Abstract
Purpose.
Dynamic color and brightness adaptation are crucial for visual functioning. The effects of glaucoma on retinal ganglion cells (RGCs) could compromise these functions. We have previously used slow dynamic changes of light at moderate intensities to measure the speed and magnitude of subtractive adaptation in RGCs. We used the same procedure to test if RGC abnormalities cause slower and weaker adaptation for patients with glaucoma when compared to age-similar controls. We assessed adaptation deficits in specific classes of RGCs by testing along the three cardinal color axes that isolate konio, parvo, and magno RGCs.
Methods.
For one eye each of 10 primary open-angle glaucoma patients and their age-similar controls, we measured the speed and magnitude of adapting to 1/32 Hz color modulations along the three cardinal axes, at central fixation and 8° superior, inferior, nasal, and temporal to fixation.
Results.
In all 15 comparisons (5 locations × 3 color axes), average adaptation was slower and weaker for glaucoma patients than for controls. Adaptation developed slower at central targets than at 8° eccentricities for controls, but not for patients. Adaptation speed and magnitude differed between affected and control eyes even at retinal locations showing no visual field loss with clinical perimetry.
Conclusions.
Neural adaptation is weaker in glaucoma patients for all three classes of RGCs. Since adaptation abnormalities are manifested even at retinal locations not exhibiting a visual field loss, this novel form of assessment may offer a functional insight into glaucoma and an early diagnosis tool.
Keywords: glaucoma, adaptation, ganglion cells
Adaptation deficits caused by glaucoma were identified by a procedure that targets each of the three types of retinal ganglion cells.
Introduction
Adapting to changing brightness and color, especially of the ambient illumination, is important for functioning effectively in the world.1,2 Neurodegenerative damage to the visual system could be expected to impair neural mechanisms of adaptation. Glaucoma is a neurodegenerative retinal disorder that is expected to affect 80 million people by the end of this decade.3 It is characterized clinically by a characteristic optic neuropathy. Morphological studies have shown that retinal ganglion cells (RGCs) are the most affected cell type and progressively degenerate over the course of the disease.3 Consequently, there is a broad concerted effort to find noninvasive measures that can detect early RGC compromise.4,5 Zaidi et al.6 used psychophysics and in vivo single-cell recordings to show that RGCs adapt in a subtractive fashion to slow changes in color and brightness at light intensities considerably below photoreceptor bleaching levels, providing the locus for color afterimages and for discounting changes in ambient illumination. Thus, it seemed natural to ask whether patients with glaucoma exhibit deficits in adaptation due to RGC dysfunction,7–11 possibly even before clinical measures, such as visual field tests, reveal loss of RGC function. If this is true, we could gain an improved understanding of functional difficulties faced by glaucoma patients,12 and develop an early screening tool for diagnosis.
Early histomorphometric evidence suggested that glaucoma preferentially affected the larger parasol RGCs that project to the magno layers in the LGN,13,14 but beginning with Greenstein et al.,15 accumulating psychophysical and anatomical evidence in human clinical glaucoma and monkey experimental glaucoma has shown that all three classes of RGCs are affected.16–22 Our electrophysiological results revealed that all three primary classes of primate RGCs (M, P, and K cells) exhibit subtractive adaption with much slower time-constants than those expected of photoreceptors.6 Hence, we assessed whether any specific adaptation abnormalities would suggest preferential degeneration of specific classes of RGCs by testing along the three cardinal color directions (S or “Blue-Yellow,” L-M or “Red-Green,” L+M+S or “Light-Dark”) that we have shown previously to isolate the three classes of primate RGCs.23
To isolate functional abnormalities in all three classes of ganglion cells, we used our new method for measuring RGC adaptation.6 Beginning and ending at mid-gray, sinusoidal half-cycles slowly modulated the colors of two halves of a disk to opposite ends of one of the cardinal axes. Observers perceived the difference between the two colors as first increasing and then decreasing to identity, followed by a negative afterimage of increasing and then decreasing differences between the complementary colors in reversed locations. Due to ganglion cell adaptation, the identity point preceded the end of the physical modulation, so the physical contrast at that instant (nulling contrast) provided an estimate of the magnitude of adaptation.6 The adapting stimulus, thus, was also the measuring stimulus, which is different from procedures where the measuring probe can perturb the effect of the adaptation stimulus. The stimulus was viewed either at central fixation or 8° superior, inferior, nasal, and temporal to fixation. Across all 15 conditions (5 locations × 3 color axes), identity points for the affected eyes of 10 primary open-angle glaucoma (POAG) patients showed slower and weaker adaptation than the eyes of 10 age-similar controls. Consequently, neural adaptation is weaker in glaucoma patients for all three classes of RGCs.
Methods
Stimuli and Procedures
We used stimuli similar to Zaidi et al.,6 and Bachy and Zaidi.24 Well-established methods25 were used to produce modulations around a mid-gray (56.59 cd/m2) along the three cardinal color axes that isolate responses of primate RGCs.23 On a cathode ray tube (CRT) at a distance of 83 cm, a central gray disk subtending 4° was divided into two halves. Sinusoidal half-cycles at a frequency of 1/32 Hz slowly modulated the colors of the two hemi-disks to opposite ends of a color axis over 16 seconds, for example, one-half changed gray > purple > gray, while the other changed gray > lime-green > gray in the complementary color direction (Fig. 1). A second circle subtending 2° was presented as a clock-face either in the center, superimposed on the stimulus, or at 8° peripheral from the stimulus, to the top, bottom, left, or right. In this manner the same stimulus in the center of the screen was seen by the observer at either the center of the fovea, or at the four 8° peripheral retinal locations. Observers were instructed to fixate on the central dot of the clock, irrespective of its location. The movement of the clock hand began 12 seconds after the beginning of each 16-second stimulus presentation. When the presentation was finished, the clock hand was pointing at 12 o'clock. Each observer viewed five blocks, each of which contained the five fixation point locations for each of the three cardinal color directions, making for 15 trials per block in randomized order, and 75 total trials per observer.
Figure 1.
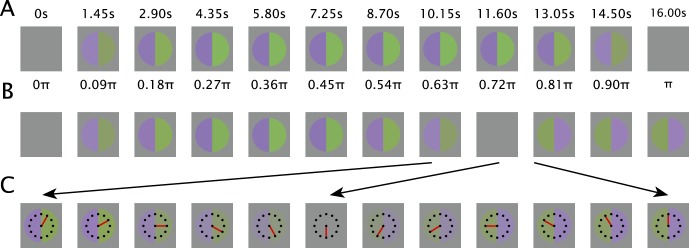
Psychophysical procedure. (A) Half cycle of sinusoidal stimulus modulation (1/32 Hz presented at 88 frames/sec depicted for this figure at 1.45 seconds and 0.09 π radian intervals). (B) Approximate percepts corresponding to the stage of stimulus modulation immediately above. The two halves reach perceptual identity before they become physically identical. The afterimage lasts for a significant period of time after the cessation of the stimulus modulation. (C) Segment of trial around the point of identity expanded to show how the clock face is used to make the measurement.
Observers were instructed to fixate on the dot at the center of the clock, while attending to the colored disk in the center of the screen. Perceptually, the two halves of the disk began as gray, gradually increasing in color/brightness contrast, and then decreasing to appear equally gray. This was followed by a negative afterimage, consisting of an apparent reversal of colors, after which the colors would again fade and appear equally gray. For example, if one-half changed gray > violet > gray, while the other changed gray > lime-green > gray in the complementary color direction, the percepts of the hemi-disks initially followed the stimulus but then accelerated past it, reaching gray before the stimulus physically did, and then continuing in the opposite directions to negative afterimages; for example, when the physical modulations returned to gray, the half modulated through violet appeared lime green and the half modulated through lime green appeared violet. The afterimage persisted after the cessation of the stimulus modulation and gradually faded to gray. Observers were instructed to press a button at the two times that the halves appeared identically gray. In other words, the two button presses occurred at the beginning and end of the afterimage, providing an estimate of afterimage duration and ensuring that each trial started after the previous trial's afterimage had faded. For the first button press, the observers were requested to attend to the time on the clock and to report this time at the end of the trial. We refer to the time on the clock reported by the observers as the “identity point,” which represents the time at which the neural signals are equated by the visual system despite the physical difference between the two hemi-disks. At the identity point, the physical difference between the two halves of the disk is called the “nulled contrast,” and provides a direct estimate of adaptation magnitude.
Equipment and Apparatus
Stimuli were displayed on a 20-inch Mitsubishi DiamondPro 2070SB CRT monitor (Mitsubishi Corp., Tokyo, Japan), which was driven by a Bits++ (CRS Ltd., Rochester, UK). The Bits++ was connected to a GeForce GTS250 graphics card (NVidia, Santa Clara, CA, USA) and provided 14-bit color resolution on each of the monitor's guns. The monitor resolution was set to 1280 × 960 and it was driven at a refresh rate of 88 Hz. The stimuli were written in Matlab (Mathworks, Natick, MA, USA) via the Psychtoolbox package on a computer running Windows XP Pro SP3 (Microsoft Corp., Redmond, WA, USA). The computer had an Intel Core 2 Duo E8400 CPU, running at 3.0 GHz, and 3.5Gb RAM. The age of our patients precluded them from viewing a fixation target without optical correction (presbyopia), so observers viewed the monitor through a combination of correcting spectacles lenses mounted in a lens holder.
Observers
Patients and controls were recruited from the University Eye Center clinics at the SUNY College of Optometry. All had prior experience with psychophysical testing. We tested one eye in each of 20 observers: 10 patients with stable POAG and 10 age-similar controls. All observers were screened for color vision defects with HRR Pseudo-isochromatic Plates. To meet the diagnostic criteria for POAG, patients were required to have open anterior chamber angles, glaucomatous field loss, glaucomatous optic neuropathy, and be under active care of a treating clinician. Patient clinical field data are summarized in Table 1. Each patient had reliable visual fields (fixation losses < 10%, false-positive < 10%, and false-negative < 10%). Each patient also had at least two repeatable contiguous points with reduced sensitivity (P < 0.01) within the central 10° of fixation. Mean deviations ranged from −1.79 to −22.21, pattern SDs between 2.12 and 14.00. In all but one instance, the glaucoma hemifield test was outside of normal limits (ONL).
Table 1.
Visual Field Characteristics of the POAG Subjects
|
POAG ID |
MD |
P < |
PSD |
P < |
GHT |
| 1 | −12.5 | 0.005 | 14 | 0.005 | ONL |
| 2 | −6.38 | 0.005 | 7.05 | 0.005 | ONL |
| 3 | −2.16 | 0.05 | 2.12 | 0.05 | ONL |
| 4 | −2.19 | 0.05 | 5 | 0.005 | ONL |
| 5 | −1.79 | 0.1 | 2.66 | 0.02 | Borderline |
| 6 | −3.23 | 0.02 | 3.91 | 0.005 | ONL |
| 7 | −2.57 | 0.05 | 3.53 | 0.01 | ONL |
| 8 | −10.63 | 0.005 | 8.96 | 0.005 | ONL |
| 9 | −7.23 | 0.005 | 4.09 | 0.005 | ONL |
| 10 | −22.21 | 0.005 | 5 | 0.005 | ONL |
MD, mean deviation; PSD, pattern standard deviation; GHT, glaucoma hemifield test; ONL, outside of normal limits.
To determine if there were measurable sensitivity differences between patients and age-similar controls in regions of the visual field which conventional perimetric analyses failed to identify, Table 2 shows the mean sensitivity (dB) of the 10 most sensitive points in each observer's visual field, a measure of diffuse loss across the visual field.26 Sensitivities of the 10 most sensitive points were lower for patients than age-similar controls by 2.5 dB on average, suggestive of diffuse losses caused by glaucoma.5,26
Table 2.
Age and Sensitivity (dB) of the 10 Most Sensitive Visual Fields Points of POAG Patients and Their Age-Similar Controls
|
POAG ID |
POAG Age |
POAG Mean, dB |
Control Mean, dB |
Control Age |
Control ID |
| 1 | 56 | 27.7 | 33.9 | 55 | 1c |
| 2 | 59 | 30.7 | 33.8 | 59 | 2c |
| 3 | 65 | 31.2 | 32.4 | 66 | 3c |
| 4 | 69 | 31.6 | 32.4 | 70 | 4c |
| 5 | 71 | 28.6 | 30.8 | 73 | 5c |
| 6 | 73 | 32.2 | 33.5 | 74 | 6c |
| 7 | 77 | 27.3 | 31.3 | 74 | 7c |
| 8 | 78 | 31.1 | 32.2 | 74 | 8c |
| 9 | 79 | 30.2 | 33.5 | 79 | 9c |
| 10 | 84 | 26.5 | 29.7 | 81 | 10c |
| Mean | Overall mean | Overall mean | Mean | ||
| 71.1 | 29.7 | 32.4 | 70.5 | ||
| SD | SD | SD | SD | ||
| 9.01 | 2.01 | 1.4 | 8.3 |
Inclusion and Exclusion Criteria
Common inclusion criteria for both groups were best corrected visual acuity of 20/20 or better (20/30 over age 70), spherical equivalent within −6 to +2 diopters (D), cylinder correction within 3 D, clear ocular media, and absence of known eye disease during a comprehensive eye examination within 2 years (except for glaucoma in the patient group). An additional inclusion criterion was IOP < 21 mm Hg for controls, and < 30 mm Hg for patients. Patients had IOP ranging from 12 to 21 mm Hg with an average of 16.5.
Common exclusion criteria for both groups were ocular or systemic disease known to affect retinal sensitivity (e.g., diabetic retinopathy, prior vein occlusion, macular degeneration), except for glaucoma in the patient group; history of intraocular surgery (except for uncomplicated cataract surgery more than a year before enrollment, or glaucoma surgery in the patient group); and use of medications known to affect retinal function (e.g., hydroxychloroquine). An additional exclusion criterion for controls was a first-degree relative with glaucoma.
This study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board at SUNY College of Optometry. Informed consent was obtained from each participant before testing began, after explaining the procedures and goals of the study.
Each session consisted of informed consent, visual acuity, contrast sensitivity, and color vision measures, in addition to explaining the protocol, setting the patient in the device, and pretesting trial runs, followed by data collection. The total session time was one hour. Typical data collection time varied between 35 and 40 minutes.
Results
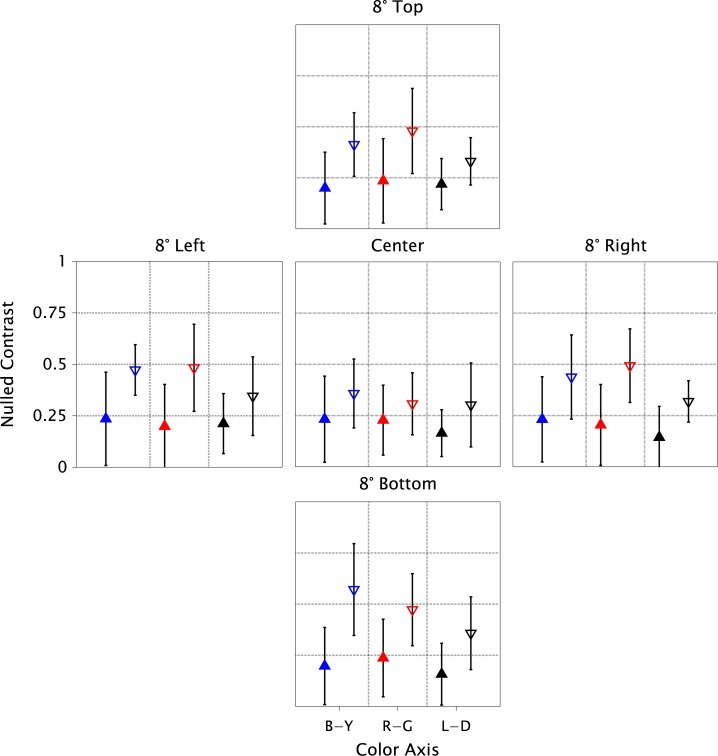
For each location and color axis, Figure 2 shows the adaptation magnitude measured as physical contrast at the identity point (Nulled-Contrast) for each color axis, averaged over the group of patients, and paired with the average for the group of age-similar controls. A smaller Nulled-Contrast represents weaker and slower adaptation. The differences in each comparison were small, but in 15 of 15 comparisons, adaptation was weaker and slower for the glaucoma group. If there were no actual difference in adaptation between the groups, the chance of this happening would be 1/32,768 (calculated from the binomial cumulative distribution function with P = 0.5), providing strong evidence against the assumption of no difference. In addition, the results showed that controls adapt slower for central stimuli than for peripheral presentations. This is the classic Troxler effect and has been shown to be due to the effects of fixational eye-movements causing transient responses in ganglion cells.24 An interesting aspect of the results is that the foveal slowing does not happen for glaucoma patients, so that the difference between the groups is reduced. The cause remains to be investigated systematically, but since detection thresholds for all 3 color axes have been shown to be elevated in the fovea of glaucoma patients,15 it is possible that compromised macular ganglion cells do not generate the normal transients from miniature eye movements that are required to improve sensitivity to low temporal frequencies.27 The peripheral results, being less affected by eye movements, may thus provide better comparisons of neuronal adaptation. To support our inferences, we also ran an ANOVA on the Nulled-Contrasts (Table 3). The marginal effect of Group (Patients versus Controls) was highly significant (P < 2e-16). There was a small significant effect of color axis, due to adaptation being weaker for the L-D axis than the other two, consistent with previous results.6 The effects of retinal location and all interactions were nonsignificant.
Figure 2.
Effect of glaucoma on retinal adaptation (groups): Mean Nulled-Contrast of patient group (filled triangles pointing up) and age-similar control group (empty triangles pointing down). Symbol colors: blue, red, and black represent Blue-Yellow, Red-Green, and Light-Dark, respectively. Panels are labeled by retinal locations. Error bars depict SEMs.
Table 3.
ANOVA Results: Nulled-Contrast Tested as a Linear Function of Retinal Location, Color Axis, Patient Versus Control Groups, and the Interactions Between the Independent Variables
|
Factor |
DF |
SumSq |
MeanSq |
F Value |
Prob |
| Location | 4 | 0.1638 | 0.04096 | 1.2830 | 0.27695 |
| Color | 2 | 0.4189 | 0.20945 | 6.5613 | 0.00165 |
| Group | 1 | 3.0965 | 3.09646 | 96.9992 | <2e-16 |
| Location × color | 8 | 0.0942 | 0.01178 | 0.3689 | 0.93637 |
| Location × group | 4 | 0.1977 | 0.04943 | 1.5485 | 0.18847 |
| Color × group | 2 | 0.1007 | 0.05034 | 1.5771 | 0.20847 |
| Location × color × group | 8 | 0.1359 | 0.01699 | 0.5321 | 0.83195 |
| Residuals | 270 | 8.6191 | 0.03192 |
The patient versus controls difference is highly significant.
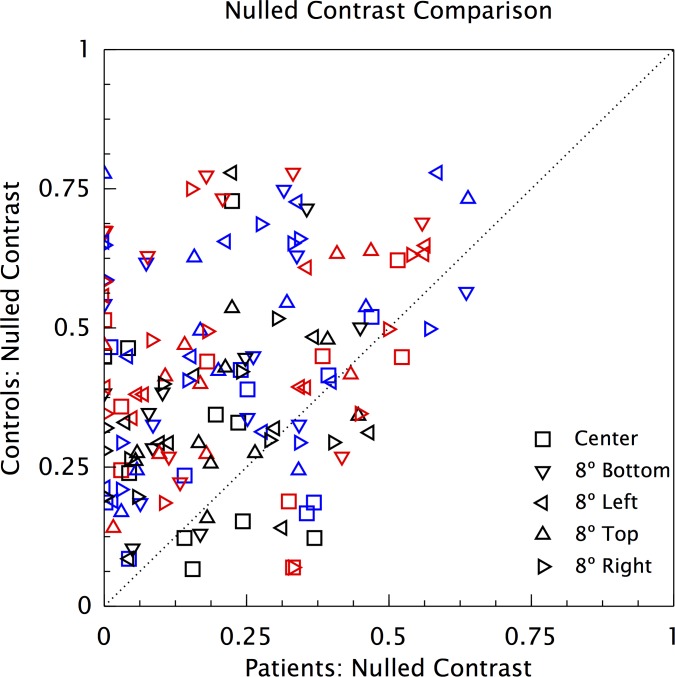
To visualize the individual data points, we have plotted the mean Nulled-Contrast of each patient for each color axis and retinal location against the mean of the age-similar control (Fig. 3). There are 150 points (10 pairs of observers × 3 color axes × 5 locations), and only 25 are below the unit diagonal, indicating generally weaker adaptation for the patients. If there were no actual difference in adaptation between each patient and the age-similar control, the chance of this happening, calculated from the binomial cumulative distribution function, is 1.7 × 10−17, providing strong evidence against the assumption of no difference. This is despite the almost equal distribution of the foveal comparison results around the unit diagonal, a result that is in concordance with the comparison of the group means in Figure 2.
Figure 3.
Effect of glaucoma on retinal adaptation (Individuals). Mean Nulled-Contrast of each patient for each color axis, plotted against the corresponding mean for the age-similar control. Symbol colors: blue, red, and black represent Blue-Yellow, Red-Green, and Light-Dark stimuli, respectively.
Reported afterimage durations were much more variable than reported identity points. It was not possible to establish a difference between patients and controls due to the high variance. The reports did demonstrate that all patients saw prolonged afterimages, providing confirmation that they saw the stimuli well and adapted to them.
Discussion
In our observers, the presence of adaptation abnormalities effectively differentiated POAG patients from their age-similar controls, especially for peripheral locations (Fig. 3). There are a few earlier psychophysical reports of adaptation deficits caused by glaucoma,7–11 but the present results have the advantage of being based on a psychophysical method for targeting ganglion cell function that was validated by single-cell electrophysiology recordings. Our results also indicate that all three classes of RGC are affected in the presence of glaucomatous damage and that no one type is preferentially affected. This finding is consistent with a body of recent experimental and clinical evidence.15–22 There are sporadic anecdotal accounts of glaucoma patients reporting trouble with visual adaptation, but in light of our results, it will be worthwhile to systematically investigate functional consequences for glaucoma patients, and even glaucoma suspects, of deficits in slow ganglion cell adaptation. These consequences may include a lack of color constancy across changing illumination.28
We are currently exploring the possibility that adaptation deficits are present before clinical visual field losses, in a longitudinal study of glaucoma suspects that includes 10-2 visual field measurements. As part of the clinical evaluation, the patients in this study were tested using standard 24-2 visual perimetry, which does not provide an ideal comparison to the adaptation results. Test points on the 24-2 are separated by 6°, and only 12 points are located within the central 10° of the visual field. Consequently, the circumference of the 4° central disk was 2.4° from the closest test point on the visual field (in 10-2 perimetry there would be 4 points overlapping the disc in this location). The remaining 4° disks were all 1.6° away from the closest test points on the 24-2 visual field. Therefore, none of the disks had a visual field point within their boundaries (assuming perfect fixation stability). That said, repeatable contiguous sensitivity deficits that are adjacent to the disk locations and significant at P < 0.1 or less, cannot be ignored. Table 4 shows the number of adjacent visual field test points at or below the P < 0.1 level within 2° of disk location. If we extend this to 2.5°, then the central locations also would include areas of measured loss of sensitivity. Of the 16 locations revealing no clinically measured loss (represented as zeros in Table 4), the patient's adaptation was faster than the age-similar controls in 42/48 of the adaptation measurements. A much larger data set is needed, but these limited results definitely suggest that RGC adaptation may be compromised in a retinal location before clinical measurements reveal a visual field loss.
Table 4.
Number of Adjacent Visual Field Test Points at or Below the P < 0.1 Level Within 2° of Adaptation Disk Location (Within 2.5° Central) for POAG Patients
|
Patient |
Up |
Down |
Left |
Right |
Center |
| 1 | 2 | 0 | 2 | 1 | 1 |
| 2 | 1 | 2 | 0 | 2 | 1 |
| 3 | 2 | 4 | 4 | 1 | 3 |
| 4 | 1 | 0 | 0 | 1 | 0 |
| 5 | 2 | 1 | 2 | 2 | 3 |
| 6 | 0 | 2 | 0 | 0 | 0 |
| 7 | 0 | 2 | 2 | 0 | 0 |
| 8 | 2 | 3 | 2 | 2 | 4 |
| 9 | 2 | 0 | 2 | 1 | 1 |
| 10 | 1 | 0 | 0 | 1 | 0 |
The testing methods in this study were suitable for a clinical population, but much needs to be done before translation into a clinical procedure is possible. First, all observers in the present study were experienced with functional testing with clinical perimetry, so we intend to investigate the appropriateness of these testing procedures in a more naïve patient population. Additionally, the duration of each session is longer than what would likely be acceptable as a clinical procedure, so optimizing the method to reduce test time will be important. The most common form of functional assessment in glaucoma, clinical perimetry (visual fields) is a lengthy procedure with high test–retest variability. It remains to be seen whether adaptation testing has less test–retest variability over a broad spectrum of stages of glaucoma, so that it can be a viable supplement to visual field testing. We intend to explore the usefulness of the method in differential diagnosis of patients classified as glaucoma suspects and in monitoring progression of glaucoma suspects and patients.
Retinal ganglion cells are the most affected cell type in glaucoma and progressively degenerate over the course of the disease. The RGC axons exit the eye and enter the optic nerve by passing through the optic nerve head (ONH), which is an important site of initial damage. Higher IOP is an important risk factor for glaucoma, but the molecular links between elevated IOP and axon damage in the ONH have not yet been identified.3 Recent studies in experimental glaucoma provide evidence that RGCs undergo morphological changes before cell death, as cell volume is reduced in surviving cells with corresponding reductions in the size of the axon and dendritic tree.3 It also is likely that widespread changes in the retinal ganglion cell population precede cell death and affect the physiological behavior of these cells.29 The results in this study show that the cell machinery that performs important adaptation functions may be compromised fairly early. Consequently, measures of adaptation may provide an early indicator for the disease, and an insight into the consequences of the disease for daily functioning.
Acknowledgments
Supported by National Institutes of Health (Bethesda, MD, USA) Grants EY007556, EY013312, EY007716, and T35EY020481.
Disclosure: M. Dul, None; R. Ennis, None; S. Radner, None; B. Lee, None; Q. Zaidi, None
References
- 1. Zaidi Q. The role of adaptation in color constancy. In: CW Clifford, Rhodes G. eds Fitting the Mind to the World: Adaptation and After-Effects in High-Level Vision. 1st ed. Oxford, UK: Oxford University Press; 2005; 2: 103–131. [Google Scholar]
- 2. Webster MA. Evolving concepts of sensory adaptation. F1000 Biol Rep. 2012; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci. 2012; 35: 153–179. [DOI] [PubMed] [Google Scholar]
- 4. Sharma P, Sample PA, Zangwill LM, Schuman JS. Diagnostic tools for glaucoma detection and management. Surv Ophthalmol. 2008; 53: S17–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hood DC, Raza AS, de Moraes CGV, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013; 32: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaidi Q, Ennis R, Cao D, Lee B. Neural locus of color afterimages. Curr Biol. 2012; 22: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eisner A, Samples JR, Campbell HM, Cioffi GA. Foveal adaptation abnormalities in early glaucoma. J Opt Soc Am A Opt Image Sci Vis. 1995; 12: 2318–2328. [DOI] [PubMed] [Google Scholar]
- 8. McKendrick AM, Badcock DR, Morgan WH. Psychophysical measurement of neural adaptation abnormalities in magnocellular and parvocellular pathways in glaucoma. Invest Ophthalmol Vis Sci. 2004; 45: 1846–1853. [DOI] [PubMed] [Google Scholar]
- 9. Lek JJ, Vingrys AJ, McKendrick AM. Rapid contrast adaptation in glaucoma and ageing. Invest Ophthalmol Vis Sci. 2014; 55: 3171–3178. [DOI] [PubMed] [Google Scholar]
- 10. McKendrick AM, Sampson GP, Walland MJ, Badcock DR. Impairments of contrast discrimination and contrast adaptation in glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 920–927. [DOI] [PubMed] [Google Scholar]
- 11. Sun H, Swanson WH, Arvidson B, Dul MW. Assessment of contrast gain signature in inferred magnocellular and parvo- cellular pathways in patients with glaucoma. Vision Res. 2008; 48: 2633–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson P, Aspinall P, Papasouliotis O, Worton B, O'Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma. 2003; 12: 139–150. [DOI] [PubMed] [Google Scholar]
- 13. Quigley HA, Sanchez RM, Dunkelberger GR, L. 'Hernault NL, Baginski TA Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987; 28; 913–920. [PubMed] [Google Scholar]
- 14. Glovinsky Y, Quigley HA, Dunkelberger GR. Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci. 1991; 32; 484–491. [PubMed] [Google Scholar]
- 15. Greenstein V, Halevy D, Zaidi Q, Koeng KL, Ritch RH. Chromatic luminance systems deficits in glaucoma. Vision Res. 1996; 36: 621–629. [DOI] [PubMed] [Google Scholar]
- 16. Greenstein VC, Seliger S, Zemon V, Ritch R. Visual evoked potential assessment of the effects of glaucoma on visual subsystems. Vision Res. 1998; 38: 1901–1911. [DOI] [PubMed] [Google Scholar]
- 17. Alvarez SL, Pierce GE, Vingrys AJ, Benes SC, Weber PA, King-Smith PE. Comparison of red-green, blue-yellow and achromatic losses in glaucoma. Vision Res. 1997; 37: 2295–2301. [DOI] [PubMed] [Google Scholar]
- 18. Osborne NN, Wood JPM, Chindlow JHB, Melena J, Nash MS. Ganglion cell death in glaucoma: what do we really know? Br J Ophthalmol. 1999; 83: 980–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morgan JE, Hideya U, Caprioli J. Retinal ganglion cell death in experimental glaucoma. Br J Ophthalmol. 2000; 84: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vickers JC, Schumer RA, Podos SM, Wang RF, Riederer BM, Morisson JH. Differential vulnerability of neurochemically identified subpopulation of retinal neurons in a monkey model of glaucoma. Brain Res. 1995; 680: 23–35. [DOI] [PubMed] [Google Scholar]
- 21. Yucel YH, Zhang Q, Gupta N, Kaufman PL, Weinreb RN. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000; 118: 378–384. [DOI] [PubMed] [Google Scholar]
- 22. Pearson P, Swanson WH, Fellman RL. Chromatic and achromatic defects in patients with progressing glaucoma. Vision Res. 2001; 41: 1215–1227. [DOI] [PubMed] [Google Scholar]
- 23. Sun H, Smithson HE, Zaidi Q, Lee BB. Specificity of cone inputs to macaque retinal ganglion cells. J Neurophysiol. 2006; 95: 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bachy R, Zaidi Q. Factors governing the speed of color adaptation in foveal versus peripheral vision. J Opt Soc Am A Opt Image Sci Vis. 2014; 31: A220–A225. [DOI] [PubMed] [Google Scholar]
- 25. Zaidi Q, Halevy D. Visual mechanisms that signal the direction of color changes. Vision Res. 1993; 33: 1037–1051. [DOI] [PubMed] [Google Scholar]
- 26. Henson DB, Artes PH, Chauhan BC. Diffuse loss of sensitivity in early glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 3147–3151. [PubMed] [Google Scholar]
- 27. Ennis R, Lee B, Zaidi Q. Eye-movements and the neural basis of context effects on visual sensitivity. J Neurosci. 2014; 34: 8119–8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smithson H, Zaidi Q. Color constancy in context: roles for local adaptation and levels of reference. J Vis. 2004; 4: 693–710. [DOI] [PubMed] [Google Scholar]
- 29. Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002; 11: 365–370. [DOI] [PubMed] [Google Scholar]