | Dead | Dead | Dead | Dead |
| 0.1 | Alive | Alive | Alive | Alive | Alive | Alive | Dead |
| 0.01 | Alive | Alive | Alive | Alive | Alive | Alive | Dead |
| 0.001 | Alive | Alive | Alive | Alive | Alive | Alive | Alive |
| Control | Alive | Alive | Alive | Alive | Alive | Alive | Alive |
Human skin fibroblasts (passage 2) were exposed to FARs for 24 hours followed by a 48-hour recovery in fresh cell media.
Discussion
In this study, we used both intact cornea and cut scleral tissue pieces to test the cross-linking efficacy of compounds known as FARs, comparing the effects against two higher-order nitroalcohols (HONAs), BP and HNPD.35 Three of the FARs were found to be significantly more effective as TXL agents when compared to the HONAs, showing both pH- and concentration-dependent effects. The FARs are a group of compounds commonly used as preservatives in cosmetics and PCPs and as fabric cross-linkers in the textile industry (i.e., for making wrinkle-free clothing) and include BP, which is a well-known compound. They are known to release formaldehyde in a pH- and concentration-dependent manner as determined by 13C nuclear magnetic resonance (NMR) equilibrium studies, in which formaldehyde release among FARs popularly used in cosmetics, including DAU, IMU, DMDM, and SMG, was compared.31
Formaldehyde releasers in commercial use include O- and N-formal compounds.31 An O-formal group is a formaldehyde entity linked to the rest of the compound via an oxygen atom. An N-formal group is a formaldehyde entity linked to the rest of the compound via a nitrogen atom and can be of two types: amide based (the nitrogen is a part of an amide) and amine based (the nitrogen is a part of an amine). The type of group attached to the N-formal group confers different release properties. Slower release occurs with the amide-based N-formals (such as DAU, IMU, and DMDM), which can act as formaldehyde reservoirs, whereas amine-based N-formals like SMG have been reported to decompose completely under alkaline conditions and max allowed concentration.31
Based on chemical structure alone, DAU should be the most effective cross-linking agent with the ability to release 4 mol formaldehyde (contains four N-formal groups), followed by HNPD (3 mol), with SMG being the least effective (1 mol). The amount of formaldehyde actually released in solution by each FAR, however, is not as easily predictable as evidenced by the pH- and concentration-dependent effects noted earlier. The release of formaldehyde is reported to be facilitated at acid pH for SMG, in contrast to the other FARs and nitroalcohols, which are facilitated by alkaline pH. Once released from FARs, formaldehyde can react in a number of ways, including reactions with starting material or polymerizing, which can occur under equilibrium conditions as reported by Emeis and colleagues.31 In addition, the availability of reactive substrates under nonequilibrium conditions (such as in the presence of tissue amines from cornea and sclera) can drive the reaction toward formaldehyde release. When used at max allowed concentration (0.5%) as in this study, formaldehyde release from SMG has been reported to be rapid at pH 8.5, which is consistent with its structure as an amine-based N-formal compound.31
We also compared chemical TXL using FARs with riboflavin-mediated photochemical collagen cross-linking (CXL), which is regarded as the “gold standard” of therapeutic corneal cross-linking. Our value for the increase in thermal denaturation temperature following CXL is slightly lower than the shift in the onset of thermal shrinkage (ΔTi) reported by Spoerl et al.34 following CXL of the anterior portion of porcine cornea: ΔTm = 1.733 ± 0.487°C versus ΔTi = 2.5°C (originally reported as ΔTi = 5°C but confirmed by DCP to be 2.5°C). We have previously reported a 1.9°C shift in Ti for porcine cornea cross-linked using the UVA-riboflavin method.19 Therefore, our values for ΔTm induced by CXL are similar to the shifts in Ti induced by CXL as reported previously even considering the differences in species used (i.e., rabbit versus porcine cornea).
Corneal epithelial permeability is another consideration that should be borne in mind. Our results are favorable since the ex vivo setup simulates conditions that would be encountered in a living system. Of particular interest is the fact that cross-linking effects were induced with the corneal epithelium intact, suggesting that some of these compounds may be able to pass through the epithelial barrier (i.e., SMG MWt = 104 Da). The ability to induce a cross-linking effect without the need for epithelial removal, if possible, would be a significant advantage over riboflavin-mediated collagen cross-linking (CXL). Differences in transepithelial permeability for IMU, for example, may explain the lack of cross-linking effect seen in the intact cornea (Fig. 1). Imidazolidinyl urea is the largest of the compounds tested at 388 Da, and its size may have hindered passage into the corneal stroma, accounting for the lack of effect in cornea, while positive cross-linking effects were observed for the same compound with cut scleral pieces where permeability was not hindered by an intact corneal epithelium (Figs. 2, 3). Molecular size is well known to affect transcorneal permeability, especially for hydrophilic compounds such as the ones under consideration here.36
Figure 3.
Comparison of scleral TXL potency of seven FARs. Porcine scleral tissue was cross-linked using FAR solution at 25 mM in 0.1 M NaHCO3 buffer for 30 minutes. Control samples were treated identically with vehicle. ΔTm indicates average shifts in the denaturation temperature of scleral tissue after TXL compared to the control as measured by DSC. Dark blue bars depict shifts at pH 8.5, and light blue bars depict shifts at pH 7.4. Error bars represent standard error. Asterisks indicate significant changes in Tm following TXL based on nonpaired t-tests from data on three independent trials (P ≤ 0.05).
Regarding thermal denaturation as an assay for TXL, several methods have been used previously to evaluate cross-linking changes intentionally induced in collagenous tissues by either chemical or photochemical means; these include mechanical testing (either uniaxial strip10 or inflation testing37), enzymatic digestion,27 gel electrophoresis,38 and thermal denaturation.39 We have previously used thermal denaturation (as thermal shrinkage temperature) as an assay measure of chemically and UVA-riboflavin-induced cross-linking of collagenous tissue.18 In this study, we evaluated TXL efficacy using an automated differential scanning calorimeter, which is an instrument that measures change in heat flow over time and can be used to determine the thermal transition (or denaturation) temperature (Tm) of a given substance. Thermal transition temperature is a concept familiar to the biomaterials industry where it has been used as a means to evaluate the efficacy of TXL for decades. Differential scanning calorimetry produces a denaturation curve, which depicts a major endotherm with the Tm value at its peak. In the case of collagenous tissue, the major endotherm reflects collagen denaturation, which involves triple helical uncoiling and tissue shortening. In addition to collagen cross-linking, it is also possible for proteoglycans to be modified in the TXL procedure since they contain potential reactive sites. However, this is not expected to alter or contribute to the thermal denaturation of collagen, since removal of proteoglycans has been shown not to alter the Tm of collagenous soft tissue.26
Differential scanning colorimetry has been used successfully in many tissue types including tendon,40 bone,41 cartilage,42 and skin,43 but there are few reports regarding cornea.28,44,45 An additional advantage of DSC is that tissue samples are hermetically sealed, preventing tissue dehydration, which can introduce experimental error into these measurements. Changes that can occur in the water content of tissue are particularly relevant in the case of cornea, which has a large capacity to swell and/or shrink. Finally, the ease of analyzing DSC data using the Pyris software adds to the effectiveness of using DSC for cross-link analysis.
In order to directly assess the toxicity of these chemicals, we conducted an in vitro cell toxicity experiment using HSFs and these FARs in studies similar to recently published work.33 The toxicity threshold of all FARs tested was determined to be below 1 mM with the exception of BP, which was the most toxic (toxicity threshold below 0.01 mM). Our past cell toxicity studies using HSFs indicated that genipin and glutaraldehyde both have toxic thresholds on par with BP.33 In the same study, we showed glyceraldehyde to be the least toxic cross-linking agent for HSFs, with a toxic threshold of 1 mM. Therefore, the toxicity of FARs lies between the toxicity of glutaraldehyde and glyceraldehyde, with glyceraldehyde being the least toxic. Here, we would like to point out that the cell toxicity thresholds determined are not designed to provide direct clinical information regarding potentially applicable concentrations, but rather as a means to compare toxicity between compounds.
Finally, for a word on safety, owing to their widespread use in cosmetics and by the textile industry, where workplace hazards are closely scrutinized, the FARs have been extensively tested in European safety studies by the Scientific Committee on Cosmetics and Non-Food Products32,46 following the commission of Cosmetic Products Directive 76/768/EC by the Council of the European Communities in 1976.47 The result of the Cosmetic Directive was a delineation of which FARs can appear in cosmetics and PCPs and at what concentrations. For this study, we adapted the maximum allowed concentrations of FARs as defined in the Cosmetic Directive since we believed that working within the maximum allowed value would be a good starting point in evaluating effects that could be induced in patients.
Keeping this in mind, the literature values for the dermal toxicity of FARs reported above (Table 1) should be viewed with some discretion, since the effect on skin might not translate to ocular tissues. Some data highlighting the effects of FARs on ocular tissues are present in the literature, though variations exist between studies, specifics of experimental design are lacking in some cases, and the reports are directed toward irritation rather than toxicity per se. Diazolidinyl urea, when administered in the conjunctival sac of albino rabbits at a concentration of 5% (solvent not specified), was reported to be nonirritating to ocular tissues over a seven-day period, without a water rinse.48 Imidazolidinyl urea was shown to be nonirritating to ocular tissues in aqueous solutions at concentrations of 5%, 10%, and 20% with a seven-day follow-up in albino rabbits.49 Albino rabbits treated with a 0.5% solution of DMDM (with and without water rinse) were reported to have minimal, transient irritation over a seven-day period.50
Other studies have indicated that a 2% solution of BP is irritating to rabbit eyes51 while aqueous solutions of 53.1% to 56.8% of HNPD showed only slight irritation in the same species.52 Data on the effect of OCT and SMG on ocular tissues are not readily available in the literature. Information from Material Safety Data Sheets (MSDS) indicates that OCT causes serious eye damage or irritation in rabbits,53 and SMG has shown a mildly irritating effect on rabbit eyes (dosage not specified).54 Interestingly, SMG has been used as a preservative in ophthalmic solutions including BLUgelA multidose artificial tears manufactured by SOOFT italia (Montegiorgio, Italy) at a concentration of 0.002% (0.157 mM),55 which suggests that SMG may be safe to use on ocular tissues. This concentration, however, is much lower than the highest concentration of SMG that we have proposed for therapeutic TXL (39.06 mM). Information from the literature thus generally supports the safety of FARs as cross-linking agents for the cornea and sclera. It is noted that studies more directly assessing the effect of FARs on the viability of ocular tissues are needed to better judge the therapeutic potential of the new TXL agents. This question will be more carefully addressed in future studies.
In conclusion, the present study has demonstrated a novel therapeutic application for FARs commonly employed in consumer PCPs. Two of these agents, DAU and SMG, have shown effective cross-linking abilities in intact cornea and cut scleral pieces as indicated by shifts in thermal denaturation temperature (Tm). In light of the current growing therapeutic cross-linking application in both the cornea and sclera, FARs may have potential in the treatment of diseases such as keratoconus and myopia. Continued screening of FARs from the compiled registry could lead to the identification of additional potent cross-linking agents.
Acknowledgments
Supported in part by Research to Prevent Blindness; National Institutes of Health National Center for Research Resources (NCRR) Grant UL1RR024156 and National Eye Institute (NEI) Grants P30 Y019007 and R01EY020495 (DCP); and the Bjorg & Stephen Olendorf Fund.
Disclosure: N. Babar, None; M. Kim, None; K. Cao, None; Y. Shimizu, None; S.-Y. Kim, None; A. Takaoka, None; S.L. Trokel, P; D.C. Paik, P
References
- 1. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003; 135: 620–627. [DOI] [PubMed] [Google Scholar]
- 2. Phillips JR, Khalaj M, McBrien NA. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000; 41: 2028–2034. [PubMed] [Google Scholar]
- 3. Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linking of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006; 32: 837–845. [DOI] [PubMed] [Google Scholar]
- 4. Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006; 17: 356–360. [DOI] [PubMed] [Google Scholar]
- 5. Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J Cataract Refract Surg. 2008; 34: 796–801. [DOI] [PubMed] [Google Scholar]
- 6. Wittig-Silva C, Whiting M, Lamoureux E, Lindsay RG, Sullivan LJ, Snibson GR. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008; 24: S720–S725. [DOI] [PubMed] [Google Scholar]
- 7. Vinciguerra P, Albe E, Trazza S, et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology. 2009; 116: 369–378. [DOI] [PubMed] [Google Scholar]
- 8. Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011; 37: 149–160. [DOI] [PubMed] [Google Scholar]
- 9. Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007; 33: 2035–2040. [DOI] [PubMed] [Google Scholar]
- 10. Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998; 66: 97–103. [DOI] [PubMed] [Google Scholar]
- 11. Wollensak G, Aurich H, Wirbelauer C, Pham DT. Potential use of riboflavin/UVA cross-linking in bullous keratopathy. Ophthalmic Res. 2009; 41: 114–117. [DOI] [PubMed] [Google Scholar]
- 12. Al-Sabai N, Koppen C, Tassignon MJ. UVA/riboflavin crosslinking as treatment for corneal melting. Bull Soc Belge Ophtalmol. 2010: 13–17. [PubMed] [Google Scholar]
- 13. Makdoumi K, Mortensen J, Crafoord S. Infectious keratitis treated with corneal crosslinking. Cornea. 2010; 29: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 14. Wollensak G, Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg. 2004; 30: 689–695. [DOI] [PubMed] [Google Scholar]
- 15. Wollensak G, Iomdina E, Dittert DD, Salamatina O, Stoltenburg G. Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol Scand. 2005; 83: 477–482. [DOI] [PubMed] [Google Scholar]
- 16. Danilov NA, Ignatieva NY, Iomdina EN, et al. Stabilization of scleral collagen by glycerol aldehyde cross-linking. Biochim Biophys Acta. 2008; 1780: 764–772. [DOI] [PubMed] [Google Scholar]
- 17. Avila MY, Navia JL. Effect of genipin collagen crosslinking on porcine corneas. J Cataract Refract Surg. 2010; 36: 659–664. [DOI] [PubMed] [Google Scholar]
- 18. Paik DC, Wen Q, Airiani S, Braunstein RE, Trokel SL. Aliphatic beta-nitro alcohols for non-enzymatic collagen cross-linking of scleral tissue. Exp Eye Res. 2008; 87: 279–285. [DOI] [PubMed] [Google Scholar]
- 19. Paik DC, Wen Q, Braunstein RE, Airiani S, Trokel SL. Initial studies using aliphatic beta-nitro alcohols for therapeutic corneal cross-linking. Invest Ophthalmol Vis Sci. 2009; 50: 1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Li Y, Kim M, Trokel SL, Turro NJ, Paik DC. Aliphatic beta-nitroalcohols for therapeutic corneoscleral cross-linking: chemical stability studies using 1H-NMR spectroscopy. Photochem Photobiol. 2014; 90: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Groot A, White IR, Flyvholm MA, Lensen G, Coenraads PJ. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. Part 2. Patch test relationship to formaldehyde contact allergy, experimental provocation tests, amount of formaldehyde released, and assessment of risk to consumers allergic to formaldehyde. Contact Dermatitis. 2010; 62: 18–31. [DOI] [PubMed] [Google Scholar]
- 22. de Groot AC, Flyvholm MA, Lensen G, Menne T, Coenraads PJ. Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers. Contact Dermatitis. 2009; 61: 63–85. [DOI] [PubMed] [Google Scholar]
- 23. Thomas K. The “No More Tears” shampoo, now with no formaldehyde. New York Times. January 18, 2014: A1. [Google Scholar]
- 24. de Groot AC, Le Coz CJ, Lensen GJ, Flyvholm MA, Maibach HI, Coenraads PJ. Formaldehyde-releasers: relationship to formaldehyde contact allergy. Part 2. Formaldehyde-releasers in clothes: durable press chemical finishes. Contact Dermatitis. 2010; 63: 1–9. [DOI] [PubMed] [Google Scholar]
- 25. Xu Y. Thermal stability of collagen triple helix. Methods Enzymol. 2009; 466: 211–232. [DOI] [PubMed] [Google Scholar]
- 26. Bailey AJ, Sims TJ, Avery NC, Miles CA. Chemistry of collagen cross-links: glucose-mediated covalent cross-linking of type-IV collagen in lens capsules. Biochem J. 1993; 296 (pt 2): 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sung HW, Liang IL, Chen CN, Huang RN, Liang HF. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J Biomed Mater Res. 2001; 55: 538–546. [DOI] [PubMed] [Google Scholar]
- 28. Sionkowska A. Thermal stability of UV-irradiated collagen in bovine lens capsules and in bovine cornea. J Photochem Photobiol B. 2005; 80: 87–92. [DOI] [PubMed] [Google Scholar]
- 29. Wiegand N, Vamhidy L, Kereskai L, Lorinczy D. Differential scanning calorimetric examination of the ruptured Achilles tendon in human. Thermochim Acta. 2010; 498: 7–10. [Google Scholar]
- 30. Louis KS, Siegel AC. Cell viability analysis using trypan blue: manual and automated methods. Methods Mol Biol. 2011; 740: 7–12. [DOI] [PubMed] [Google Scholar]
- 31. Emeis D, Anker W, Wittern KP. Quantitative 13C NMR spectroscopic studies on the equilibrium of formaldehyde with its releasing cosmetic preservatives. Anal Chem. 2007; 79: 2096–2100. [DOI] [PubMed] [Google Scholar]
- 32. The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. Opinion concerning the determination of certain formaldehyde releasers in cosmetic products. SCCNFP; 2002. Available at: http://ec.europa.eu/food/fs/sc/sccp/out188_en.pdf. Accessed September 15, 2013. [Google Scholar]
- 33. Kim M, Takaoka A, Hoang QV, Trokel SL, Paik DC. Pharmacologic alternatives to riboflavin photochemical corneal cross-linking: a comparison study of cell toxicity thresholds. Invest Ophthalmol Vis Sci. 2014; 55: 3247–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spoerl E, Wollensak G, Dittert DD, Seiler T. Thermomechanical behavior of collagen-cross-linked porcine cornea. Ophthalmologica. 2004; 218: 136–140. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Li Y, Kim M, Trokel SL, Turro NJ, Paik DC. Aliphatic beta-nitroalcohols for therapeutic corneoscleral cross-linking: chemical stability studies using 1H-NMR spectroscopy. Photochem Photobiol. 2014; 90: 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998; 87: 1479–1488. [DOI] [PubMed] [Google Scholar]
- 37. Wong FF, Lari DR, Schultz DS, Stewart JM. Whole globe inflation testing of exogenously crosslinked sclera using genipin and methylglyoxal. Exp Eye Res. 2012; 103: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simionescu A, Simionescu D, Deac R. Lysine-enhanced glutaraldehyde cross-linking of collagenous biomaterials. J Biomed Mater Res. 1991; 25: 1495–1505. [DOI] [PubMed] [Google Scholar]
- 39. Wollensak G. Thermomechanical stability of sclera after glyceraldehyde crosslinking. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 399–406. [DOI] [PubMed] [Google Scholar]
- 40. Miles CA, Avery NC, Rodin VV, Bailey AJ. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J Mol Biol. 2005; 346: 551–556. [DOI] [PubMed] [Google Scholar]
- 41. Lozano LF, Pena-Rico MA, Heredia A, et al. Thermal analysis study of human bone. J Mater Sci. 2003; 38: 4777–4782. [Google Scholar]
- 42. Than P, Vermes C, Schaffer B, Lorinczy D. Differential scanning calorimetric examination of the human hyaline cartilage - a preliminary study. Thermochim Acta. 2000; 346: 147–151. [Google Scholar]
- 43. McClain PE, Wiley ER. Differential scanning calorimeter studies of the thermal transitions of collagen. Implications on structure and stability. J Biol Chem. 1972; 247: 692–697. [PubMed] [Google Scholar]
- 44. Kampmeier J, Radt B, Birngruber R, Brinkmann R. Thermal and biomechanical parameters of porcine cornea. Cornea. 2000; 19: 355–363. [DOI] [PubMed] [Google Scholar]
- 45. Monti D, Chetoni P, Burgalassi S, Najarro M, Saettone MF. Increased corneal hydration induced by potential ocular penetration enhancers: assessment by differential scanning calorimetry (DSC) and by desiccation. Int J Pharm. 2002; 232: 139–147. [DOI] [PubMed] [Google Scholar]
- 46. The Scientific Committee on Consumer Safety's Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation. 7th revision. 2010. http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_s_004.pdf. Accessed September 15, 2013. [Google Scholar]
- 47. Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products. OJEC. 1976; 262: 169. [Google Scholar]
- 48. 7 Final report on the safety assessment of diazolidinyl urea. Int J Toxicol. 1990; 9: 229–245. [Google Scholar]
- 49. Cosmetic Ingredients Review Expert Panel final report of the safety assessment for imidazolidinyl urea. In: Cosmetic Ingredients: Their Safety Assessment. Forest Park South, IL: Pathotox Publishers; 1980: 133–146. [Google Scholar]
- 50. 1 Final report on the safety assessment of DMDM hydantoin. Int J Toxicol. 1988; 7: 245–277. [Google Scholar]
- 51. 5 Addendum to the final report on the safety assessment of 2-bromo-2-nitropropane-1,3-diol. Int J Toxicol. 1984; 3: 139–155. [Google Scholar]
- 52. United States Environmental Protection Agency. Reregistration eligibility decision (R.E.D): tris (hydroxymethyl)-nitromethane. Available at: http://www.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-083902_1-Sep-93.pdf. September, 1993. Accessed April 8, 2013. [Google Scholar]
- 53. Sigma-Aldrich. 5-Ethyl-1-aza-3,7-dioxabicyclo[3.3.0]octane [Material Safety Data Sheet]. Available at: http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=417793&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Finterface%3DAll%26term%3D5-Ethyl-1-aza-3%252C7-dioxabicyclo%255B3.3.0%255Doctane%26N%3D0%26mode%3Dmatch%2520partialmax%26focus%3Dproduct%26lang%3Den%26region%3DUS. Revised April 4, 2014. Accessed August 21, 2014. [Google Scholar]
- 54. International Specialty Products. Sodium hydroxymethylglycinate [Material Safety Data Sheet]. Available at: http://naturalingredient.org/Articles/msds_suttocide_A.pdf. Revised September 25, 2003. Accessed December 15, 2014. [Google Scholar]
- 55. Ghelardi E, Celandroni F, Gueye SA, Salvetti S, Campa M, Senesi S. Antimicrobial activity of a new preservative for multiuse ophthalmic solutions. J Ocul Pharmacol Ther. 2013; 29: 586–590. [DOI] [PubMed] [Google Scholar]
- 56. SciFinder, Chemical Abstracts Service; Columbus, OH; RN 78491-02-8 Available at: https://scifinder.cas.org. Accessed December 22, 2013. [Google Scholar]
- 57. SciFinder, Chemical Abstracts Service; Columbus, OH; RN 39236-46-9 Available at: https://scifinder.cas.org. Accessed December 22, 2013. [Google Scholar]
- 58. Sigma-Aldrich. Sodium hydroxymethylglycinate [Material Safety Data Sheet]. Available at: http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=CDS003712&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2Fcds003712%3Flang%3Den. Revised October 1, 2013. Accessed October 2, 2013. [Google Scholar]
- 59. SciFinder, Chemical Abstracts Service; Columbus, OH; RN 6440-58-0 Available at: https://scifinder.cas.org. Accessed December 22, 2013. [Google Scholar]
- 60. SciFinder, Chemical Abstracts Service; Columbus, OH; RN 7747-35-5 Available at: https://scifinder.cas.org. Accessed December 22, 2013. [Google Scholar]
- 61. SciFinder, Chemical Abstracts Service; Columbus, OH; RN 52-51-7 Available at: https://scifinder.cas.org. Accessed December 22, 2013. [Google Scholar]
- 62. SciFinder, Chemical Abstracts Service; Columbus, OH; RN 126-11-4 Available at: https://scifinder.cas.org. Accessed December 22, 2013. [Google Scholar]
- 63. Sigma-Aldrich. Diazolidinyl urea [Material Safety Data Sheet]. Available at: http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=D5146&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Finterface%3DAll%26term%3Ddiazolidinyl%2Burea%26N%3D0%26mode%3Dmatch%2520partialmax%26focus%3Dproduct%26lang%3Den%26region%3DUS. Revised October 30, 2012. Accessed September 15, 2013. [Google Scholar]
- 64. Sigma-Aldrich. Imidazolidinyl urea [Material Safety Data Sheet]. Available at: http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=I5133&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2Fi5133%3Flang%3Den. Revised October 10, 2012. Accessed September 15, 2013. [Google Scholar]