Abstract
Treatment of rats in vivo with cobalt chloride stimulated heme oxidation by hepatic microsomes to levels up to 800% above controls. This treatment also caused increases in liver weight and in total microsomal protein; in contrast, marked decreases were produced in microsomal oxidation of ethylmorphine (80%), and in cytochrome P-450 (60-70%) and heme (30-50%) contents. Cobalt chloride treatment did not affect heme oxidation by the spleen heme oxygenase system.
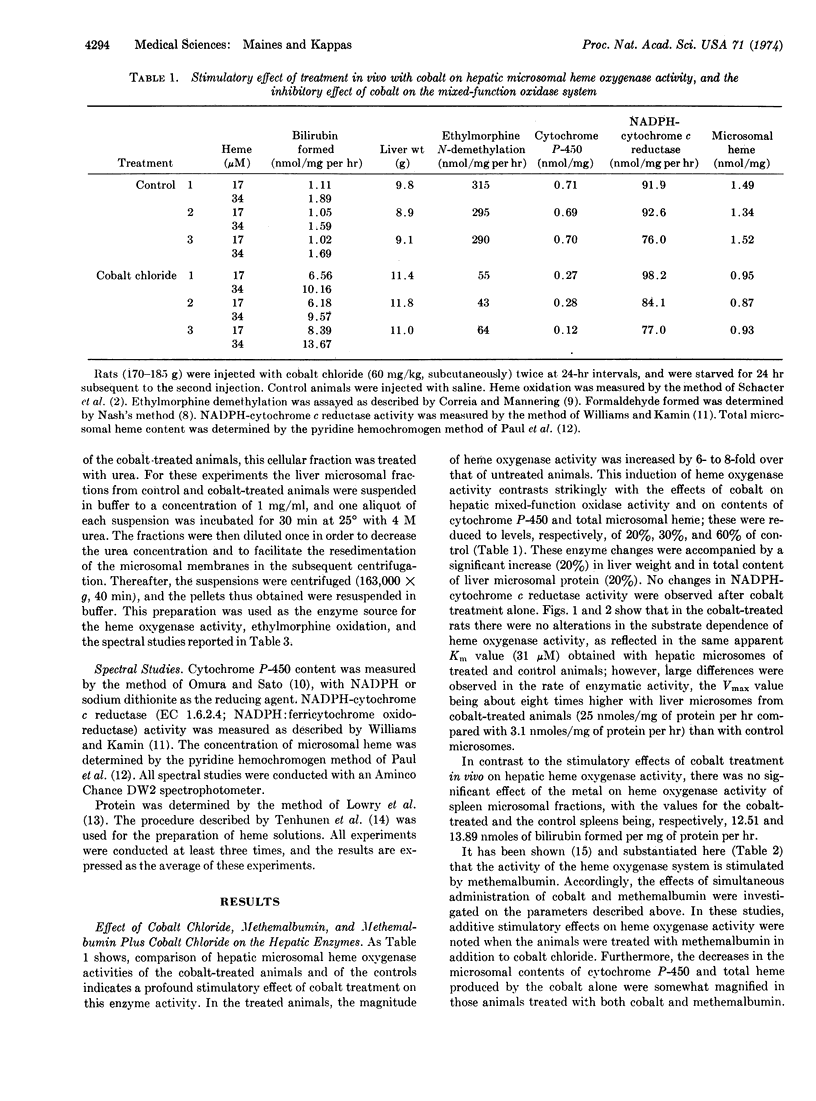
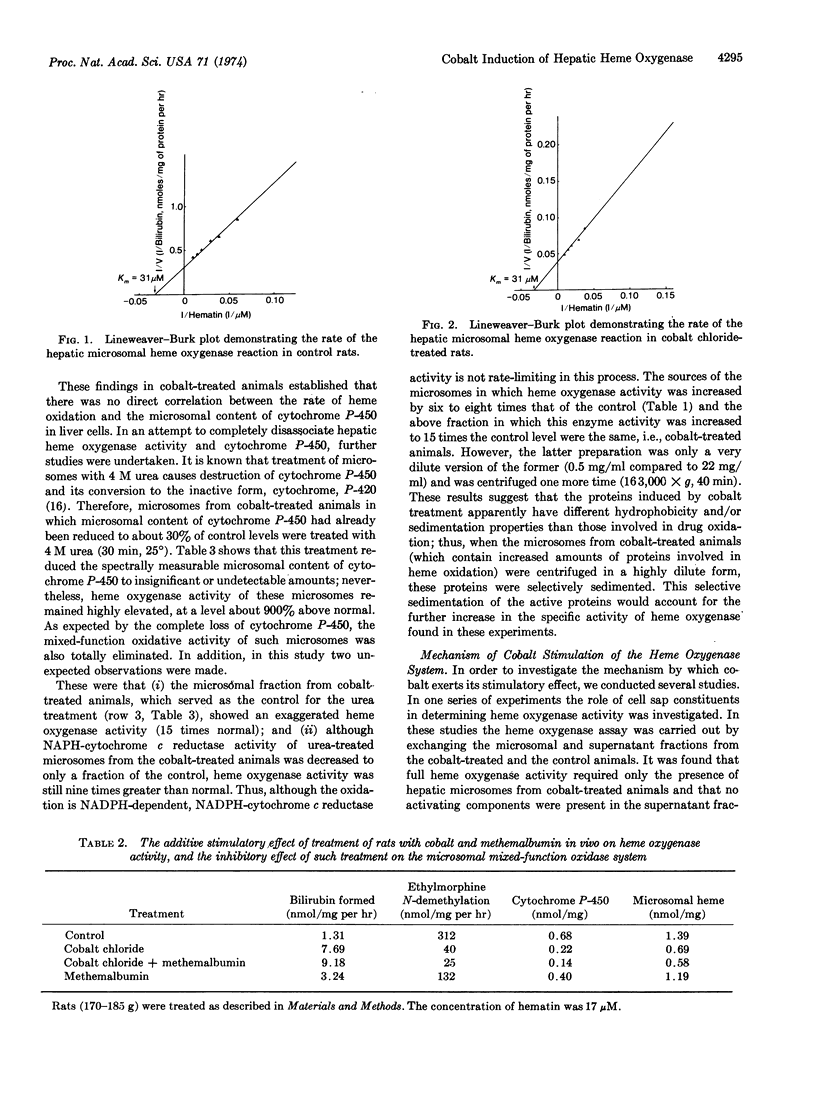
The rate of heme oxidation by hepatic microsomal enzymes and the microsomal content of cytochrome P-450 were found to be unrelated. This conclusion was reached from studies in which microsomal heme oxygenase activity from cobalt-treated animals could be increased by 900% above control levels in the same microsomal preparation in which cytochrome P-450 content was decreased to spectrally unmeasurable amounts after incubation with 4 M urea. The same treatment eliminated ehtylmorphine demethylation and decreased microsomal NADPH-cytochrome c reductase (EC 1.6.2.4) activity by 75%.
It is concluded that (i) the hepatic microsomal enzyme system that oxidizes heme compounds is not the same as that which metabolizes drugs, (ii) cytochrome P-450 is not essential for the oxidation of heme by liver cells, (iii) there is no direct relationship between the rate of heme oxidation and the level of NADPH-cytochrome c reductase activity, and (iv) the oxidation of heme is protein-dependent and the active proteins are inducible, but are different from those involved in drug metabolism.
Keywords: heme oxidation, enzyme induction, mixed-function oxidation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Correia M. A., Mannering G. J. Reduced diphosphopyridine nucleotide synergism of the reduced triphosphopyridine nucleotide-dependent mixed-function oxidase system of hepatic microsomes. I. Effects of activation and inhibition of the fatty acyl coenzyme A desaturation system. Mol Pharmacol. 1973 Jul;9(4):455–469. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lathe G. H. The degradation of haem by mammals and its excretion as conjugated bilirubin. Essays Biochem. 1972;8:107–148. [PubMed] [Google Scholar]
- Maines M. D., Anders M. W., Muller-Eberhard U. Studies on heme transfer from microsomal hemoproteins to heme-binding plasma proteins. Mol Pharmacol. 1974 Mar;10(2):204–213. [PubMed] [Google Scholar]
- Mason H. S., North J. C., Vanneste M. Microsomal mixed-function oxidations: the metabolism of xenobiotics. Fed Proc. 1965 Sep-Oct;24(5):1172–1180. [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol A. W. The formation of biliverdin by chicken macrophages in tissue culture. Observations on the effect of inhibitors. Biochim Biophys Acta. 1970 Oct 27;222(1):28–40. doi: 10.1016/0304-4165(70)90348-x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- OSTROW J. D., HAMMAKER L., SCHMID R. The preparation of crystalline bilirubin-C14. J Clin Invest. 1961 Aug;40:1442–1452. doi: 10.1172/JCI104375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B. A., Mason J. I. The effect of phenobarbital, 3-methylcholanthrene, 3,4-benzpyrene, and pregnenolone-16 alpha-carbonitrile on microsomal heme oxygenase and splenic cytochrome P-450. Arch Biochem Biophys. 1974 Jan;160(1):274–278. doi: 10.1016/s0003-9861(74)80034-2. [DOI] [PubMed] [Google Scholar]
- Schacter B. A., Nelson E. B., Marver H. S., Masters B. S. Immunochemical evidence for an association of heme oxygenase with the microsomal electron transport system. J Biol Chem. 1972 Jun 10;247(11):3601–3607. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of hemoglobin to bilirubin. Trans Assoc Am Physicians. 1969;82:363–371. [PubMed] [Google Scholar]
- Tenhunen R., Marver H., Pimstone N. R., Trager W. F., Cooper D. Y., Schmid R. Enzymatic degradation of heme. Oxygenative cleavage requiring cytochrome P-450. Biochemistry. 1972 Apr 25;11(9):1716–1720. doi: 10.1021/bi00759a029. [DOI] [PubMed] [Google Scholar]
- Tephly T. R., Hibbeln P. The effect of cobalt chloride administration on the synthesis of hepatic microsomal cytochrome P-450. Biochem Biophys Res Commun. 1971 Feb 19;42(4):589–595. doi: 10.1016/0006-291x(71)90528-6. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]



