Abstract
Background: This study was conducted to assess self-reported prevalence and management of lower urinary tract symptoms (LUTS), along with drivers of treatment seeking, among patients with multiple sclerosis (MS).
Methods: An online, cross-sectional survey was administered to US-residing participants with self-reported MS to assess presence of LUTS, including urinary incontinence (UI). Participants experiencing LUTS were asked additional questions related to management and current therapies. Multivariate logistic regression identified drivers of treatment-seeking behavior.
Results: A total of 1052 participants completed the survey; 1047 were included in the analysis. Nine hundred sixty-six participants (92%) reported at least one LUTS, the most common being post-micturition dribble (64.9%), urinary urgency (61.7%), and a feeling of incomplete emptying (60.7%). Eight hundred twenty-six (79%) reported having some type of UI. Of those with any type of LUTS, 70% (n = 680) had previously discussed urinary symptoms with a health-care provider (HCP), while only 32% (n = 311) had seen an HCP in the past year. Logistic regression found urgency (odds ratio [OR] 1.20 [95% confidence interval (CI), 1.00–1.44]), intermittent urine stream (OR 1.40 [95% CI, 1.15–1.69]), and urgency UI (OR 1.78 [95% CI, 1.22–2.60]) to be significant predictors of seeking treatment. Of those who had discussed LUTS with an HCP, 480 (70.6%) were currently receiving at least one LUTS treatment; the most common treatments were reducing fluid intake, pelvic exercises, oral anticholinergic medications, and avoiding certain foods/alcohol.
Conclusions: LUTS are commonly experienced among people with MS but are largely untreated. Proper LUTS assessment and work-up is warranted in MS patients.
Multiple sclerosis (MS) affects 58 to 95 per 100,000 people in the United States, with a predilection for women and people aged 40 to 59 years.1,2 Urologic manifestations are one of the most well-known components of the disease, with more than 90% of patients with MS experiencing urologic symptoms 10 years after disease onset.3 Symptoms relating to the bladder and bowel are often considered to be among the most distressing facets of the disease,4 and urinary symptoms have been shown to have a detrimental effect on health-related quality of life (HRQOL) in patients with MS.5–8
Although the presence of bladder dysfunction in patients with MS is well established, information on the types and prevalence of specific lower urinary tract symptoms (LUTS) is sparse, as they are not routinely or comprehensively assessed. Furthermore, it is unclear how often urinary symptoms are assessed and treated during routine evaluations of patients with MS. As health-care providers (HCPs) may be focused on treating the neurodegenerative symptoms of MS, LUTS may be overlooked, and as such, may not be adequately and properly managed.
The purpose of this study was to assess the prevalence of LUTS among a sample of US-residing individuals with MS. The overall utilization of care for LUTS in patients with MS was also investigated, including key drivers of treatment-seeking behavior, types of treatments being utilized, reasons for discontinuation, and overall satisfaction with treatment among those who sought care.
Methods
Study Overview
Between January 1, 2011, and May 1, 2011, a cross-sectional online survey was administered to a sample of US-residing individuals with MS who self-elected to participate in the study. Individuals with MS were recruited through the following Web-based patient advocacy organizations: MS World, the National Multiple Sclerosis Society (NMSS), and the Multiple Sclerosis Foundation (MSF).2,9,10 A study advertisement (which directed participants to a Web link for the online survey) was placed in the research section of the NMSS website, selected NMSS local chapter social media websites and newsletters, monthly MSF e-newsletters, and weekly MS World e-newsletters. The advertisement asked participants to voluntarily participate in an online survey asking them about their health, and did not provide specific information about its contents. To ensure that participants completed the survey only once, Internet Protocol and e-mail addresses were recorded, and each respondent was provided with a unique uniform resource locator to access the survey. All participants provided informed consent, had a 72-hour window to complete the survey, and were nominally compensated for their time upon completion of the study.
To be considered eligible to take part in this study, participants had to meet the following criteria: aged 18 to 89 years; ability to read and comprehend the English language; self-reported physician diagnosis of MS; and taking one or more prescribed medications to treat their MS (steroids, disease-modifying therapies [interferon beta-1a/b, glatiramer acetate, natalizumab, fingolimod, and mitoxantrone], immunomodulators [azathioprine, intravenous immune globulin], or plasma exchange) at the time that they completed the survey. Preliminary screener questions were asked when potential participants accessed the website to verify that they met these eligibility criteria.
Participants responded to questions pertaining to demographics, disease severity and history, HRQOL, work/activity impairment, and LUTS. Participants reporting any urinary symptoms were asked additional questions about their level of bother and urinary symptom treatment patterns. The total number of items each participant answered varied based on the presence and characteristics of their urinary symptoms and ranged from 85 to 126 items. The study was approved by and conducted in accordance with the University of Arizona Human Subjects Protection Program.
Assessment of MS Disease Severity
The Patient-Determined Disease Steps (PDDS) was used to classify participants by level of MS severity.11–13 PDDS is a self-reported disability assessment with scores ranging from 0 (normal, defined as having mild symptoms that are mostly sensory and do not limit activity) to 8 (bedridden, defined as unable to sit in a wheelchair for more than 1 hour). The PDDS has been correlated with the physician-scored Expanded Disability Status Scale, a universally used clinical scale developed to measure disability status in people with MS.11,12
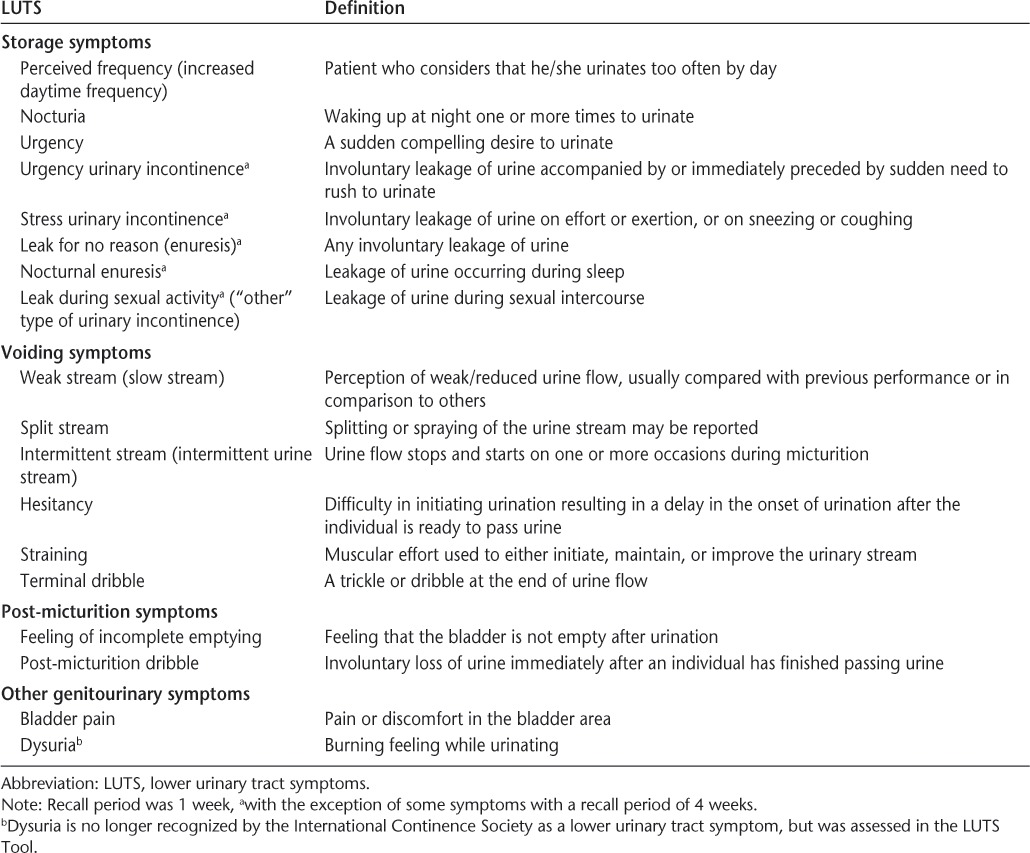
Assessments of LUTS Prevalence and Bother
The prevalence of LUTS was assessed using the LUTS Tool, a comprehensive, self-administered questionnaire developed in accordance with the International Continence Society definitions for urinary symptoms,14 and following extensive qualitative research in adults with at least one LUTS.15 This instrument provides data on the prevalence of 18 different types of LUTS, as classified according to voiding (weak stream, split stream, intermittent urine stream, hesitancy, straining, and terminal dribble), storage (perceived frequency, nocturia, urgency, urgency incontinence, stress incontinence, leak for no reason, nocturnal enuresis, and leak during sexual activity), post-micturition (feeling of incomplete emptying or post-micturition incontinence), or other (bladder pain or dysuria) symptoms (Table 1). For symptoms not associated with urinary incontinence (UI), patients could report experiencing a symptom “never,” “rarely,” “sometimes,” “often,” or “almost always” in the past week. They were considered to have a symptom if they responded that they experienced a symptom at least “sometimes.” For symptoms experienced in association with UI (urgency incontinence, stress incontinence, leaking for no reason, nocturnal enuresis, leaking during sexual activity, and post-micturition incontinence), patients were considered to have that symptom if they checked a box indicating that they had experienced the UI symptom in the preceding 4 weeks. Participants who reported experiencing symptoms (UI-associated and non-UI-associated) were then asked to what degree they were bothered by each symptom and could report that they were “not at all,” “a little bit,” “somewhat,” “quite a bit,” or “a great deal” bothered. Two definitions of bother were utilized, namely: 1) at least “somewhat” bothered, and 2) at least “quite a bit” bothered. Participants who reported experiencing UI were also asked how frequently they experienced that type of UI: “less than once a month,” “a few times a month,” “a few times a week,” “daily,” or “many times a day.”
Table 1.
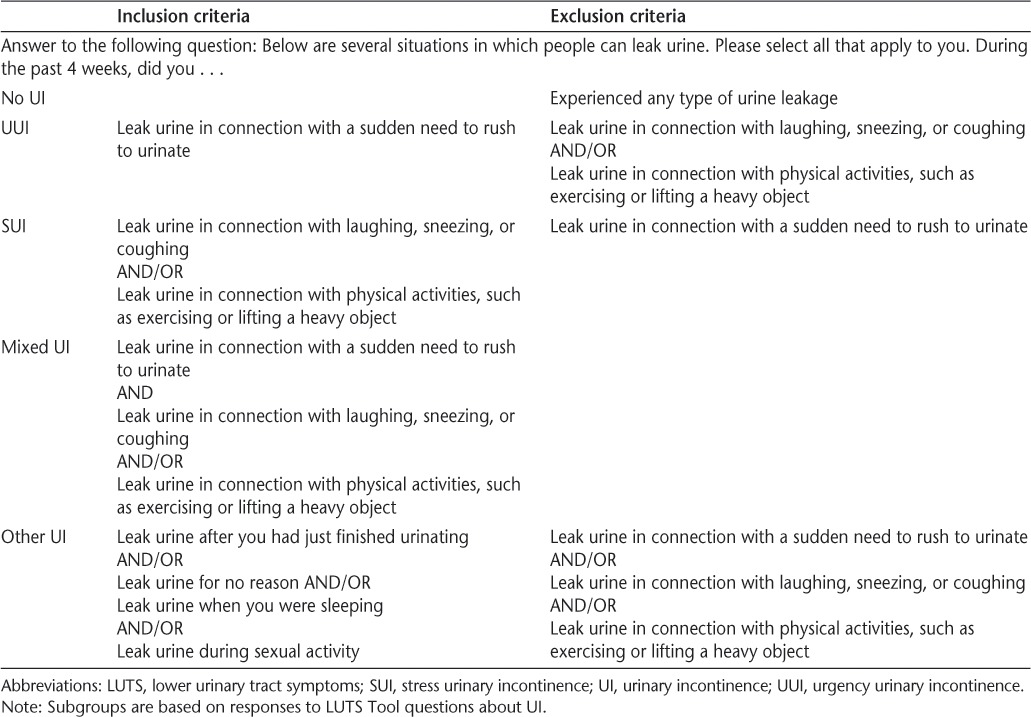
From the responses to the questions related to prevalence of the 18 LUTS described above, participants were stratified into the following five mutually exclusive subgroups based on the type of UI they experienced in the past 4 weeks: 1) no UI (did not leak urine under any circumstance); 2) urgency UI (UUI; leaking urine in connection with a sudden need to rush to urinate but not stress UI [leaking urine in connection with laughing, sneezing, or coughing and/or in connection with physical activities]; other types of UI [leaking urine after just finished urinating, for no reason, when sleeping, or during sexual activity] were permitted); 3) stress UI (SUI; SUI but not UUI; other types of UI were permitted); 4) mixed UI (both UUI and SUI; other types of UI were permitted); and 5) other UI only (as described above and not in association with UUI or SUI) (Table 2). Creation of these subgroups allowed patients to be more easily characterized with respect to their UI profile and facilitated more meaningful reporting of descriptive data.
Table 2.
Mutually exclusive UI subgroups
Assessment of Treatment-Seeking Behavior and Treatment Satisfaction
To elucidate the extent to which urinary symptoms were being addressed, participants experiencing LUTS were asked additional questions related to treatment-seeking behavior, types of treatments being sought, and attitudes about current treatments. The total number of items each participant answered varied based on the presence and characteristics of their urinary symptoms. Concepts assessed included discussion of urinary symptoms with an HCP, types of treatments prescribed (historic and current), reasons for discontinuing treatment (if applicable), and overall attitudes, satisfaction, and treatment efficacy.
Statistical Analyses
Descriptive statistics (mean, standard deviation for continuous/ordinal variables, frequency, and proportions [%] for categorical variables) were used to characterize all variables, including clinical and demographic characteristics, the overall prevalence of each type of LUTS, the degree of bother of individual LUTS, and the prevalence of each of the five types of UI. To determine prevalence of LUTS and each of the five UI groups, the number of participants categorized in each group was divided by the total sample. To evaluate differences in clinical and demographic characteristics across the five urinary groups, the χ2 test was used for categorical variables, while analysis of variance (ANOVA) with Bonferroni adjustment for post hoc multiple comparisons was conducted for continuous variables.
A series of multivariate logistic regression models were explored to identify the key drivers of treatment-seeking behavior. All models were adjusted for gender, age, MS disease severity (PDDS score), current MS exacerbation status (no or yes), and comorbid conditions present in 5% or more of the sample (anxiety, arthritis, asthma, depression, diabetes, eye disorders, fibromyalgia, hypertension, high cholesterol, irritable bowel syndrome, and migraines). The 18 LUTS variables were the key predictors of interest and were modeled as per the response options described above according to whether LUTS were experienced, how frequently LUTS were experienced, and how bothersome LUTS were perceived to be by participants. Odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated to measure the strength of the association between each covariate and treatment-seeking behavior. Diagnostics included testing for linear correlation (multi-collinearity) among the independent variables using the variance inflation factor (VIF), testing goodness of model fit using the Hosmer-Lemeshow test, and calculating the area under the receiver operating characteristic (ROC) curve to evaluate diagnostic accuracy.16 All statistical analyses were performed using Stata Data Analysis and Statistical Software, version 11 (College Station, TX), and differences were considered to be significant at α = .05.
Results
Participant Disposition and Characteristics
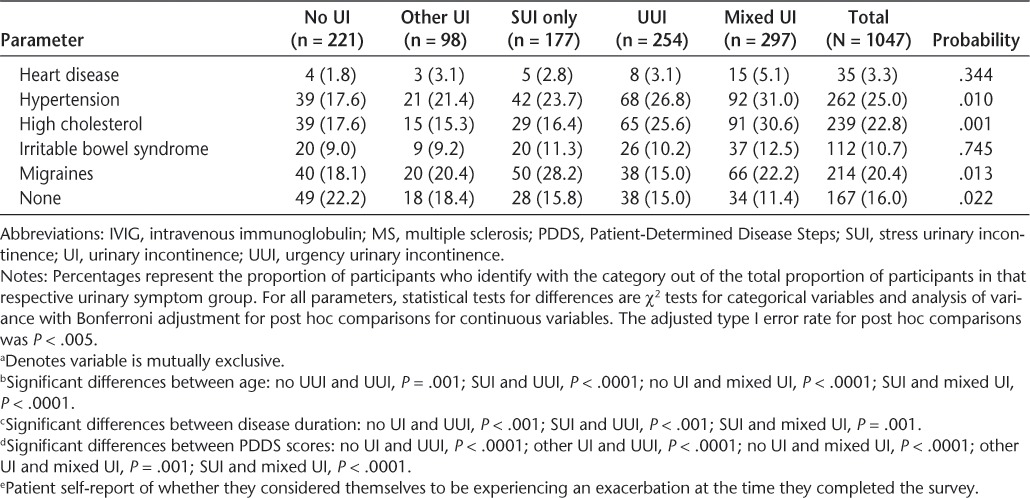
A total of 1052 participants completed the survey. Five participants did not answer enough LUTS Tool questions for placement into any of the urinary symptom subgroups, leaving a final sample of 1047 participants. The mean age of the sample was 47.8 (SD = 10.6) years (Table 3). The vast majority of respondents were women (81%) and identified themselves as non-Hispanic white (84%). More than half of all participants (60.9%) had a college or university qualification. Mean duration of MS was 8.5 (SD = 7.7) years, with most participants (80%) reporting a diagnosis of the relapsing-remitting type. Over 50% of participants reported that they were currently experiencing an exacerbation of their MS symptoms. The MS therapy most commonly used in this sample was interferon beta (36%), followed by glatiramer acetate (33%) and natalizumab injections (15%). Depression was the most common self-reported comorbid condition (42%), although a significant minority of participants also reported anxiety (25%), hypertension (25%), hyperlipidemia (23%), migraines (20%), and eye disorders (19%).
Table 3.
Demographic and clinical characteristics across UI subgroups
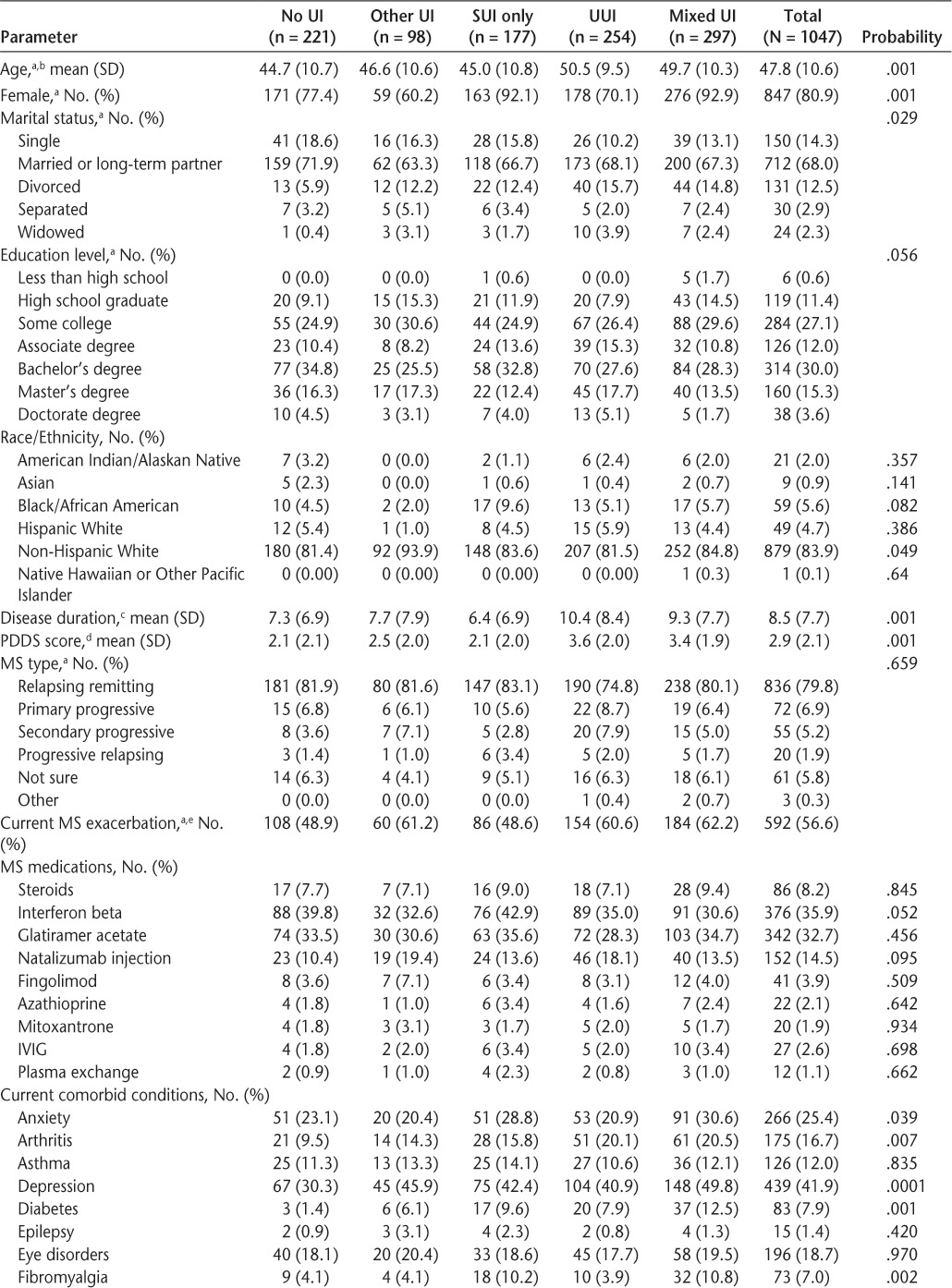
Table 3.
Demographic and clinical characteristics across UI subgroups (continued)
LUTS and Urinary Incontinence Prevalence
Of the 1047 participants, 966 (92%) reported some type of LUTS, with similar prevalence of LUTS among females (93%) and males (91%). The most commonly reported LUTS was terminal dribble (64.9%), followed by urinary urgency (61.7%) and a feeling of incomplete emptying (60.7%) (Figure 1). The most bothersome LUTS were urgency and urgency incontinence, with 34% and 29% of the overall sample being at least “quite a bit” bothered by these symptoms, respectively.
Figure 1.
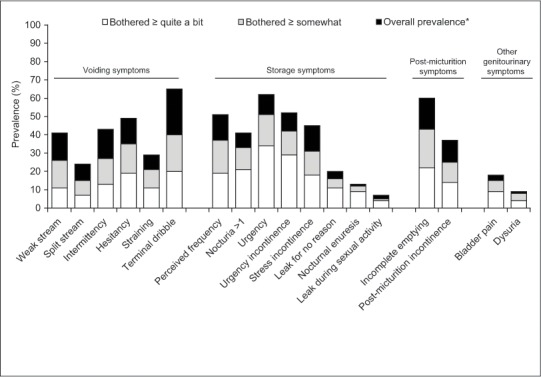
Overall LUTS prevalence and bother
LUTS, lower urinary tract symptoms.
*Total height of bar represents overall prevalence of each type of LUTS.
A total of 826 participants (79%) were classified as having some type of UI. With respect to the five mutually exclusive UI subgroups, most patients were placed into the mixed UI category (28.3%), followed by UUI (24.3%), SUI (16.9%), and other UI (9.4%) (Table 3). Demographic and clinical characteristics were similar across all UI categories; however, there were significant between-group differences in age, sex, marital status, disease duration, PDDS scores, and several comorbid conditions.
Treatment-Seeking Behavior
Among all participants with any type of LUTS (n = 966), 680 (70%) had discussed their symptoms with their HCP at some time, and 311 (32%) had seen their HCP about urinary symptoms in the past year. Patients with UUI or mixed UI were the most likely to have discussed symptoms with their HCP (79–80% of participants) or have seen their HCP for urinary symptoms (47–55%). Of the participants who had discussed their symptoms with their HCP, neurologists were the most frequently approached HCP (74%), followed by primary-care physicians (52%). Forty-four percent had seen a urologist to discuss their LUTS. This trend was similar across all UI subgroups.
The most commonly reported reasons that led participants to consult with their HCP about LUTS were increasing symptom frequency (n = 278, 41%), the HCP inquiring about any urinary symptoms (n = 277, 41%), worsening symptoms (n = 267, 39%), and embarrassing incident(s) (n = 184, 27%). Patients with UUI and mixed UI primarily consulted their HCP because of more frequent or worsening symptoms, while patients with other UI or SUI reported consulting their HCP primarily because they were asked about symptoms.
Conversely, of those participants with LUTS who never discussed their symptoms with their HCP (n = 286, 30%), the leading responses that participants cited for never discussing urinary symptoms with their HCP were a perception that symptoms were not severe enough (n = 186, 65%), feelings of discomfort discussing symptoms (n = 38, 13%), no direct questioning by the HCP (n = 29, 10%), and a belief that their HCP would not be able to help (n = 28, 10%).
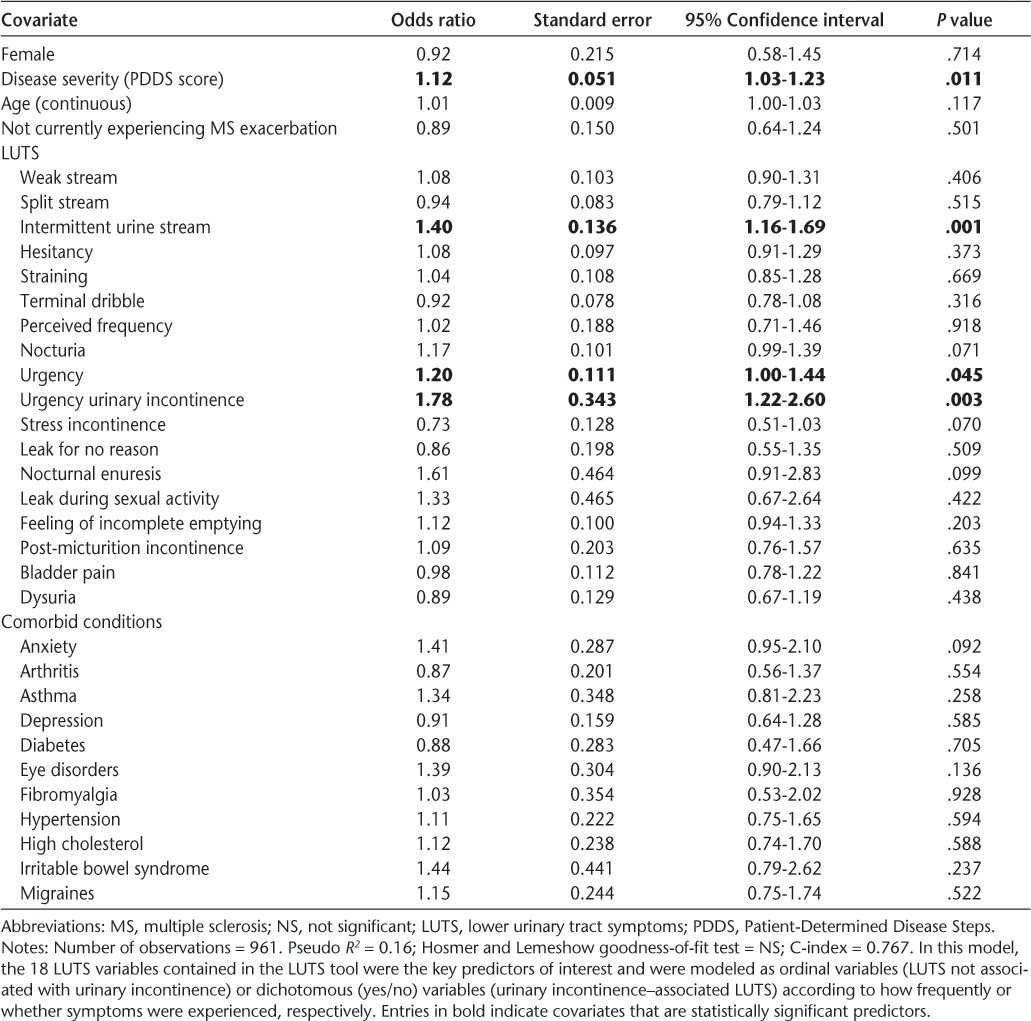
Predictors of Treatment-Seeking Behavior
Multivariate logistic regression revealed that even after adjustment for age, gender, MS disease severity (PDDS), and MS exacerbation, MS patients who experienced greater frequency of urgency (OR 1.20 [95% CI, 1.00–1.44]), greater frequency of intermittent urine stream (OR 1.40 [95% CI, 1.15–1.69]), and UUI (OR 1.78 [95% CI, 1.22–2.60]) were significantly more likely to seek treatment (C-index = 0.77) (Table 4). The VIF was less than 3 for all covariates, indicating that none of the independent variables were collinear with each other. Similar trends were seen when degree of LUTS bother and/or frequency of UI were modeled instead, with intermittent urine stream and UUI consistently being associated with a higher likelihood of seeking treatment. No notable differences were seen when the models were run separately for gender, although statistical inferences could not be drawn as easily in the male subgroup because of the markedly smaller sample size (n = 200).
Table 4.
Logistic regression results: predictors of treatment-seeking behavior
LUTS Treatment in the MS Population
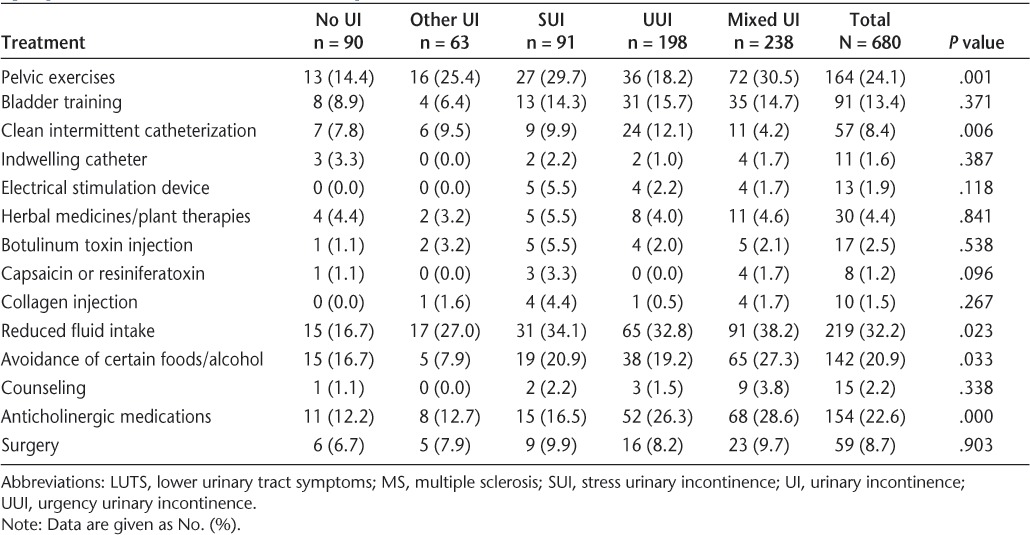
Of the 680 participants who had discussed LUTS with their HCP, 480 (70.6%) were currently receiving at least one LUTS treatment. The most frequently used current treatments included reducing fluid intake (n = 219, 32% of participants), pelvic exercises (n = 164, 24%), oral anticholinergic medications (n = 154, 23%), and avoiding certain foods/alcohol (n = 142, 21%) (Table 5). Use of other treatment modalities, such as botulinum toxin type A injections, electrical stimulation devices, clean intermittent catheterization, indwelling catheters, or collagen injections, was low. Among the five UI subgroups, there were significant differences in the use of pelvic exercises, clean intermittent catheterization, reduced fluid intake, avoidance of certain foods, and the use of anticholinergic medications. In terms of the latter, 27% to 28% of patients with UUI or mixed UI reported using anticholinergic medications versus only 13% to 17% of patients with other UI or SUI.
Table 5.
Current treatments among MS patients with LUTS who had ever discussed their symptoms with their health-care provider
One hundred twenty-one participants (17.8%) who reported having any urinary symptoms (wet or dry) had never tried any treatment. Of these, most participants stated that this was because symptoms were not serious enough (n = 68, 57%). Other reasons cited by participants for having never tried any treatment included no prescription offered (n = 45, 38%), lack of knowledge about treatment availability (n = 12, 10%), inaction (n = 8, 7%), not wanting to treat (n = 6, 5%), being unable to or unwilling to pay (n = 5, 5%), and negative stories about available treatments (n = 2, 2%).
Reasons for Treatment Discontinuation
Suboptimal efficacy was by far the most common reason for discontinuing treatment, with 29%, 58%, 35%, and 47% of participants treated with reduced fluid intake, pelvic exercises, oral anticholinergic medications, and avoidance of foods/alcohol discontinuing treatment for this reason, respectively. Adverse effects were responsible for cessation of oral anticholinergic agents in 35% of respondents. Adverse effects (28% and 32% of the time) and change of therapy (30% and 24% of the time) were the common reasons cited for discontinuing treatment with intermittent and indwelling catheters, respectively, although a further 32% and 20% of participants, respectively, ceased this treatment because of LUTS resolution.
Treatment Satisfaction
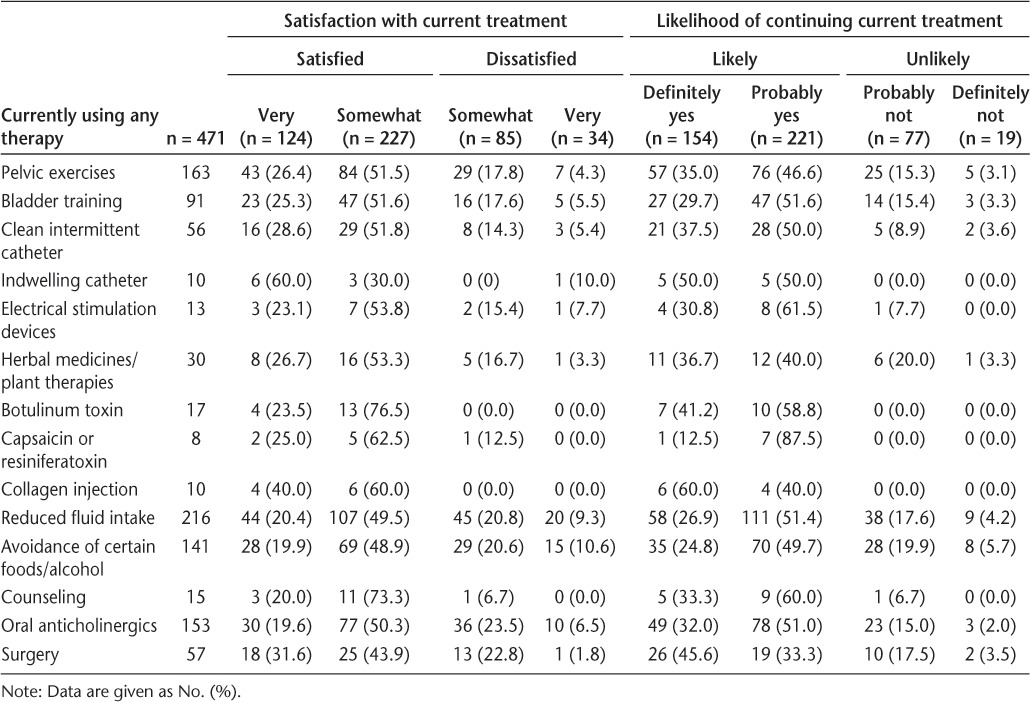
Most participants who were currently being treated were very or somewhat satisfied with their current therapy for LUTS and reported that they would probably or definitely continue treatment (Table 6). However, some patients were dissatisfied and indicated that they would not continue. For example, 22% and 30% of patients treated with pelvic exercises and oral anticholinergics, respectively, were either somewhat or very dissatisfied with their treatments, and 17% to 18% of patients treated with these therapies indicated that they would probably or definitely not continue treatment. Interestingly, all patients treated with botulinum toxin or collagen injections indicated that they were satisfied with these treatments and would definitely or probably continue them, although the overall frequency of their use was low.
Table 6.
Satisfaction with current treatments
Discussion
The presence of urinary and bladder symptoms in individuals with MS is well known but has been understudied, perhaps because of a focus on the mechanisms that underlie the progressive and irreversible neurologic impairment of the disease. This cross-sectional observational study provides the first comprehensive assessment of the prevalence and management of specific types of LUTS in patients with MS in the US primary-care setting. Urinary symptoms were found to be highly prevalent in this population. Over 90% of patients with MS reported experiencing LUTS regardless of gender, with symptoms reflective of the overactive bladder (OAB) syndrome (ie, frequency, urgency, UUI, and nocturia) being particularly common. These findings agree with those from a study by Fowler,17 which found that urinary urgency is a primary complaint among MS patients with established bladder dysfunction. A recent study by Mahajan et al.,18 which used data from the 2005 North American Research Committee on Multiple Sclerosis (NARCOMS) survey, also found that a majority (65%) of patients with MS reported one or more moderate to severe urinary symptom(s).
In addition, nearly 80% of the MS patients in our sample were classified as having some type of UI. The prevalence of LUTS in this sample, particularly UI, is substantially higher than what has been found in the general population.19 This finding is consistent with the pathology of MS, and may be an extension of the neurologic facets of the disease itself. The primary pathologic features of MS include demyelination and inflammation, but there is also a loss of axons in MS, which can lead to atrophy of the brain and spinal cord. It is believed that these lesions and/or the loss of axons from pathways that control bladder function leads to detrusor overactivity and detrusor sphincter dyssynergia.20 Such neurologic deficits give rise to symptoms of urinary urgency and UUI, which were some of the most commonly reported symptoms in this survey.
Despite the prevalence of LUTS, a notable proportion of participants (30%) who reported urinary symptoms never sought treatment from their physicians. The majority of these patients (65%) felt that their symptoms were not severe enough to discuss; however, patients also noted that they were uncomfortable discussing symptoms with their HCP and/or failed to seek treatment because their HCP did not ask or they believed that their HCP would not be able to help. Studies in the general LUTS population have found that patients often do not report their symptoms for a variety of reasons, including embarrassment, fear of being placed in a nursing home or of surgery, or a belief that it is a normal part of aging or that there is no treatment available.21–23 It is possible that this population is even more reluctant to treat their urinary symptoms because they are already taking medications for their MS and want to reduce pill or treatment burden as much as possible. Further exploration is needed to determine why patients with MS are reluctant to discuss their urinary symptoms in order to improve overall management.
Patients who sought treatment did so for a range of reasons, but primarily because their LUTS had deteriorated to the extent that a consultation was warranted (ie, increasing symptom frequency, worsening symptoms, embarrassing incidents). Urinary urgency, intermittent urine stream, and UUI were found to be the most significant predictors of seeking treatment, with patients who had these symptoms being 20%, 40%, and 78% more likely to seek treatment, respectively, than those without these symptoms. Symptoms of urgency and UUI in particular have previously been shown to significantly impair HRQOL6,24,25; thus it is not entirely surprising that they are among the key factors driving patients to seek treatment. This observation is in contrast to the EpiLUTS study, which showed that urinary frequency was the significant symptom predictor of seeking treatment in both men and women with OAB symptoms.19 It is important to note, however, that EpiLUTS was conducted in the general population. Given the substantial neurologic impairments that stem from MS, the underlying cause and presentation of symptoms should be distinguished from that of a non-MS population.
The finding that the symptom of intermittent urine stream was a significant predictor of seeking treatment in patients with MS was more surprising, given that this symptom is typically associated with an enlarged prostate, and the majority of individuals who participated in our survey were female. Although intermittent urine stream tends to be associated with bladder outlet obstruction, particularly in men,26 alterations in urine flow characteristics in both males and females may be influenced by bladder neoplasms, urethral diverticula, or neuropathic changes in the bladder.27 This last etiology may help explain this particular finding in this sample. Conversely, while the difference was not statistically significant, SUI was associated with a 27% reduced likelihood of seeking treatment. Because SUI occurs in conjunction with sneezing, coughing, or physical activity, patients may consider it to be a natural part of aging or due to other causes, rather than being related to their MS, and they may be unaware of viable treatment options. Thus, they may “self-treat” and try to stop or decrease activities associated with the UI, rather than seek treatment from an HCP.
The most commonly used treatments for LUTS among patients with MS were reducing fluid intake, pelvic exercises, anticholinergic medications, and avoidance of certain foods or alcohol, but these conventional modalities were also associated with a lack of efficacy and adverse effects, which often resulted in their discontinuation. A subset of patients in the group without UUI indicated that they utilized anticholinergic medications. These patients may have experienced other symptoms of OAB (eg, urgency, frequency, and nocturia) that could warrant treatment with anticholinergics. Newer treatments, such as botulinum toxin type A or neural stimulation, were not extensively utilized. Our findings agree with those from the 2005 ancillary analysis of NARCOMS, which also found botulinum toxin A treatment to be underused.18 This is not entirely surprising, as onabotulinumtoxinA is the only botulinum toxin product approved for the treatment of neurogenic detrusor overactivity, and approval was not received until August 2011 (after both analyses were performed).
In general, patients reported being moderately satisfied with their current treatments. Patient satisfaction is an important indicator of whether patients believe a treatment is worthwhile and whether they would choose the same treatment again,28 and higher levels of satisfaction suggest that treatment should be continued.29 However, up to almost 25% of participants indicated that they would probably or definitely not continue their therapy, which suggests that there is room for improvement in patient satisfaction and that physicians should proactively question patients on their desire to continue treatment; if they do not wish to continue, alternate treatments should be considered.
Limitations of this study were the potential for selection and recall bias, since participants were not randomly selected and had to be Internet-versed and have Internet access. Additionally, as with any self-report study, participants may have had variable ability to report information accurately. However, to obtain the prevalence of LUTS within a fairly uncommon chronic condition (along with its associated treatment patterns) across a geographically diverse population with a broad spectrum of MS disabilities in the real world, patient selection via an online questionnaire was considered the least prohibitive method. In addition, care should be taken in interpreting some of the descriptive findings, as this was a self-selected sample of individuals with MS. Finally, although there was no clinical validation that the respondents have MS and participants received a small incentive for completing the survey, the mode of recruitment and screener questions that were used to ensure that a reliable MS population was sampled appeared to be successful given that the ratio of women to men, race/ethnicity distribution, and prevalence of relapsing-remitting MS was highly consistent with estimates reported in the literature.30–32
Conclusion
We have described a large MS population with a high prevalence of LUTS. Despite the high rate of LUTS and UI, many patients did not seek treatment and most were not treated, while others discontinued treatment primarily because of suboptimal efficacy or adverse events. Proper LUTS assessment and management should be part of the clinical work-up in patients with MS if the entire spectrum of disease symptoms is to be addressed.
PracticePoints.
Over 90% of patients with MS report having lower urinary tract symptoms (LUTS), and approximately 80% of patients were classified as having some type of urinary incontinence (UI).
Despite this, many patients with MS do not discuss their urinary symptoms with a health-care provider, and most are not receiving treatment. Key drivers of seeking treatment include symptoms of urgency, intermittent urine stream, and urgency incontinence.
Proper LUTS assessment and management should be addressed with every patient with MS as part of the clinical work-up.
Acknowledgments
Writing assistance for manuscript development was provided by Linda Wychowski, PhD, of Evidence Scientific Solutions, and was funded by Allergan, Inc, Irvine, CA. Dr. Khalaf was the principal investigator for this project and was funded through an Allergan-funded fellowship with the University of Arizona.
Footnotes
Financial Disclosures: Dr. Khalaf was an employee of Allergan at the time this manuscript was accepted for publication and is currently a PhD student at the University of Arizona, as well as an employee of Xcenda. Dr. Coyne has been a paid scientific consultant to Allergan on this project. Dr. Globe was an employee of Allergan at the time this manuscript was accepted for publication. Dr. Burks is a consultant for Allergan, Novartis Pharmaceuticals, and Sanofi-Aventis Pharmaceutical, from which he receives honoraria. He is on the speakers' bureau for Bayer Pharmaceutical and Novartis Pharmaceuticals, for which he receives honoraria. Dr. Burks does not own stocks in any of the pharmaceutical companies for which he is a consultant or on the speakers' bureau. Dr. Armstrong and Dr. Malone have no conflicts of interest to disclose.
Funding/Support: This study and its analysis were sponsored by Allergan, Inc, Irvine, CA.
References
- 1.Noonan CW, Williamson DM, Henry JP et al. The prevalence of multiple sclerosis in 3 US communities. Prev Chronic Dis. 2010;7:A12. [PMC free article] [PubMed] [Google Scholar]
- 2.National Multiple Sclerosis Society. http://www.nmss.org. Accessed July 9, 2013.
- 3.Committee on Multiple Sclerosis, Board on Neuroscience and Behavioral Health. Multiple Sclerosis: Current Status and Strategies for the Future. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 4.Hemmett L, Holmes J, Barnes M, Russell N. What drives quality of life in multiple sclerosis? QJM. 2004;97:671–676. doi: 10.1093/qjmed/hch105. [DOI] [PubMed] [Google Scholar]
- 5.Nortvedt MW, Riise T, Frugård J et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler. 2007;13:106–112. doi: 10.1177/1352458506071210. [DOI] [PubMed] [Google Scholar]
- 6.Quarto G, Autorino R, Gallo A et al. Quality of life in women with multiple sclerosis and overactive bladder syndrome. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:189–194. doi: 10.1007/s00192-006-0131-9. [DOI] [PubMed] [Google Scholar]
- 7.Coyne KS, Wein AJ, Tubaro A et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103(suppl 3):4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]
- 8.Khalaf K, Globe D, Armstrong E, Malone D, Coyne K. Health-related quality of life among patients with MS and urinary symptoms. Paper presented at: Annual Meeting of the Consortium of Multiple Sclerosis Centers; June 1. 2012; San Diego, CA.
- 9.Multiple Sclerosis Assocation of America. http://www.msassociation.org. Accessed July 9, 2013.
- 10.Multiple Sclerosis World. http://www.msworld.org. Accessed July 9, 2013.
- 11.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45:251–255. doi: 10.1212/wnl.45.2.251. [DOI] [PubMed] [Google Scholar]
- 12.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a longitudinal study comparing disease steps and EDSS to evaluate disease progression. Mult Scler. 1999;5:349–354. doi: 10.1177/135245859900500508. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz CE, Vollmer T, Lee H, North American Research Consortium on Multiple Sclerosis Outcomes Study Group Reliability and validity of two self-report measures of impairment and disability for MS. Neurology. 1999;52:63–70. doi: 10.1212/wnl.52.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Abrams P, Cardozo L, Fall M et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167–178. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 15.Coyne KS, Sexton CC, Kopp Z et al. Assessing patients' descriptions of lower urinary tract symptoms (LUTS) and perspectives on treatment outcomes: results of qualitative research. Int J Clin Pract. 2010;64:1260–1278. doi: 10.1111/j.1742-1241.2010.02450.x. [DOI] [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. Hoboken, NJ: John Wiley and Sons; 2013. [Google Scholar]
- 17.Fowler CJ. Pathophysiology of micturition disturbance in multiple sclerosis. Sex Disabil. 1996;14:7–12. [Google Scholar]
- 18.Mahajan ST, Patel PB, Marrie RA. Under treatment of overactive bladder symptoms in patients with multiple sclerosis: an ancillary analysis of the NARCOMS Patient Registry. J Urol. 2010;183:1432–1437. doi: 10.1016/j.juro.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Coyne KS, Sexton CC, Thompson CL et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104:352–360. doi: 10.1111/j.1464-410X.2009.08427.x. [DOI] [PubMed] [Google Scholar]
- 20.McCombe PA, Gordon TP, Jackson MW. Bladder dysfunction in multiple sclerosis. Expert Rev Neurother. 2009;9:331–340. doi: 10.1586/14737175.9.3.331. [DOI] [PubMed] [Google Scholar]
- 21.Burgio KL, Ives DG, Locher JL, Arena VC, Kuller LH. Treatment seeking for urinary incontinence in older adults. J Am Geriatr Soc. 1994;42:208–212. doi: 10.1111/j.1532-5415.1994.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Conor RM, Johannesson M, Hass SL, Kobelt-Nguyen G. Urge incontinence: quality of life and patients' valuation of symptom reduction. Pharmacoeconomics. 1998;14:531–539. doi: 10.2165/00019053-199814050-00005. [DOI] [PubMed] [Google Scholar]
- 23. Schabert VF, Bavendam T, Goldberg EL, Trocio JN, Brubaker L. Challenges for managing overactive bladder and guidance for patient support Am J Manag Care 2009. 15( suppl) S118 S122 [PubMed] [Google Scholar]
- 24.Coyne KS, Sexton CC, Kopp ZS, Ebel-Bitoun C, Milsom I, Chapple C. The impact of overactive bladder on mental health, work productivity and health-related quality of life in the UK and Sweden: results from EpiLUTS. BJU Int. 2011;108:1459–1471. doi: 10.1111/j.1464-410X.2010.10013.x. [DOI] [PubMed] [Google Scholar]
- 25.Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology. 1999;53:1098–1103. doi: 10.1212/wnl.53.5.1098. [DOI] [PubMed] [Google Scholar]
- 26.Reynard J, Lim C, Abrams P. Significance of intermittency in men with lower urinary tract symptoms. Urology. 1996;47:491–496. doi: 10.1016/S0090-4295(99)80483-X. [DOI] [PubMed] [Google Scholar]
- 27.White JM, O'Brien DPI. Incontinence and stream abnormalities. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990. [PubMed] [Google Scholar]
- 28.Brubaker L, Shull B. EGGS for patient-centered outcomes. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:171–173. doi: 10.1007/s00192-005-1300-y. [DOI] [PubMed] [Google Scholar]
- 29.Wiklund I. Assessment of patient-reported outcomes in clinical trials: the example of health-related quality of life. Fundam Clin Pharmacol. 2004;18:351–363. doi: 10.1111/j.1472-8206.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs LD, Cookfair DL, Rudick RA et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 31.Orton SM, Herrera BM, Yee IM. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. et al.; for the Canadian Collaborative Group. [DOI] [PubMed] [Google Scholar]
- 32.Weinshenker BG. The natural history of multiple sclerosis. Neurol Clin. 1995;13:119–146. [PubMed] [Google Scholar]


