Abstract
Background: The main clinical determinants of quality of life (QOL) 5 years after clinically isolated syndrome (CIS) are Expanded Disability Status Scale (EDSS) score and conversion to clinically definite multiple sclerosis (CDMS). The aim of this study was to determine the demographic, clinical, and magnetic resonance imaging (MRI) factors associated with QOL 10 years after CIS.
Methods: Controlled High Risk Avonex® Multiple Sclerosis Prevention Study in Ongoing Neurologic Surveillance (CHAMPIONS) 10-year patients were assessed for CDMS, EDSS score, MRI T2 activity, brain parenchymal fraction, and patient-reported QOL. Associations were evaluated using analysis of variance models.
Results: A second clinical event consistent with CDMS and higher EDSS scores at years 5 and 10 were associated with lower 36-item Short Form Health Status Survey (SF-36) Physical Component Summary scores at year 10 (P < .01). Patients with earlier onset of CDMS had worse patient-reported Physical Component Summary, SF-36 Mental Component Summary, fatigue, and pain scores at year 10 than patients with later or no onset of CDMS. Neither initial randomization group nor any MRI metrics assessed at baseline or during follow-up were associated with QOL at 10 years.
Conclusions: These results support the development of therapies for patients with CIS that significantly reduce the risk of conversion to CDMS and the progression of physical disability to milestones as low as EDSS scores of 2.0.
Multiple sclerosis (MS) is a chronic progressive disease of the nervous system that can lead to significant increases in physical and cognitive-behavioral disabilities over time, but patient-reported symptoms tend to dominate the early years of the illness, which often spans more than 5 decades. A common first indication of MS is the occurrence of clinically isolated syndrome (CIS), characterized by an initial episode of demyelination typically involving the optic nerve, brainstem, cerebellum, or spinal cord.1,2 Patients with a CIS diagnosis and characteristic white matter lesions on magnetic resonance imaging (MRI) are at high risk for clinically definite MS (CDMS),1,2 and several clinical trials have demonstrated that current first-line therapies reduce the rate of conversion to CDMS.3–6 However, little is known about the long-term impact of treatment initiated at CIS onset on disability or patient-reported outcomes (PROs).
The beneficial effect of early intervention with weekly intramuscular interferon beta-1a (IFNβ-1a) on the rate of CDMS development, first demonstrated in the Controlled High-Risk Subjects Avonex® Multiple Sclerosis Prevention Study (CHAMPS), persisted in the Controlled High Risk Avonex® Multiple Sclerosis Prevention Study in Ongoing Neurologic Surveillance (CHAMPIONS) 5- and 10-year studies, in which the comparison group was patients who initiated therapy later (a median of 2.5 years after CIS onset).6,7 Few patients in CHAMPIONS, regardless of treatment group, developed significant disability or progressive disease over 10 years, although there was a modest reduction in the proportion of patients in the immediate-treatment (IT) group developing a second clinical event consistent with CDMS.4,6,7
Patients followed in CHAMPIONS completed the Multiple Sclerosis Quality of Life Inventory (MSQLI), a battery including the 36-item Short Form Health Status Survey (SF-36) and nine disease-specific scales as outcome measures, at study years 5 and 10. We previously reported that MSQLI scores, particularly on the SF-36 Physical Component Summary (PCS), the Modified Fatigue Impact Scale (MFIS), the Pain Effects Scale (PES), and the Bladder Control Scale, were significantly worse in patients who developed CDMS by 5 years than in patients still classified as having CIS at 5 years.8 These worsening quality of life (QOL) scores were also significantly associated with an increase in Expanded Disability Status Scale (EDSS) scores in patients developing CDMS by 5 years, whereas patients without CDMS at 5 years reported QOL (SF-36) scores that were similar to the scores of controls.8,9
This study examined whether patient conversion to CDMS remained associated with QOL at 10 years and whether other baseline or on-study demographic, clinical, or MRI characteristics were associated with QOL in the CHAMPIONS 10-year extension.
Methods
This study was primarily a cross-sectional analysis of data from CHAMPIONS patients at their 5- and 10-year evaluations (approximately 5 and 10 years after their enrollment into CHAMPS). Additional longitudinal analyses were performed to assess changes in SF-36 summary scores between 5 and 10 years.
Study Design and Outcomes
The CHAMPIONS study design has previously been described.6 Patients were divided into an IT group, which initiated intramuscular IFNβ-1a treatment at the onset of CIS symptoms (CHAMPS initiation), and a delayed-treatment (DT) group, which initiated treatment at a later point (a median of 30 months after symptom onset [CHAMPS initiation]; interquartile range, 24–35 months).
Previous analyses comparing patient baseline demographic and clinical characteristics between the IT and DT groups showed no statistically significant differences between the two groups.7 Similarly, there were no significant differences in MSQLI scores between the IT and DT groups at CHAMPIONS study years 5 and 1010 or between these two groups among patients who completed the CHAMPIONS 10-year study. Therefore, IT and DT group data from patients who had MSQLI scores at both 5 and 10 years and completed the CHAMPIONS 10-year study were combined for the current analyses.
Clinical Outcome Measures
The development of CDMS required a second clinical event in a location different from the initial event and was determined by review of suspected cases by an independent blinded outcomes committee that maintained the same outcome definitions used in CHAMPS.4,6,7 Changes on MRI were not used to determine CDMS. The number of relapses and neurologic disability as measured by the EDSS were determined by an unmasked site neurologist at each annual visit as in CHAMPS and CHAMPIONS.4,6,7,11 The number of new or enlarging T2 lesions and T2 lesion volume and brain parenchymal fraction (BPF) at years 5 and 10 in CHAMPIONS were assessed by the MRI reading center (blinded to previous/current treatment) and by Elizabeth Fisher at the Cleveland Clinic Foundation (Cleveland, OH), respectively, using previously reported methods.4,6,7,12
PRO Measures
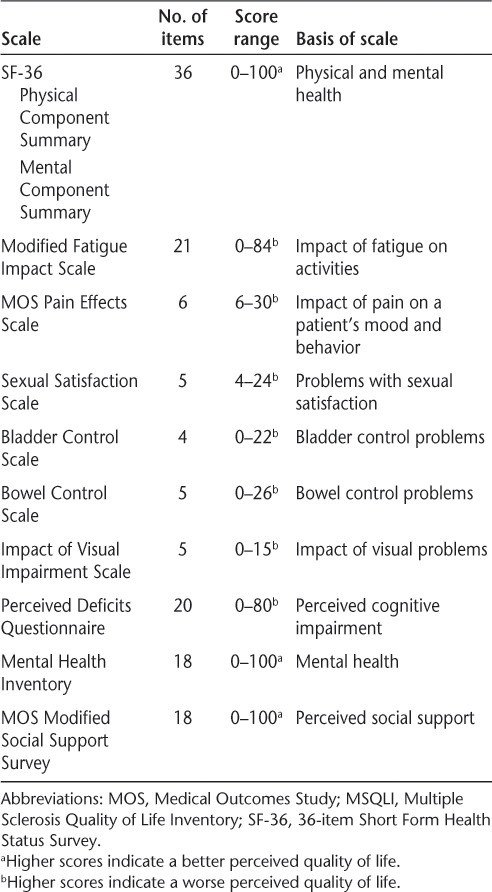
The MSQLI13 consists of ten scales designed to assess current health status from the patient's perspective (Table 1). The MSQLI includes disease-specific measures and the SF-36, which measures a patient's physical and mental well-being. The MSQLI does not provide a single overall number or score summarizing QOL; rather, each scale is scored separately. The SF-36 is reported by PCS and Mental Component Summary (MCS) scores. Patient responses to the MSQLI as a measure of QOL were collected during CHAMPIONS study years 5 and 10 but were not collected at baseline in CHAMPS.
Table 1.
MSQLI individual component scales13
Statistical Analyses
Analysis of variance (ANOVA) was used to compare the IT and DT groups (for patients who completed the CHAMPIONS 10-year study) for any of the baseline variables. Also, ANOVA was used to evaluate patient-reported SF-36 (PCS and MCS) scores at 10 years (and change in SF-36 scores between 5 and 10 years) and their association with each variable. Differences in SF-36 scores among baseline, clinical, and MRI variables were analyzed.
Associations between SF-36 scores at 10 years and EDSS scores at 5 and 10 years were analyzed across EDSS score subgroups (0.0, 1.0–1.5, 2.0–2.5, and ≥3.0) and separately for patients with and without CDMS by CHAMPIONS study year 10. Similarly, possible associations among patient EDSS scores, baseline characteristics, and MSQLI scores were evaluated at CHAMPIONS study year 10 using ANOVA.
Each of the MSQLI subscales was also analyzed by CDMS status within certain intervals; this was either across subgroups (CDMS between 0 and 5 years, CDMS between 5 and 10 years, and no CDMS by 10 years) or between the CDMS-by-10-years and no-CDMS-by-10-years groups.
Spearman correlation coefficients were used to evaluate correlations between age and EDSS scores at the 5- and 10-year follow-up visits; between each QOL measure and each of the subscales of the Multiple Sclerosis Functional Composite (Timed 25-Foot Walk test, Nine-Hole Peg Test, and Paced Auditory Serial Addition Test) as well as the composite score of the Multiple Sclerosis Functional Composite; between all QOL measures and BPF measures at 5 and 10 years; and between changes in all QOL measures and percentage change in BPF measures from 5 to 10 years.
Because multiple comparisons were conducted during these analyses, probability values between .05 and .01 were considered suggestive, and only probability values less than or equal to .01 were regarded as significant.
Results
Patients
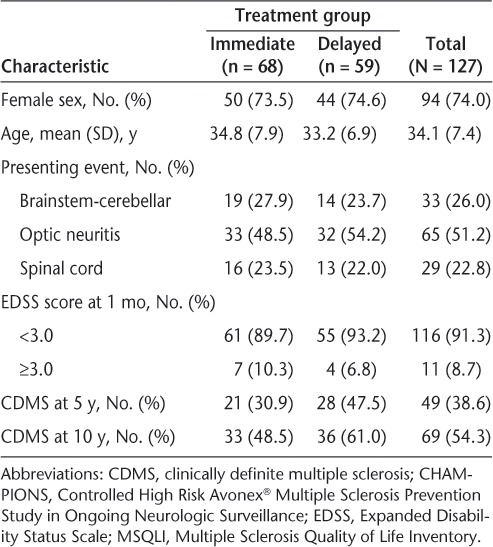
The IT and DT groups exhibited similar baseline demographic and clinical characteristics, with no statistically significant differences (Table 2; additional data not shown). A total of 155 patients enrolled (IT, n = 81; DT, n = 74), and 127 patients (IT, n = 68; DT, n = 59) completed the CHAMPIONS 10-year examination. From this subset of 127 patients, 125 (IT, n = 67; DT, n = 58) completed the MSQLI at 5 and 10 years.
Table 2.
Demographic and baseline clinical characteristics and CDMS status at 5 and 10 years for CHAMPIONS extension participants who completed the MSQLI at 10 years
Baseline Determinants of QOL After CIS
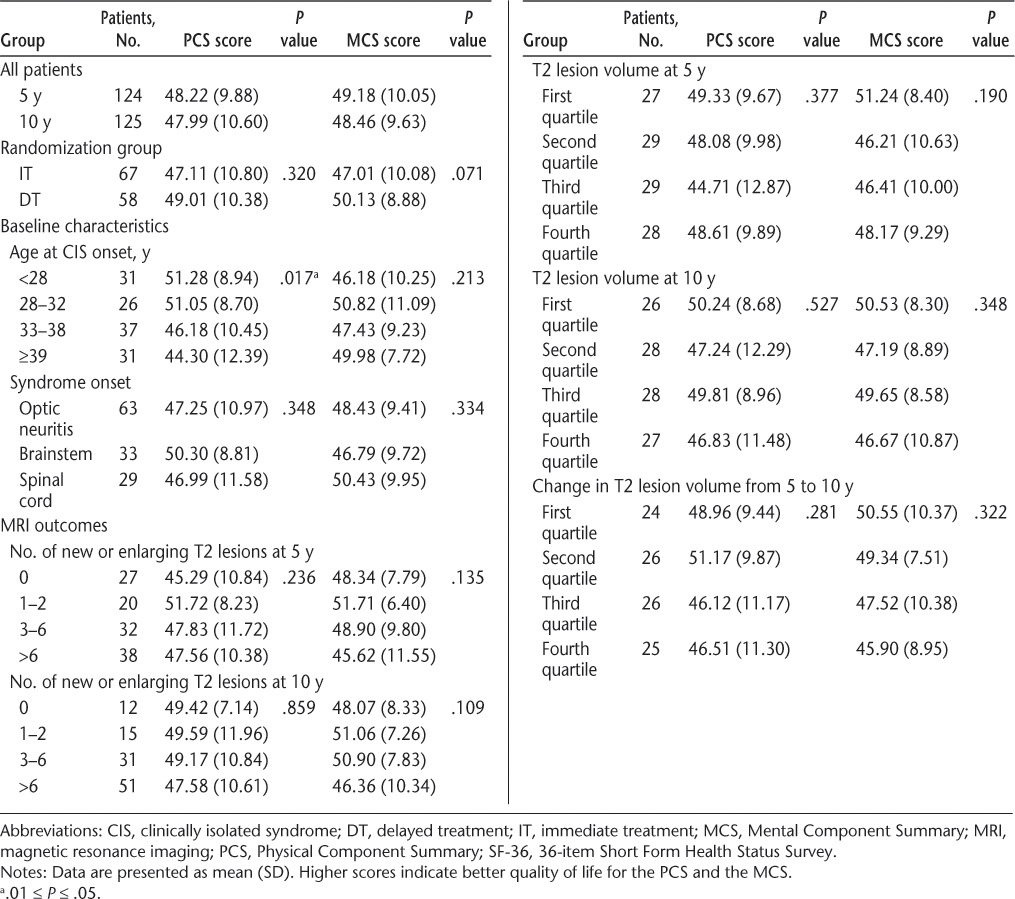
Associations between baseline characteristics and SF-36 summary scores revealed that there were no significant differences in SF-36 scores at 10 years (Table 3) or in change in SF-36 scores from 5 to 10 years (data not shown) between the randomization groups (IT and DT). There were also no significant differences in SF-36 scores by syndrome type at onset. There was a trend suggesting lower SF-36 PCS scores at 10 years with increased age at CIS onset (P = .017). This result was likely related to the significant association between age at CIS onset and EDSS score at 10 years (Spearman correlation r = 0.17; P = .047). Baseline T2 lesion number and volume and gadolinium lesion number were not associated with SF-36 PCS or MCS scores at 5 or 10 years (Table 3).
Table 3.
Association of baseline characteristics and MRI measures with SF-36 summary scores at 10 years
On-Study Determinants of QOL After CIS
The development of CDMS by study year 5 or 10 was significantly associated with worse SF-36 PCS scores (P = .004 and P = .005, respectively). Similarly, the development of CDMS by 5 years was marginally associated with lower SF-36 MCS scores at 10 years (P = .015), and the development of CDMS by 10 years was more significantly associated with lower MCS scores at 10 years (P = .002).
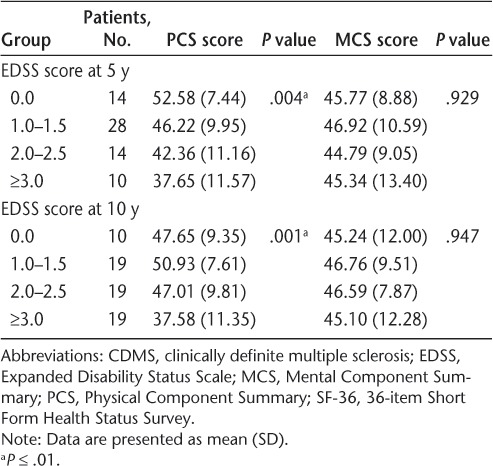
The EDSS scores at years 5 and 10 were significantly associated with worse SF-36 PCS scores at 10 years (P < .001 for both), although few patients had EDSS scores of 3.0 or greater at either 5 years (9.8%) or 10 years (18.4%) (Table 4). This association remained strong when data from only patients with CDMS by 10 years were included in the analysis (Table 5). However, no statistically significant relationship was detected between EDSS and SF-36 MCS scores. The SF-36 PCS scores showed considerable variability among patients with EDSS scores of 0.0 to 2.5, although PCS scores at 10 years were consistently low in patients with EDSS scores of 3.0 or greater at 10 years (data not shown).
Table 4.
Association between clinical measures and SF-36 summary scores at 10 years
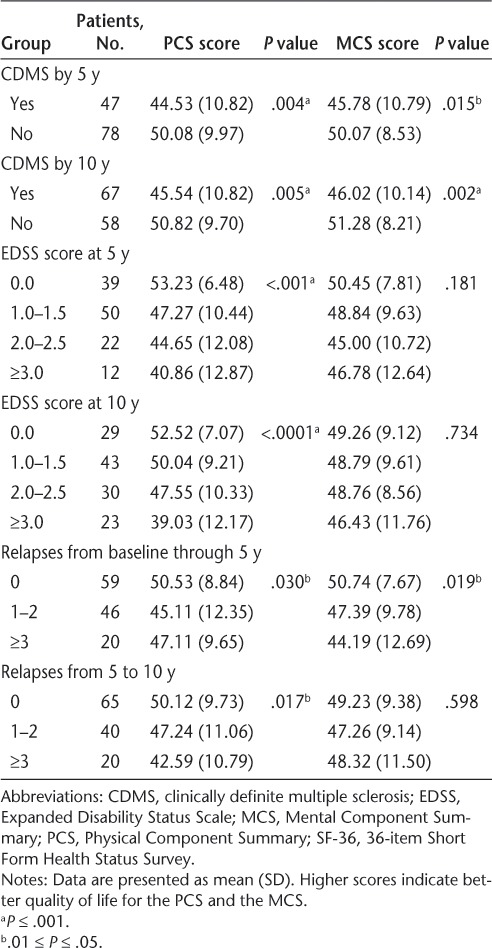
Table 5.
Association between SF-36 summary and EDSS scores at 10 years in patients reaching CDMS by 10 years
The number of relapses between CIS onset and 5 years and the number of relapses between 5 and 10 years were marginally, but not significantly, associated with lower SF-36 PCS scores (P = .030 and .017, respectively) (Table 4). Similarly, a nonsignificant association was found between a greater number of relapses between CIS onset and 5 years and lower SF-36 MCS scores at 10 years (P = .019) (Table 4).
The number of new or enlarging T2 lesions between CHAMPS baseline and 5 or 10 years was not associated with SF-36 scores (Table 3). Similarly, neither T2 lesion volume at 5 or 10 years nor change in T2 lesion volume between baseline and 10 years or between 5 and 10 years was associated with SF-36 PCS or MCS scores (Table 3; additional data not shown). There were also no significant associations between T2 lesion volume at 10 years and any of the 11 MSQLI subscores in patients with or without CDMS at 10 years. The BPF at 10 years was not associated with any MSQLI measure.
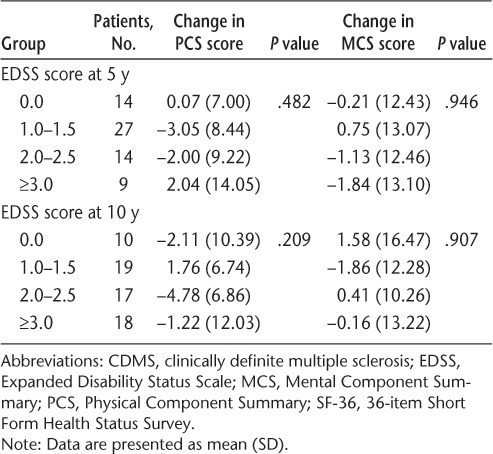
There were no univariate associations between change in mean SF-36 scores from 5 to 10 years and any baseline or interval clinical or MRI variables (Tables 6–8).
Table 6.
Association of baseline characteristics and MRI measures with SF-36 summary score changes between 5 and 10 years
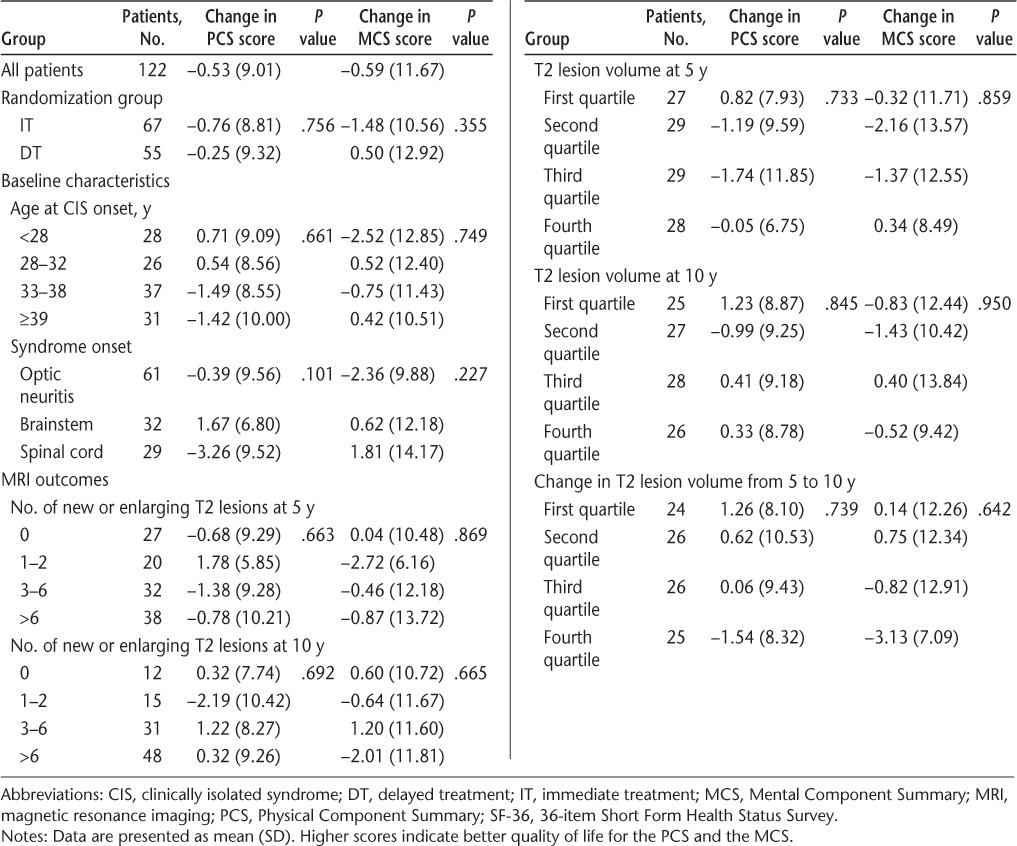
Table 7.
Association between clinical measures and SF-36 summary score changes between 5 and 10 years
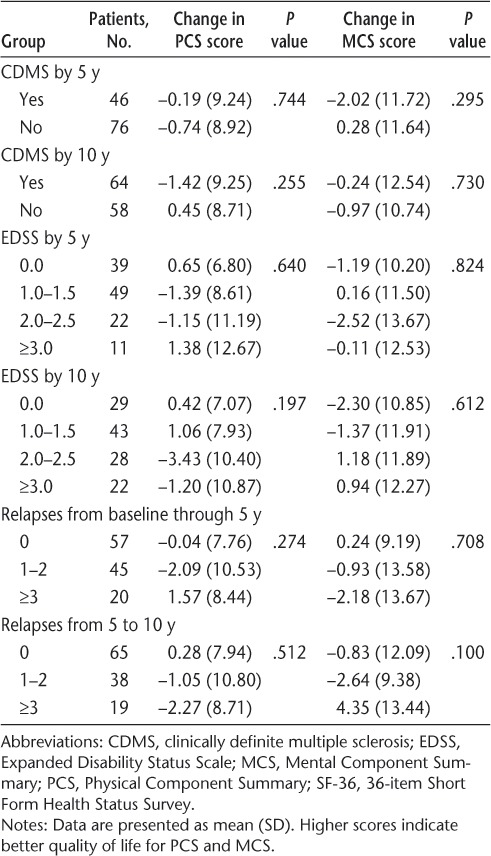
Table 8.
Association between SF-36 summary score changes between 5 and 10 years and EDSS scores at 10 years in patients reaching CDMS by 10 years
Further Analysis of QOL by Interval CDMS Status
To better understand the relationship between the time from onset of symptoms to a second clinical event consistent with CDMS and both generic and disease-specific QOL outcomes at 10 years, MSQLI subscale scores were categorized by interval CDMS status and were evaluated (Table 9). Specifically, MSQLI comparisons were performed between patient subgroups that developed CDMS between baseline and 5 years, those that developed CDMS between 5 and 10 years, and those that did not develop CDMS by 10 years. An MSQLI score comparison was also performed between patients who did and those who did not develop CDMS by 10 years.
Table 9.
MSQLI subscale scores at 10 years by interval CDMS status
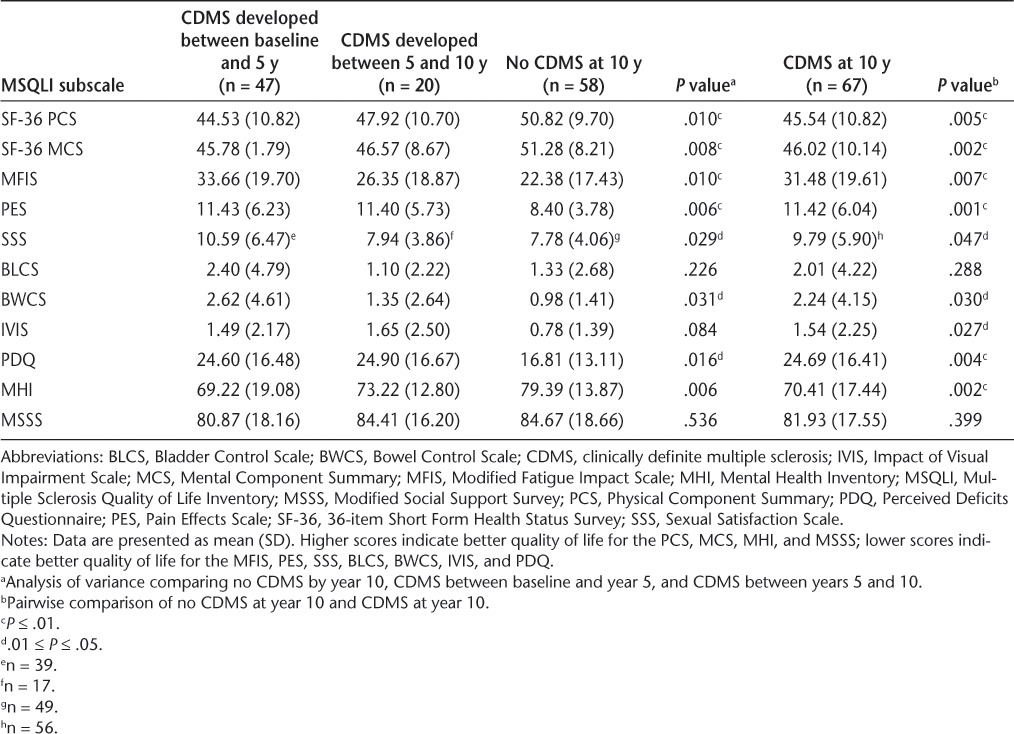
The SF-36 PCS, SF-36 MCS, MFIS (fatigue), PES (pain), and Mental Health Inventory scores at 10 years were significantly worse for patients with CDMS compared with patients without a CDMS diagnosis. Greater worsening in patient-reported QOL measures at 10 years was seen in patients with earlier CDMS diagnosed between baseline and 5 years than in patients who developed CDMS between 5 and 10 years. Scores on the Perceived Deficits Questionnaire (a self-reported measure of perceived cognitive impairment) at 10 years were also significantly worse in patients with CDMS than in patients without a CDMS diagnosis at 10 years, with no clear time-dependent relationship. Sexual Satisfaction Scale and Bowel Control Scale scores exhibited nonsignificant trends toward greater worsening in patients with CDMS than in those without CDMS. No significant associations were detected between conversion to CDMS and change in MSQLI subscale scores from 5 to 10 years (data not shown).
Discussion
Several phase 3 studies reported a reduction in the rate of a second clinical event consistent with CDMS in patients with high-risk CIS who started treatment with IFNβ shortly after onset of symptoms.3–5 A modest continued reduction in conversion to CDMS for IT patients persisted for 5 to 10 years, even after placebo-treated patients were switched to active treatment, but two studies did not demonstrate convincing evidence that early treatment with IFNβ after a CIS episode reduces long-term disability or improves MRI outcomes compared with delayed initiation of therapy.6,7,14 To date, the only direct evidence of a prolonged benefit from IFNβ treatment is a report of increased long-term survival 21 years after the start of the original phase 3 subcutaneous IFNβ-1b trial.15 Although ascertainment was high for this follow-up study, there was no evidence of reduced disability among survivors, and neither was it possible to control for relevant comorbid medical conditions that may have been unequally distributed between groups. Without a potential mechanism for the enhanced survival, which was primarily related to a higher rate of pulmonary infections in the group originally randomized to receive placebo, this potential treatment benefit will require further confirmation.15
Our previous report demonstrated that the development of CDMS and the EDSS score at 5 years are the only disease-related variables associated with a lower QOL at 5 years.8 This report extends the 5-year CHAMPIONS results, supporting the somewhat intuitive finding that patient-reported QOL is higher in patients who do not develop CDMS within 10 years of CIS onset. However, it is important to interpret these results in light of the diagnostic criteria for CDMS used in this study, which were based solely on clinical criteria; in fact, 96% of patients in the CHAMPIONS 10-year study eventually met current MS diagnostic criteria, which encompass relapses and new MRI lesions.2,7
Patients who developed CDMS earlier reported worse QOL at 10 years than those who developed CDMS later, as confirmed by the analysis distinguishing between patients who developed CDMS within 5 years and those who developed CDMS between 5 and 10 years (Table 4). These results provide further validation of the CDMS outcome measure commonly used in CIS treatment trials.
The SF-36 PCS score at 10 years was highly associated with the level of disability as measured by the EDSS. This association was noticeable at modest levels of disability (EDSS score ≥2.0) but was most pronounced with greater disability (EDSS score ≥3.0). There was also a relationship between lower SF-36 PCS scores and older age at CIS onset that was at least partly explained by the relationship between age at CIS onset and higher EDSS scores at 10 years. At each EDSS score level from 0.0 to 3.5, SF-36 PCS scores varied considerably, likely reflecting the pronounced interrater variability of EDSS scores between 0.0 and 3.5 and the inability of the EDSS to adequately characterize symptoms, patient concerns, and perceived disability at these lower EDSS scores.16,17 Hence, the use of patient-reported QOL measures can complement EDSS disability measures.
The SF-36 MCS score was lower in patients with CDMS than in those without CDMS at 10 years, but unlike the SF-36 PCS score, this was not associated with a significantly lower level of disability as measured by the EDSS. These results are consistent with a previous report that showed significant associations between PCS scores and EDSS scores and no significant associations between MCS scores and EDSS scores from a large international cohort of patients with CIS and MS.18
Disease-specific QOL measures particularly sensitive to worsening with the development of CDMS included the MFIS, PES, Perceived Deficits Questionnaire, and Mental Health Inventory. These QOL measures were also associated with CDMS status in the CHAMPIONS 5-year study and reflect commonly reported symptoms that are known to uniquely affect QOL early in MS and are not captured by typical neurologic impairment or disability scales, such as the EDSS.19–21
There were no significant associations between QOL at 10 years and any of the baseline MRI metrics or change in MRI metrics over any time span. There were also no significant associations between these measures when the analysis was performed separately in patients with and without CDMS at 10 years. This is perhaps not surprising because MSQLI measures were not associated with new or enlarging T2 lesions in the CHAMPIONS 5-year study.8 In contrast, Rudick et al.22 found associations between SF-36 subscores (PCS and MCS) and T1 and T2 lesion volumes in a combined analysis of patients who received natalizumab for 2 years. This was most likely due to the large number of patients in their combined analysis, the large effect of natalizumab treatment on these patients, and differences in the populations studied (patients with relapsing-remitting MS rather than CIS at treatment initiation).
Despite the association between the rate of developing CDMS and worsening SF-36 scores at 5 and 10 years, no effect of initial randomization group (DT vs. IT) was observed on either QOL scores at 5 or 10 years or change in QOL scores between 5 and 10 years. Therefore, we are not able to determine in our study whether the previously reported reduction in the rate of developing CDMS associated with earlier initiation of treatment is associated with improved QOL outcomes at 10 years.7 It is unclear whether this inability to see an effect on QOL at 10 years by initial randomization group is a result of the small sample size in this post hoc analysis or the treatment effect on the CDMS outcome is too small when comparing our IT and DT groups. The lack of an observed difference in EDSS scores by randomization group at 10 years suggests that the modest benefits of earlier initiation of therapy with IFN in slowing the development of CDMS and reducing early relapses do not translate into large differences in QOL at 10 years. Further studies are required to determine whether smaller yet meaningful improvements in long-term QOL occur with early initiation of therapy, especially under circumstances in which the control group experiences a longer delay in therapy initiation.
The results presented herein support the development of therapies that reduce the risk of a second clinical event consistent with CDMS and, more importantly, delay or prevent the attainment of certain EDSS milestones, perhaps as low as an EDSS score of 2.0, after CIS onset. Future CIS studies may consider a composite outcome that combines time to development of CDMS and a sustained EDSS score of 2.0 or greater, an outcome that likely reflects a significant reduction in QOL after CIS. Such a study would be feasible only if enriched for patients with CIS at high risk for short-term disease activity and disability progression.
Further work is required to develop clinical impairment measures more closely associated with PROs in patients with milder levels of disability early in the course of MS because most patients have been shown to maintain an EDSS score of less than 3.0 for up to 10 years after CIS regardless of whether IFN therapy is initiated at onset or at a later date (usually within 2.5 years of CIS onset).7 In addition to the use of CDMS and sustained low levels of disability as a primary measure, greater emphasis should be placed on PROs as an addition to primary or secondary outcome measures in earlier stages of the disease to assess the potential positive and negative effects of treatment.
PracticePoints.
Multiple Sclerosis Quality of Life Inventory scores, reflecting the perceived impact of fatigue, pain, mental health, and cognition on quality of life (QOL), 10 years after clinically isolated syndrome are significantly worse in patients who experience a second clinical event consistent with clinically definite MS (CDMS) by 10 years, with greater perceived impact in those who develop CDMS in the first 5 years.
Expanded Disability Status Scale (EDSS) score increases, even to as low as 2.0, at years 5 and 10 are associated with lower 36-item Short Form Health Status Survey (SF-36) Physical Component Summary scores at year 10. Patients with earlier onset of CDMS report worse QOL (as indicated by SF-36 scores) at year 10 than patients with later or no onset of CDMS.
In the Controlled High Risk Avonex® Multiple Sclerosis Prevention Study in Ongoing Neurologic Surveillance (CHAMPIONS), no demonstrated effect was observed for immediate initiation of therapy with interferon beta-1a (immediate-treatment group) versus delayed initiation of therapy (delayed-treatment group) on 10-year QOL measures.
Future studies of therapies for patients with high-risk clinically isolated syndrome should aim to reduce the risk of a second attack consistent with CDMS, delay the progression of physical disability to milestones as low as EDSS scores of 2.0, and improve long-term QOL.
Acknowledgments
The authors gratefully acknowledge Elizabeth Fisher, PhD, of the Cleveland Clinic for her work on the BPF analyses. We also thank Kit Bonin, PhD, and Joshua Safran of Infusion Communications for providing writing support under the direction of the authors. Funding for medical writing support was provided by Biogen Idec.
Footnotes
Financial Disclosures: Dr. Kinkel is a consultant for Biogen Idec, Teva Neuroscience, and Genzyme. He has received research support from Biogen Idec and Genzyme in the past 5 years. Dr. Laforet was an employee of Biogen Idec at the time this work was performed and is now an employee of Genzyme. Dr. You is an employee of Biogen Idec.
Funding/Support: This work was supported by Biogen Idec.
References
- 1.Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012;11:157–169. doi: 10.1016/S1474-4422(11)70274-5. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comi G, Filippi M, Barkhof F et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357:1576–1582. doi: 10.1016/s0140-6736(00)04725-5. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs LD, Beck RW, Simon JH. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. et al.; CHAMPS Study Group. [DOI] [PubMed] [Google Scholar]
- 5.Kappos L, Polman CH, Freedman MS et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- 6.Kinkel RP, Kollman C, O'Connor P et al. IM interferon beta-1a delays definite multiple sclerosis 5 years after a first demyelinating event. Neurology. 2006;66:678–684. doi: 10.1212/01.wnl.0000200778.65597.ae. [DOI] [PubMed] [Google Scholar]
- 7.Kinkel RP, Dontchev M, Kollman C, Skaramagas TT, O'Connor PW, Simon JH. Association between immediate initiation of intramuscular interferon beta-1a at the time of a clinically isolated syndrome and long-term outcomes: a 10-year follow-up of the Controlled High-Risk Avonex Multiple Sclerosis Prevention Study in Ongoing Neurological Surveillance. Arch Neurol. 2012;69:183–190. doi: 10.1001/archneurol.2011.1426. [DOI] [PubMed] [Google Scholar]
- 8.Miller DM, Kollman C, Kalajian A, O'Connor PW, Kinkel RP. Comparison of quality of life in clinically isolated syndrome patients who convert and do not convert to clinically definite multiple sclerosis. Int J MS Care. 2009;11:17–24. [Google Scholar]
- 9.Ware JE., Jr. SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 10. Kinkel RP, Laforet G, You X, Hyde R. Impact of intramuscular interferon beta-1a therapy timing after clinically isolated syndrome onset on quality of life 10 years later [abstract] Int J MS Care 2011. 13( suppl 3) S71 [Google Scholar]
- 11.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 12.Fisher E, Rudick RA, Simon JH et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology. 2002;59:1412–1420. doi: 10.1212/01.wnl.0000036271.49066.06. [DOI] [PubMed] [Google Scholar]
- 13.Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG, editors. Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York, NY: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 14.Kappos L, Freedman MS, Polman CH et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8:987–997. doi: 10.1016/S1474-4422(09)70237-6. [DOI] [PubMed] [Google Scholar]
- 15.Goodin DS, Reder AT, Ebers GC et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology. 2012;78:1315–1322. doi: 10.1212/WNL.0b013e3182535cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodkin DE, Cookfair D, Wende K. Inter- and intrarater scoring agreement using grades 1.0 to 3.5 of the Kurtzke Expanded Disability Status Scale (EDSS) Neurology. 1992;42:859–863. doi: 10.1212/wnl.42.4.859. et al.; Multiple Sclerosis Collaborative Research Group. [DOI] [PubMed] [Google Scholar]
- 17.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45:251–255. doi: 10.1212/wnl.45.2.251. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez O, Baumstarck-Barrau K, Simeoni MC, Auquier P. Patient characteristics and determinants of quality of life in an international population with multiple sclerosis: assessment using the MusiQoL and SF-36 questionnaires. Mult Scler. 2011;17:1238–1249. doi: 10.1177/1352458511407951. [DOI] [PubMed] [Google Scholar]
- 19.Brunet DG, Hopman WM, Singer MA, Edgar CM, MacKenzie TA. Measurement of health-related quality of life in multiple sclerosis patients. Can J Neurol Sci. 1996;23:99–103. doi: 10.1017/s0317167100038798. [DOI] [PubMed] [Google Scholar]
- 20.Rudick RA, Miller D, Clough JD, Gragg LA, Farmer RG. Quality of life in multiple sclerosis: comparison with inflammatory bowel disease and rheumatoid arthritis. Arch Neurol. 1992;49:1237–1242. doi: 10.1001/archneur.1992.00530360035014. [DOI] [PubMed] [Google Scholar]
- 21.Ruet A, Deloire M, Hamel D, Ouallet JC, Petry K, Brochet B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: a 7-year longitudinal study. J Neurol. 2013;260:776–784. doi: 10.1007/s00415-012-6705-1. [DOI] [PubMed] [Google Scholar]
- 22.Rudick RA, Miller D, Hass S et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:335–346. doi: 10.1002/ana.21163. [DOI] [PubMed] [Google Scholar]


