Abstract
Context:
Turmeric is obtained from the plant Curcuma longa L; its major constituent, curcumin, is a polyphenol with multiple effects which can modulate some signaling pathways.
Evidence Acquisition:
Insulin resistance is a major risk factor for chronic diseases such as type 2 diabetes, atherosclerotic, metabolic syndrome and cardiovascular disease. In addition, Insulin resistance in peripheral tissue is one of the most important reasons of hyperglycemia which can cause global or systemic effects. The present study reviewed studies published in PubMed from 1998 to 2013, indicating the role of curcumin in attenuation of many pathophysiological processes involved in development and progression of hyperglycemia and insulin resistance.
Results:
Curcumin can reduce blood glucose level by reducing the hepatic glucose production, suppression of hyperglycemia-induced inflammatory state, stimulation of glucose uptake by up-regulation of GLUT4, GLUT2 and GLUT3 genes expressions, activation of AMP kinase, promoting the PPAR ligand-binding activity, stimulation of insulin secretion from pancreatic tissues, improvement in pancreatic cell function, and reduction of insulin resistance.
Conclusions:
Curcumin has antihyperglycemic and insulin sensitizer effects. Thereby, more studies evaluating the effects of curcumin on hyperglycemic state and insulin resistance in related disorders such as diabetes are recommended.
Keywords: Turmeric, Curcumin, Curcuminoids, Curcuma longa, Hyperglycemia, Blood Glucose, Insulin Resistance, Hyperinsulinemia
1. Context
1.1. Turmeric or Curcuma longa
1.1.1. Health Benefits
Turmeric is obtained from the plant Curcuma longa L, which is a member of the ginger family. It is a rhizome consumed as a popular food ingredient in Iran as well as a key component of Indian, Chinese, Polynesian, Malaysian, and Thai curries, and western mustard preparing. The plant can be cultivated in tropical climate (1-3).
Turmeric has been used as a medical treatment in Asian countries for at least 2500 years (3). It has been used for centuries in Ayurvedic medicine and Unani systems of medicine as a therapy for inflammatory disorders, including hepatitis and arthritis, a blood purifier, a remedy for liver, stomach and dental problems, contraception, bactericide, germicide, beauty, deodorizing agent, disinfectant, a cure for leach and insect bites, cough, cold, sneeze, various types of skin diseases and related ailments such as Tinea versicolor, patches, itching, dermatitis, scabies, acne, psoriasis, rash, eruptions, and burn injuries. Based on these traditional applications, dietary supplements containing turmeric rhizome and extracts are also being used in western countries to prevent and treat arthritis (1, 3-6)
1.2. Curcumin
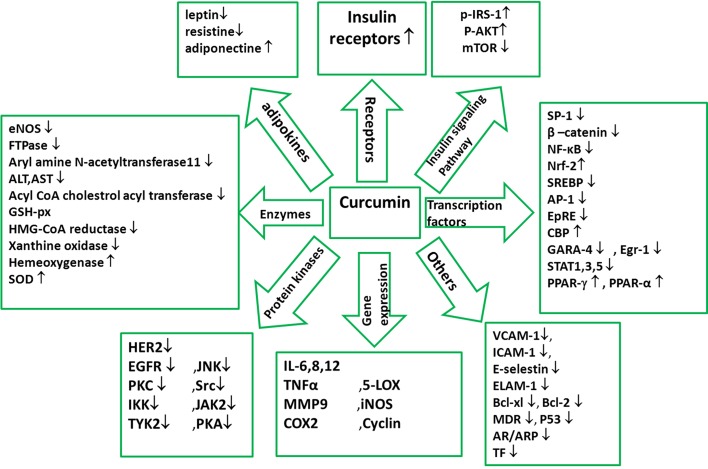
Curcumin is a lipophilic polyphenol approximately insoluble in water, but is quite stable in the acidic pH of stomach (5). During the recent decades, polyphenol intakes have been getting greater attention, possibly because of their protective roles against various degenerative diseases such as cardiovascular diseases and cancer (7). Curcumin is a polyphenol with multiple effects which can modulate the biological activity of a number of signaling pathways. Chemical structure of curcumin is 1, 7-bis [4-hydroxy-3-methoxyphenyl]-1, 6-heptadiene-3,5-dione (Figures 1-3) (4, 6, 8). Chemical structure of curcumin as a component of turmeric or a single supplement plays a role in suppression of platelet aggregation, tumor genesis, metastasis, oxidative processes, inflammatory cytokine production, and myocardial infarction. Curcumin can modulate cystic fibrosis defects, lower cholesterol, suppress diabetes, improve wound healing, enhance multiple sclerosis, and block human immunodeficiency virus (HIV) replication. Moreover, reports also demonstrate the role of curcumin in protection against cataract formation, adriamycin-induced nephrotoxicity, drug or alcohol induced liver injury, and Inflammatory Bowel Disease (IBD) (1). Extensive reports from in vitro and in vivo studies have manifested the molecular basis for pharmaceutical applications of this polyphenol against numerous chronic diseases such as cancer, autoimmune diseases, neurological diseases, metabolic disorders, and pulmonary, liver and cardiovascular diseases (6). Molecular targets of curcumin are summerized in Figure 2 and details follow.
Figure 1. Chemical Structure of Curcumin.
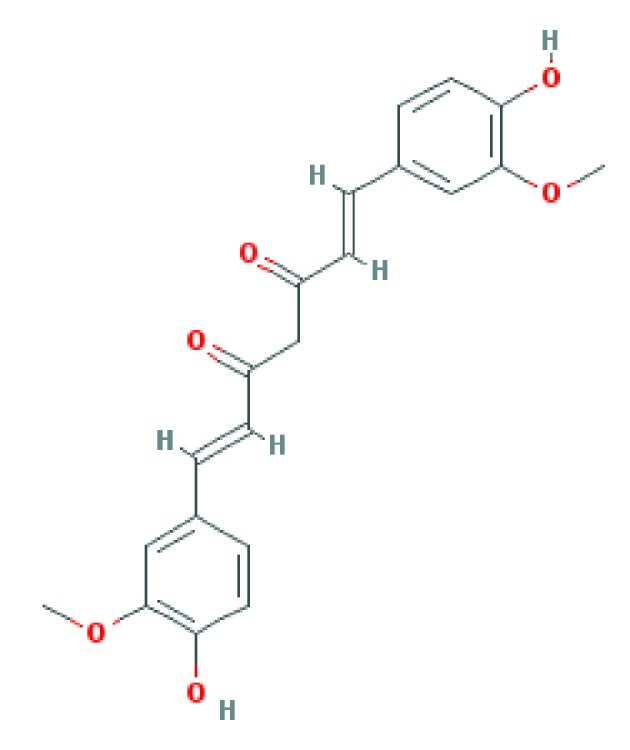
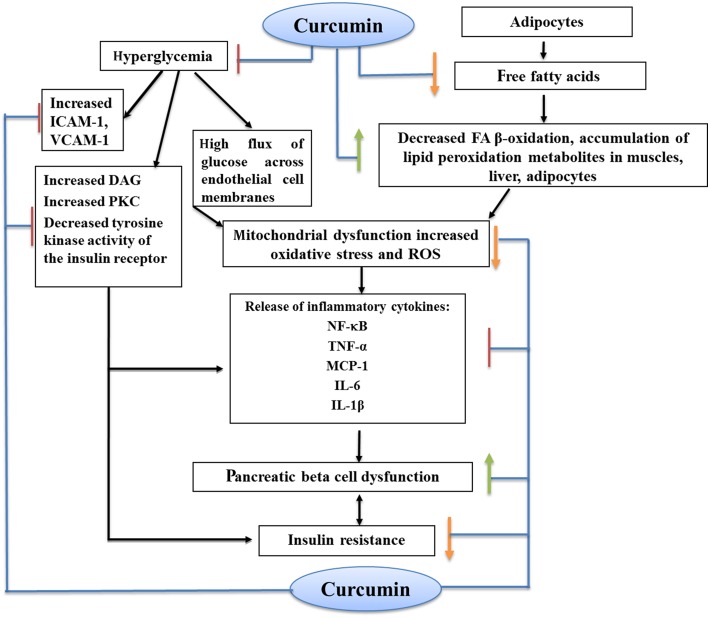
Figure 3. Antihyperglycemic and Anti-Insulin Resistance Effects of Curcumin.
Figure 2. Molecular Targets of Curcumin.
1.2.1. Anti-Inflammatory and Antioxidant Effects of Curcumin
Curcumin is constantly approved as an antioxidant and an anti-inflammatory agent. It seems that the hydroxyl and methoxy groups of curcumin are responsible for such effects (9). Curcumin performs these effects by modulating signaling pathways with many molecular targets (Figure 2) (10). Curcumin down-regulates signaling through the JAK/STAT pathway, which leads to negative regulation of proinflammatory interleukins (IL-1, -2, -6, -8, -12), and cytokines (tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1)). Curcumin modulates the inflammatory response by down-regulating the activity of cyclooxygenase-2 (COX-2), lipoxygenase, xanthine oxidase, and inducible nitric oxide synthase (iNOS) enzymes, resulting in inhibition of STAT3 phosphorylation and consequent STAT3 nuclear translocation (8-13) Curcumin inhibitions of COX-2 and iNOS are possibly contributed to suppression of the nuclear factor kappaB (NF-B) activation by this polyphenol. NF-B increases the expression of proinflammatory cytokines genes such as IL-1 and TNF-α and also stimulates the expression of inflammatory process enzymes including iNOS and COX-2 (9). TNF-α up-regulates the gene expression of various inflammatory cytokines which have causal association with hypertension, obesity, and high fasting glucose, decrease insulin sensitivity, and may lead to insulin resistance (IR), type 2 diabetes, and cardiovascular disease (14). Some studies have indicated that the beneficial anti-inflammatory effect of curcumin is mediated by up-regulation of peroxisome proliferator-activated receptor-γ (PPAR-γ) activation (15).
Curcumin stimulates the expression of Nrf2 and HO-1. Nrf2 is expressed in a wide range of tissues, many of which are sites of expression for phase 2 detoxification genes; It binds to the nuclear factor-erythroid derived 2 (NF-E2) binding sites, consisted of a subset of antioxidant response elements (ARE), which is a critical mechanism of protection against free radicals (9).
1.3. Hyperglycemia and Hyperinsulinemia
1.3.1. Insulin Function and Insulin Resistance
Insulin is a hormone with multiple effects. Binding of insulin to the α-subunit of the insulin receptor molecule induces rapid auto-phosphorylation of the β-subunit, which lead to increase of its tyrosine kinase activity. Tyrosine phosphorylation of insulin receptor proteins induces the cytoplasmic binding activity of insulin receptor substrate-1 (IRS-1) to insulin receptor. IRS-1 plays a key role in transmitting signals from insulin receptors to intracellular PI3K/Akt and MAP kinase pathways, which eventually results in the second intracellular step of insulin action (16-18), protein-tyrosine phosphatase 1B (PTP1B), a negative regulator of insulin signaling, which is elevated in obesity, targeting tyrosine-phosphorylated insulin receptor β and IRS-1. Therefore, PTP1B inhibition causes elevated insulin sensitivity and glucose tolerance; thus, inhibition of PTP1B can be a new target in treatment of impaired glucose tolerance and insulin signaling in diabetic patients (16). Some of the substances possibly affecting or playing roles in insulin signaling are shown in Table 1.
Table 1. Some Substances With Possible Roles in Insulin Signaling a (11, 12, 16, 21).
| Substance Name | Role in Insulin Signaling |
|---|---|
| GLUT | Translocated to plasma membrane for glucose influx |
| IL-6 | Impairs/inhibits insulin signaling; suppresses adipogenesis and secretion of adiponectin |
| NF-κB | Influences gene transcription; inhibited by adiponectin |
| PPAR-γ | Stimulates free fatty acid (FFA) catabolism; thiazolidinediones (TZDs) are PPAR-γ agonists |
| TNFα | Main factor for stimulating the secretion of FFAs from adipose tissue into circulation; impairs insulin signaling |
| MCP-1 | Impairs insulin-stimulated glucose uptake; promotes atherosclerosis |
| FFAs | Adipocytes decrease glucose uptake in peripheral tissues by releasing free fatty acids |
| Akt | A key regulator of insulin action and glucose uptake in mammals |
| IRS-1 | Plays a key role in transmitting signals from insulin receptor to intracellular PI3K/Akt pathways and Ras/mitogen activated protein kinase, which eventually mediate various actions of insulin |
| PTP1B | A negative regulator of insulin signaling which targets tyrosine-phosphorylated insulin receptor β and IRS-1 |
| mTOR | Inhibits IRS-1 tyrosine phosphorylation |
aGLUT, Glucose transporter; IL-6, Interleukin-6; NF-κB, Nuclear transcription factor κB; PPAR-γ, Peroxisome proliferator-activated receptor-gamma; TNFα, Tumor necrosis factor alpha; MCP-1,Monocyte chemoattractant protein-1; FFAs, free fatty acids; Akt, protein kinase B; IRS-1, insulin receptor substrate-1; PTP1B, protein–tyrosine phosphatase 1B; mTOR, mammalian target of rapamycin.
IR is a condition in which defects in the action of insulin are such that normal levels of insulin do not operate as the signal for glucose uptake. Pancreas compensates for the decreased insulin response by increasing the insulin secretion; however, the result is hyperinsulinemia to maintain euglycemia. This process will continue until the reserve capacity is surpassed by metabolic demands and insulin secretion is no more sufficient; then, blood glucose concentration rises and glucose intolerance and type 2 diabetes develop (18-20).
Adipocytes decrease glucose uptake in peripheral tissues by release of free fatty acids (FFAs). It seems that FFAs induce IR in muscle at the level of insulin-dependent glucose transport by impairing the insulin-signaling pathway (17, 18). On the other hand, when the serum FFAs concentration rises, signs of chronic inflammation are observed with the release of inflammatory cytokines including TNF-α, IL-6, and IL-1β, which have been implicated in pathogenesis of insulin resistance (17). Furthermore, increased oxidative stress and accumulation of lipid peroxidation metabolites in body, pancreatic beta cell dysfunction, mitochondrial dysfunction, and decreased FA β-oxidation are other factors which can lead to insulin resistance (17, 19).
Therefore, insulin resistance, even in the absence of obesity, is also a major risk factor for related chronic diseases such as type 2 diabetes, atherosclerotic and cardiovascular disease. Insulin resistance is a key component of metabolic syndrome, consisted of a series of risk factors such as abdominal obesity, hypertension, insulin resistance, and dyslipidemia (20)
1.3.2. Hyperglycemia
The process of glucose homeostasis maintains the plasma glucose levels within a narrow range, usually between 60 and 150 mg/dL (17, 18). GLUT-4 is located on cell membranes of muscle cells and adipocytes. Insulin binds to its receptor and initiates a signaling cascade which leads to translocation of GLUT-4 to plasma membrane, thus, permitting the influx of glucose; in the absence of insulin, GLUT-4 in cytosol is surrounded by membrane vesicles (18). Insulin resistance of peripheral tissue, discharge of glycogen storage, changed digestion and absorption of dietary carbohydrate, increased gluconeogenesis and over output hepatic glucose, and β-cell dysfunction are the most important reasons of hyperglycemia (22).
1.3.3. Hyperglycemia-Induced Tissue Damage
High flux of glucose across endothelial cell membranes is the initial point in the process of hyperglycemia-induced tissue damage. Increased glucose levels induce excess production of ROS, promote the protein kinase C (PKC) activity, and elevate the hexosamine pathway activation. Therefore, the generated oxidative stress can be responsible for the harmful effects of chronic hyperglycemia on pancreatic β-cell function. Additionally, pancreatic islets are chiefly composed of β cells and have the lowest antioxidant capacity among all metabolic tissues. Generally, it seems that over-productions of ROS and PKC mediate hyperglycemia-induced insulin resistance (23, 24). Moreover, hyperglycemia causes global or systemic effects such as vascular inflammation and immune system impairment by stimulating inflammatory cytokines and cell adhesion molecules and by inhibiting leukocyte function (18). This review summarized what is currently known about the effects of turmeric and its components on plasma glucose and insulin levels. Recent literature suggested that turmeric and its major component, curcumin, have antihyperglycemic and insulin sensitizer effects. We therefore aimed to present the putative mechanisms linking curcumin to blood glucose and insulin resistance.
2. Evidence Acquisition
The present study reviewed studies published from 1998 to 2013, indicating that curcumin attenuates many of the pathophysiological processes involved in development and progression of hyperglycemia and insulin resistance. It was based on a PubMed and Science Direct literature search using the fallowing MeSH terminology and keywords: turmeric, curcumin, curcuminoids, Curcuma longa, hyperglycemia, blood glucose, insulin resistance, and hyperinsulinemia.
3. Results
3.1. Studies Conducted on the Effects of Curcumin on Blood Glucose Levels and Insulin Resistance
3.1.1. In Vivo/in Vitro Experiments
In vitro and in vivo studies are summarized in Table 2.
Table 2. Beneficial Effects of Supplementary Curcumin on Hyperglycemic Animal Models and High Glucose State Cell Culturea.
| In Vivo/in Vitro experiment | Dose, Duration | Results In the End of the Study in Treatment Group | Overall Conclusion | Authors/Publication year | Reference Number |
|---|---|---|---|---|---|
| STZ induced Diabetic Wistar rats (55 mg/kg) | 60 mg/kg Curcumin 14 days | ↑ Gene expression of: acetylcholine esterase, Glut3 Muscarinic M1, M3, α7 nicotinic acetylcholine and insulin receptors | Curcumin plays an important role in regulating the activity of cholinergic and insulin receptors and mechanism of glucose transportation through Glut3, which lead to normalizing the diabetes-mediated cerebellar disorders. Thus, Curcumin has a significant role in a therapeutic application for the prevention or progression of diabetic complications in the cerebellum. | Peeyush,KT and collegues/2009 | (25) |
| high-fat diet+30 mg/kg STZ induced Diabetic rats (in vivo study) | 3 groups 50, 150, or 250mg/kg Curcumin. 7 wk | plasma lipids and glucose ↓,glucose uptake↑, phosphorylated acetyl COA carboxylase in L6 myotubes ↑, phosphorylated AMPK ↑, carnitine palmitoyl transferase 1 ↑, pyruvate dehydrogenase 4 ↓, phosphorylated glycogen synthase (GS) ↓, glycogen synthesis in skeletal muscle ↑, in both in vivo and in vitro studies. | Curcumin enhances muscular insulin resistance by promoting oxidation of fatty acid and glucose, which is, at least in part, mediated through LKB1-AMPK pathway. | Na,LX and collegues/2011 | (26) |
| L6 myotubes (in vitro experiment) | Curcumin at 5, 10, 20, or 40 mol/L | ||||
| high fat diet induced Diabetic Sprague Dawley rats | Curcumin (80 mg/kg) 15 days | hyperglycemia ↓, Improve glucose tolerance, Improve lipid profile, Fasting insulin ↑, Insulin sensivity ↑ | Curcumin exhibited an anti-hyperglycemic effect and improved insulin sensitivity | El-Moselhy,MA and collegues/2011 | (27) |
| Alloxan induced diabetic rats | Turmeric 1 g/kg and Curcumin 0.08 g/kg 21 days | blood sugar ↓, Hb and glycosylated hemoglobin levels ↓ , diabetic oxidative stress ↓ | Curcumin was more effective in attenuating diabetes mellitus related changes than turmeric | Arun,N and colleagues /2002 | (28) |
| Wild-type and ob/ob C57BL/6J mice | Dietary Curcumin (3%) 10 wks. | HbA1c levels ↓, improves glucose tolerance blood glucose levels ↓, adipose, hepatic, and systemic inflammation ↓, Serum adiponectin levels ↑, serum levels of liver and adipocytes MCP-1 ↓ | Curcumin in high oral doses safely treat diabetes in several mouse models of obesity-associated diabetes. Curcumin also greatly improved inflammation at the cellular and biochemical level in white adipose tissue of obese mice. | Weisberg,SP and colleagues /2008 | (29) |
| C57BL/ KsJ-ob/obmice | Curcumin (0.02%, wt/wt) 6 wks | blood glucose ↓, HbA1c levels ↓, improved HOMA-IR and glucose tolerance, plasma insulin ↑, plasma free fatty acid, cholesterol, and triglyceride concentrations hepatic glycogen ↓, skeletal muscle lipoprotein lipase ↑, lipid peroxidation. ↓ | It seems that Curcumin is a potential glucose-lowering agent and antioxidant in type 2 diabetic ob/obmice, but had no effect in non-diabetic db/+ mice. | Seo,KI and colleagues /2008 | (30) |
| type 2 diabetic KK-Ay mice | Turmeric Et-OH Extract 0.2 or 1.0 g/100 g diet 4 Weeks | blood glucose level ↓ | Turmeric Et-OH extract has PPAR-γ ligand-binding activity which probably leads to Adipocyte that finally result in the suppression of an increase in blood glucose levels in type 2 diabetic KK-Ay mice | Kuroda,M and colleagues /2005 | (31) |
| In Vitro Experiments | human pre-adipocytes were cultured with the Turmeric Et-OH Extract 2.0, 5.0, 10, and 20 g/ml | Stimulated adipocyte differentiation | |||
| STZ induced Diabetic rats | 60 mg/kg oral Curcumin 28 days | The expression of both TNF-α and TNF-α receptor 1 ↓, Hyperglycemia ↓ , body weight improved | Curcumin seems to relieve diabetic hyperalgesia, probably through an inhibitory effect on TNF-α and TNF-α receptor 1. | Li,Y and colleagues /2013 | (32) |
| type 2 diabetic KK-Ay mice | turmeric extracts:, 0.1 or 0.5 g of hexane extraction/100 g of diet, 0.1 or 0.5 g of ethanol, extraction from hexane-extraction residue/100 g of diet 0.2 or 1.0 g of ethanol extraction/100 g of diet 4 weeks | E-ext stimulated human adipocyte differentiation turmeric extracts and Curcumin had human peroxisome proliferator-activated receptor-γ (PPAR-γ) ligand-binding activity | Both Curcuminoids and sesquiterpenoids in turmeric exhibit hypoglycemic effects via PPAR-γ activation as one of the mechanisms, and suggest that E-ext including Curcuminoids and sesquiterpenoids has the additive or synergistic effects of both components. | Nishiyama,T and colleagues /2005 | (33) |
| Cell culture of Pancreas and muscle tissues of adult mice | aqueous extract of turmeric (100 g of ground turmeric in 1 L of water) various dose | insulin secretion from mouse pancreatic tissues ↑, glucose uptake ↑ | Water soluble constituents of turmeric exhibit insulin releasing and stimulating actions within in vitro tissue culture conditions | Mohankumar,S and colleagues /2010 | (34) |
| Skeletal muscle isolated from Wistar rats. | 0.1 -1 m Curcumin on cultured C2C12 cells | glucose uptake into skeletal muscle isolated ↑, the membrane protein level of GLUT4 ↑ | Curcumin can activate muscarinic M-1 cholinoceptor (M1-mAChR) at concentrations lower than to scavenge free radicals for increase of glucose uptake into skeletal muscle through PLC-PI3-kinase pathway | Cheng,TC and colleagues /2009 | (35) |
| In vivo: STZ induced Diabetic Sprague–Dawley rats | synthetic analogues of Curcumin )C66) (0.2, 1.0 or 5.0 mg·kg-1), 6 wks. | TNF-a levels and renal inflammatory gene expression ↓ improved histological abnormalities and fibrosis of diabetic kidney | This novel compound is a potential anti-inflammatory agent and might be beneficial for the prevention of diabetic nephropathy; but did not affect the hyperglycemia in these diabetic rats. | Pan,Y and colleagues /2012 | (36) |
| In vitro :Primary peritoneal macrophages (MPM), prepared from C57BL/6 mice | Treated with HG (high glucose) in the presence or absence of C66. | Inhibited HG-stimulated production of TNF-α , NO, IL-1β, IL-6, IL-12, COX-2 and iNOS mRNA transcription, and the activation of JNK/NF-κB signalling | |||
| Male Golden-Syrian hamsters | Curcumin (0.05-g/100-g diet) on a high-fat diet 10 weeks. | levels of free fatty acid, total cholesterol, triglyceride ↓, leptin ↓, HOMA-IR ↓, HDL-C ↑, apolipoprotein (apo) A-I ↑, paraoxonase activity in plasma ↑, fatty acid β-oxidation activity ↑, fatty acid synthase ↓, 3-hydroxy-3-methylglutaryl coenzyme A reductase ↓, acyl coenzyme A:cholesterol acyltransferase ↓, lipid peroxide levels in the erythrocyte and liver ↓ | Curcumin showes an obvious hypolipidemic effect by increasing plasma paraoxonase activity, ratios of high-density lipoprotein cholesterol to total cholesterol and of apo A-I to apo B, and hepatic fatty acid oxidation activity with simultaneous inhibition of hepatic fatty acid and cholesterol biosynthesis in high-fat–fed hamsters. | Jang,EM and colleagues /2008 | (37) |
| low dose of STZ + high energy intake induced diabetic wistar rats | Curcumin 100 or 200 mg/kg/ 16 weeks | myocardial dysfunction ↓, cardiac fibrosis ↓ , AGEs accumulation ↓ ,oxidative stress ↓,inflammation ↓,apoptosis ↓, phosphorylation of Akt and GSK-3b ↑ Activation Akt/GSK-3b Signaling Pathway ↑ blood glucose levels ↓, TG ↓ | Curcumin may have great therapeutic effect in the treatment of DCM, and perhaps other cardiovascular disorders, by enhancing fibrosis, oxidative stress, inflammation and cell death | Yu,W and colleagues /2012 | (38) |
| STZ (65 mg/kg) induced Diabetic rats | Curcumin (15 and 30 mg/kg) 2 weeks | renal dysfunction ↓, oxidative stress ↓ | Perhaps anti-oxidative mechanism being responsible for the nephroprotective effect of Curcumin. | Sharma,S and colleagues /2006 | (39) |
| C57BL6 mice (5-6 weeks old) | 24 weeks either a high-fat diet (45% fat) or a low-fat diet (10% fat) together with CNB-001 (40 mg/kg/d) | a novel neuroprotective curcuminoid CNB-001: body weight gain ↓, serum triglycerides ↓, IL-6 ↓, Improved insulin signaling: p-Akt (phosphoprotein kinase B) ↑, p-IR (phosphoinsulin receptor) β ↑, endoplasmic reticulum (ER) stress ↓, proteintyrosine phosphatase1B (PTP1B) ↓ | CNB-001 alleviates obesity-induced glucose intolerance and represents a potential candidate for further development as an anti-diabetic agent | Panzhinskiy E and colleagues /2014 | (16) |
a STZ, Streptozotocin; HG, high glucose; p-Akt, phosphoprotein kinase B ; p-IR, phosphoinsulin receptor β; ER, endoplasmic reticulum; PTP1B, protein–tyrosine phosphatase1B; TG,Triglyceride.
Elmoselhy et al. showed that prophylactic oral administration of curcumin (80 mg/kg) was comparable to rosiglitazone (1 mg/kg), which may indicate that they mostly act similarly (27).
Six-week administration of curcumin (0.02%, wt/wt) in ob/ob mice could increase the hepatic glucokinase activity and decrease glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activities significantly. In ob/ob mice, curcumin significantly lowered the hepatic activities of fatty acid synthase, -oxidation, 3-hydroxy-3-methylglutaryl coenzyme reductase, and acyl-CoA: cholesterol acyltransferase. Curcumin significantly lowered plasma free fatty acids, cholesterol, and triglyceride concentrations and increased the hepatic glycogen and skeletal muscle lipoprotein lipase in ob/ob mice. Curcumin normalized erythrocyte and hepatic antioxidant enzyme activities (superoxide dismutase, catalase, glutathione peroxidase) in ob/ob mice which resulted in a significant reduction in lipid peroxidation (30). In an experimental model of streptozicine (STZP)-induced diabetes, oral administration of curcumin at a dose of 100 mg/kg/day for 8 weeks, significantly corrected all the abnormalities induced by STZ injection and hyperglycemia, such as renal dysfunction, reduced creatinine clearance, increased blood glucose, blood urea nitrogen and proteinuria, and significant reduction in the body weight. Curcumin treatment significantly decreased macrophage infiltration in kidneys of diabetic rats, inhibited the expression of proinflammatory cytokines such as TNF-α and IL-1, and degradation of I Bα. Additionally, it significantly decreased ICAM-1, MCP-1 and TGF-1 protein expressions. Moreover, at nuclear level, curcumin inhibited the NF-B activity (40).
Fujiwara et al. showed that after 120 minutes of exposure to 25 mM curcumin, hepatic gluconeogenesis and glycogenolysis were inhibited significantly. Curcumin (25 mM) showed an additive inhibitory effect with insulin on both hepatic gluconeogenesis and glycogenolysis, indicating that it inhibits hepatic glucose production in an insulin-independent manner. After 120 minutes of exposure to 25 mM curcumin, hepatic glucose-6-phosphatase (G6Pase) activity and phosphoenolpyruvate carboxykinase (PEPCK) activity were both inhibited by 30%. After 120 minutes of exposure to 25 mM curcumin, phosphorylation of AMP kinase a-Thr172 increased. They concluded that the antidiabetic effects of curcumin might be partly due to a reduction in hepatic glucose production caused by activation of AMP kinase and inhibition of G6Pase activity and PEPCK activity (41).
Pretreatment of pancreatic islets from C57/BL6J mice with curcumin (10 mM) protected the islets from cytokine-induced islet death by scavenging ROS and normalized the cytokine production induced by NF-B translocation through inhibiting phosphorylation of inhibitor of kappa B alpha (I Bα). In addition, curcumin prevented STZ-induced diabetes, as manifested by sustained normoglycemia, had normal glucose clearance performance, and maintained pancreatic GLUT2 levels. Proinflammatory cytokine concentrations in serum and pancreas rose in STZ-treated animals, but not in animals pretreated with curcumin before STZ injection (42).
3.1.2. Curcumin, Cell Function and Insulin
Curcumin activated the volume-regulated anion channels in cells. This effect was accompanied by depolarization of the cell membrane potential, generation of electrical activity, and enhanced insulin release. Curcumin also decreased the cell volume, presumably reflecting loss of Cl (and hence water) as a result of anion channel activation (43). In a recent study, administration of a new neuroprotective curcuminoid, CNB-001, improved intracellular insulin signaling, which was revealed by increased level of phosphoprotein kinase B (p-Akt), phosphor-insulin receptor (p-IR) β, as well as decreased level of endoplasmic reticulum (ER) stress, protein–tyrosine phosphatase 1B (PTP1B), and glucose uptake in gastrocnemius muscle of HFD fed mice (16).
3.1.3. The Curcumin Reduction Effects on Hyperglycemia-Induced Circulating ICAM-1, VCAM-1
Endothelial cells release multiple inflammatory mediators and express various adhesion molecules such as intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 (ICAM-1, VCAM-1). An acute increase of plasma glucose may lead to an oxidative stress, resulting in increased cellular expression of ICAM-1 (44). In another study, hyperglycemia triggered an inflammatory response in the retina of normolipidemic mice and up-regulated VCAM-1 in retinal vessels (45). Curcumin inhibits inflammation by blocking the adhesion of monocytes to endothelial cells. by inhibiting the activation of cell adhesion molecules ICAM-1 and VCAM-1 (11, 40).
3.2. Human Clinical Trials of Curcumin
Human clinical trials evaluating the effects of curcumin supplementation on blood glucose and insulin resistance are summarized in Table 3.
Table 3. Human Clinical Trials Evaluating the Effects of Curcumin Supplementation on Blood Glucose and Insulin Resistancea.
| patients | Dose, Duration | Results In the End of the Study in Treatment Group | Overall Conclusion | Authors/Publication year | Reference Number |
|---|---|---|---|---|---|
| 100 Overweight/obese type 2 diabetic patients | Curcuminoids (300 mg/day) 3 months | fasting blood glucose ↓, insulin resistance index (HOMA-IR) ↓, serum total FFAs ↓, serum triglycerides ↓, LPL activity ↑ | These findings indicate a glucose-lowering effect of Curcuminoids in type 2 diabetes, which is partially due to decrease in serum FFAs, which may result from promoting fatty acid oxidation and utilization | Na LX and colleagues/ 2012 | (46) |
| subjects (n = 240) with criteria of prediabetes | six Curcumin capsules per day (Each capsule has Curcuminoid content of 250mg) 9months | 16.4% of subjects in the placebo group were diagnosed with T2DM, whereas none were diagnosed with T2DM in the Curcumin-treated group the Curcumin-treated group showed: a better overall function of βcells HOMA-β ↑, C-peptide ↓, HOMA-IR ↓, Adiponectin ↑ | Curcumin intervention in a pre-diabetic population significantly lowered the number of pre diabetic individuals who eventually developed T2DM. In addition, the Curcumin treatment appeared to improve overall function of β-cells, with very minor adverse effects. | Chuengsamarn Sand colleagues /2012 | (47) |
| Eleven healthy subjects, aged 21–38 y with normal fasting blood glucose (100 mg/dL) and total cholesterol (200 mg/dL) concentrations | a randomly assigned, crossover design: 6 subjects 3 .0g cinnamon 5subjects 2.8 g turmeric per day each treatment 4-wk | no significant changes in fasting plasma glucose or lipids in conjunction with the 4-wk periods of each treatment. | Maybe turmeric supplementation in healthy nondiabetic subjects did not reflect blood glucose-lowering effect | Tang M and colleagues/ 2008 | (48) |
| 60 subjects, 20 years old and above, who were diagnosed mild to moderate elevated ALT levels with normal glucose levels(91.9 ± 9.7) | fermented turmeric powder 3.0 g per day 12 weeks | reduction in ALT,AST levels no significant changes in blood glucose level | FTP is effective and safe, generally well-tolerated without severe Adverse events, in the treatment of subjects with elevated ALT levels over a 12 weeks period | Kim SW and colleagues /2013 | (49) |
| Fourteen healthy subjects in a crossover trial. | 75 g oral glucose tolerance test (OGTT) together with capsules containing a placebo or C. longa (6 g) | no significant effect on the glucose response The insulin AUCs were significantly higher | The ingestion of 6 g C. longa increased postprandial serum insulin levels, but did not seem to affect plasma glucose levels or GI, in healthy subjects. The results indicate that C. longa may have an effect on insulin secretion | Wickenberg J and colleagues/ 2010 | (47) |
a HOMA-IR, insulin resistance index; OGTT,glucose tolerance test.
3.3. Schematic Roles of Curcumin in Reduction of Blood Glucose Level and Serum FFAs, Improvement of Inflammatory State and Decreasing Insulin Resistance
3.3.1. Reduction of Blood Glucose Level by Curcumin
AMPK pathway mediates the regulatory effect of curcumin on lipid and glucose oxidation and utilization, inhibits hepatic gluconeogenesis and glycogenolysis:
Inhibits hepatic G6Pase and phosphoenolpyruvate carboxykinase (PEPCK) activity. Increases phosphorylation of AMP kinase (which leads to phosphorylation of GEF (GLUT4 enhance factor)). Inhibits PDK4 expression.Decreases glycogen synthesis. Reduces hepatic glucose production.Increases PPAR ligand-binding activity. Increases phosphorylation of AKT (PKB), insulin receptor and IRS-1. Decreases PTP1B and HOMA-IR. Increases HOMA and improves cell function.Stimulates insulin secretion from pancreatic tissues. Increase GLUT4 and stimulates of glucose uptake.Reduces the oxidative stress and inflammatory state. Increases the adiponectin levels.Decreases HbA1c and blood glucosylation toward improving the insulin sensitivity and glucose control in skeletal muscle.Anti hyperglycemic and anti insulin resistance effects of curcumin are shown in Figure 3.
4. Conclusions
Curcumin can reduce blood glucose and HbA1c level by reduction in hepatic glucose production and glycogen synthesis and stimulation of glucose uptake by increasing GLUT4, GLUT2 and GLUT3 gene expressions, increasing activation of AMP kinase, promoting PPAR γ ligand-binding activity, suppressing hyperglycemia-induced inflammatory state, stimulation of insulin secretion from pancreatic tissues, improvement in pancreatic cell function, Increasing phosphorylation of AKT (PKB), insulin receptor β and reduction of insulin resistance. In human clinical trials conducted on diabetic and prediabetic patients, glucose lowering effect of turmeric and curcumin have been observed. However, no effect was seen in patients with normal baseline levels of blood sugar. More studies evaluating the effects of curcumin on hyperglycemic state and insulin resistance in related diseases such as diabetes are recommended.
Footnotes
Authors’ Contributions:Study concept and design, analysis and interpretation of data, drafting of the manuscript and revision of the manuscript for important intellectual content: Zeinab Ghorbani, Azita Hekmatdoost, and Parvin Mirmiran.
References
- 1.Agrawal S, Chakrabarti A. Potential Nutraceutical Ingredients from Plant Origin. In: editor Handbook of Nutraceuticals, editor. Pathak Y,. CRC Press; 2010. pp. 30–1. [Google Scholar]
- 2.Kotwal GJ. A Versatile Nutraceutical and an Inhibitor of Complemen. In: Boca R, editor. Kotwal GJ. Curcumin. Nutraceuticals: CRC Press; 2010. pp. 217–20. [Google Scholar]
- 3.Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol Nutr Food Res. 2013;57(9):1510–28. doi: 10.1002/mnfr.201100741. [DOI] [PubMed] [Google Scholar]
- 4.Tilak JC, Banerjee M, Mohan H, Devasagayam TP. Antioxidant availability of turmeric in relation to its medicinal and culinary uses. Phytother Res. 2004;18(10):798–804. doi: 10.1002/ptr.1553. [DOI] [PubMed] [Google Scholar]
- 5.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–53. [PubMed] [Google Scholar]
- 6.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39(1):2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 7.Sohrab G, Hosseinpour-Niazi S, Hejazi J, Yuzbashian E, Mirmiran P, Azizi F. Dietary polyphenols and metabolic syndrome among Iranian adults. Int J Food Sci Nutr. 2013;64(6):661–7. doi: 10.3109/09637486.2013.787397. [DOI] [PubMed] [Google Scholar]
- 8.Maradana MR, Thomas R, O'Sullivan BJ. Targeted delivery of curcumin for treating type 2 diabetes. Mol Nutr Food Res. 2013;57(9):1550–6. doi: 10.1002/mnfr.201200791. [DOI] [PubMed] [Google Scholar]
- 9.Rahman I, Biswas SK. Regulation of Inflammation, Redox, and Glucocorticoid Signaling by Dietary Polyphenols. In: Surh YJ, Dong Z, Cadenas E, Packer L, editors. Boca Raton: CRC Press; 2009. [Google Scholar]
- 10.Grynkiewicz G, Slifirski P. Curcumin and curcuminoids in quest for medicinal status. Acta Biochim Pol. 2012;59(2):201–12. [PubMed] [Google Scholar]
- 11.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206–17. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 12.Sahebkar A. Why it is necessary to translate curcumin into clinical practice for the prevention and treatment of metabolic syndrome? BioFactors. 2013;39(2):197–208. doi: 10.1002/biof.1062. [DOI] [PubMed] [Google Scholar]
- 13.Soetikno V, Suzuki K, Veeraveedu PT, Arumugam S, Lakshmanan AP, Sone H, et al. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov Today. 2013;18(15-16):756–63. doi: 10.1016/j.drudis.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Asghari G, Sheikholeslami S, Mirmiran P, Chary A, Hedayati M, Shafiee A, et al. Effect of pomegranate seed oil on serum TNF-alpha level in dyslipidemic patients. Int J Food Sci Nutr. 2012;63(3):368–71. doi: 10.3109/09637486.2011.631521. [DOI] [PubMed] [Google Scholar]
- 15.Jacob A, Wu R, Zhou M, Wang P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-gamma Activation. PPAR Res. 2007;2007:89369. doi: 10.1155/2007/89369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panzhinskiy E, Hua Y, Lapchak PA, Topchiy E, Lehmann TE, Ren J, et al. Novel curcumin derivative CNB-001 mitigates obesity-associated insulin resistance. J Pharmacol Exp Ther. 2014;349(2):248–57. doi: 10.1124/jpet.113.208728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martyn JA, Kaneki M, Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109(1):137–48. doi: 10.1097/ALN.0b013e3181799d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul S, Jellinger. Metabolic consequences of hyperglycemia and insulin resistance. Clinical Cornerstone. 2007;8:S30–42. doi: 10.1016/s1098-3597(07)80019-6. [DOI] [PubMed] [Google Scholar]
- 19.Bahadoran Z, Mirmiran P, Azizi F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J Med Food. 2013;16(5):375–82. doi: 10.1089/jmf.2012.2559. [DOI] [PubMed] [Google Scholar]
- 20.Hekmatdoost A, Mirmiran P, Hosseini-Esfahani F, Azizi F. Dietary fatty acid composition and metabolic syndrome in Tehranian adults. Nutrition. 2011;27(10):1002–7. doi: 10.1016/j.nut.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0028784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12(1):43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 24.Campos C. Chronic hyperglycemia and glucose toxicity: pathology and clinical sequelae. Postgrad Med. 2012;124(6):90–7. doi: 10.3810/pgm.2012.11.2615. [DOI] [PubMed] [Google Scholar]
- 25.Peeyush KT , Gireesh G, Jobin M, Paulose CS. Neuroprotective role of curcumin in the cerebellum of streptozotocin-induced diabetic rats. Life sciences. 2009;85(19-20) doi: 10.1016/j.lfs.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Na LX, Zhang YL, Li Y, Liu LY, Li R, Kong T, et al. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. [Research Support, Non-U.S. Gov’t]. 2011;Jul;21(7) doi: 10.1016/j.numecd.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 27.El-Moselhy MA, Taye A, Sharkawi SS, El-Sisi SF, Ahmed AF. The antihyperglycemic effect of curcumin in high fat diet fed rats. Role of TNF-alpha and free fatty acids. Food Chem Toxicol. 2011;49(5):1129–40. doi: 10.1016/j.fct.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Arun N, Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum Nutr. 2002;57(1):41–52. doi: 10.1023/a:1013106527829. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149(7):3549–58. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seo KI, Choi MS, Jung UJ, Kim HJ, Yeo J, Jeon SM, et al. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008;52(9):995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 31.Kuroda M, Mimaki Y, Nishiyama T, Mae T, Kishida H, Tsukagawa M, et al. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biol Pharm Bull. 2005;28(5):937–9. doi: 10.1248/bpb.28.937. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zhang Y, Liu DB, Liu HY, Hou WG, Dong YS. Curcumin attenuates diabetic neuropathic pain by downregulating TNF-alpha in a rat model. Int J Med Sci. 2013;10(4):377–81. doi: 10.7150/ijms.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, et al. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. J Agric Food Chem. 2005;53(4):959–63. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- 34.Mohankumar S, McFarlane JR. An aqueous extract of Curcuma longa (turmeric) rhizomes stimulates insulin release and mimics insulin action on tissues involved in glucose homeostasis in vitro. Phytother Res. 2011;25(3):396–401. doi: 10.1002/ptr.3275. [DOI] [PubMed] [Google Scholar]
- 35.Cheng TC, Lin CS, Hsu CC, Chen LJ, Cheng KC, Cheng JT. Activation of muscarinic M-1 cholinoceptors by curcumin to increase glucose uptake into skeletal muscle isolated from Wistar rats. Neurosci Lett. 2009;465(3):238–41. doi: 10.1016/j.neulet.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol. 2012;166(3):1169–82. doi: 10.1111/j.1476-5381.2012.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang EM, Choi MS, Jung UJ, Kim MJ, Kim HJ, Jeon SM, et al. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism. 2008;57(11):1576–83. doi: 10.1016/j.metabol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, et al. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33(10):940–5. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 40.Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-kappaB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond). 2011;8(1):35. doi: 10.1186/1743-7075-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujiwara H, Hosokawa M, Zhou X, Fujimoto S, Fukuda K, Toyoda K, et al. Curcumin inhibits glucose production in isolated mice hepatocytes. Diabetes Res Clin Pract. 2008;80(2):185–91. doi: 10.1016/j.diabres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Kanitkar M, Gokhale K, Galande S, Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol. 2008;155(5):702–13. doi: 10.1038/bjp.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Best L, Elliott AC, Brown PD. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem Pharmacol. 2007;73(11):1768–75. doi: 10.1016/j.bcp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Ceriello A, Falleti E, Motz E, Taboga C, Tonutti L, Ezsol Z, et al. Hyperglycemia-induced circulating ICAM-1 increase in diabetes mellitus: the possible role of oxidative stress. Horm Metab Res. 1998;30(3):146–9. doi: 10.1055/s-2007-978854. [DOI] [PubMed] [Google Scholar]
- 45.Gustavsson C, Agardh CD, Zetterqvist AV, Nilsson J, Agardh E, Gomez MF. Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Na LX, Li Y, Pan HZ, Zhou XL, Sun DJ, Meng M, et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol Nutr Food Res. [Research Support, Non-U.S. Gov’t]. 2013;Sep;57(9) doi: 10.1002/mnfr.201200131. [DOI] [PubMed] [Google Scholar]
- 47.Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C, Jirawatnotai S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care. 2012;35(11):2121–7. doi: 10.2337/dc12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang M, Larson-Meyer DE, Liebman M. Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. Am J Clin Nutr. 2008;87(5):1262–7. doi: 10.1093/ajcn/87.5.1262. [DOI] [PubMed] [Google Scholar]
- 49.Kim SW, Ha KC, Choi EK, Jung SY, Kim MG, Kwon DY, et al. The effectiveness of fermented turmeric powder in subjects with elevated alanine transaminase levels: a randomised controlled study. BMC Complement Altern Med. 2013;13:58. doi: 10.1186/1472-6882-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]