Abstract
Background
Delays to intra‐arterial therapy (IAT) lead to worse outcomes in stroke patients with proximal occlusions. Little is known regarding the magnitude of, and reasons for, these delays. In a pilot quality improvement (QI) project, we sought to examine and improve our door‐puncture times.
Methods and Results
For anterior‐circulation stroke patients who underwent IAT, we retrospectively calculated in‐hospital time delays associated with various phases from patient arrival to groin puncture. We formulated and then implemented a process change targeted to the phase with the greatest delay. We examined the impact on time to treatment by comparing the pre‐ and post‐QI cohorts. One hundred forty‐six patients (93 pre‐ vs. 51 post‐QI) were analyzed. In the pre‐QI cohort (ie, sequential process), the greatest delay occurred from imaging to the neurointerventional (NI) suite (“picture‐suite”: median, 62 minutes; interquartile range [IQR], 40 to 82). A QI measure was instituted so that the NI team and anesthesiologist were assembled and the suite set up in parallel with completion of imaging and decision making. The post‐QI (ie, parallel process) median picture‐to‐suite time was 29 minutes (IQR, 21 to 41; P<0.0001). There was a 36‐minute reduction in median door‐to‐puncture time (143 vs. 107 minutes; P<0.0001). Parallel workflow and presentation during work hours were independent predictors of shorter door‐puncture times.
Conclusions
In‐hospital delays are a major obstacle to timely IAT. A simple approach for achieving substantial time savings is to mobilize the NI and anesthesia teams during patient evaluation and treatment decision making. This parallel workflow resulted in a >30‐minute (25%) reduction in median door‐to‐puncture times.
Keywords: acute ischemic stroke, endovascular stroke thrombectomy, quality improvement, stroke process improvement
Introduction
Despite the availability of intravenous tissue plasminogen activator (IV tPA) and the doubling of treatment rates over the latter half of the past decade, only a small fraction of patients arrive within the time window to receive this treatment.1 Intra‐arterial therapy (IAT), consisting of device‐mediated thrombus removal or dissolution with lytics, is an alternative treatment option for patients who present with proximal intracranial artery occlusion and are ineligible for, or do not respond to, IV thrombolysis.2–3
Several studies have demonstrated that rapid treatment initiation with IV thrombolysis is associated with better clinical outcomes.4 Based on recognition of the “golden hour” in intravenous acute stroke treatment, public health initiatives have set a target door‐to‐needle time of less than 60 minutes.5–6 Efforts have targeted pre‐ and in‐hospital delays as part of quality improvement (QI) programs.7–8 Similarly, time to angiographic reperfusion of the ischemic brain has been shown to be a significant predictor of favorable clinical outcome after IAT.9 However, unlike for IV tPA, there are no large‐scale public health initiatives to improve the delivery of IAT. Although recent guidelines for comprehensive stroke centers (CSCs) have proposed a target door‐to‐groin puncture time of less than 2 hours,10 no further performance benchmarks are specified because of the lack of data regarding systems‐level processes involved in triaging patients to the neurointerventional (NI) suite and thus potential sources of delay.11
In a multidisciplinary pilot QI project, we aimed to identify delays to the NI suite after patient arrival to our emergency department (ED) and, based on these data, prospectively implement process changes to reduce the overall door‐to‐puncture time interval. We report on the impact of this QI initiative.
Methods
IAT Selection Criteria
Per our established institutional acute stroke protocol, IAT is considered for patients who have an intracranial proximal artery occlusion (internal carotid artery, middle cerebral artery [MCA] M1, or proximal M2), National Institutes of Health Stroke Scale (NIHSS) score ≥8, and imaging completed within 7 hours from last seen well (LSW) for anterior circulation strokes; these criteria were based on institutional review of best available evidence.12 Eligible patients are evaluated by a multidisciplinary team comprised of acute stroke neurology and NI fellows as well as their attending physicians. Patients are considered for IAT if they meet the initial screening criteria outlined above and have a noncontrast computed tomography (CT) hypodensity or diffusion‐weighted imaging (DWI) hyperintensity less than 100 mL (or less than one third of MCA territory), as calculated by ellipsoid approximation (ABC/2 rule).13 Our acute stroke imaging algorithm utilizes multimodal imaging, when possible, with an emergent CT/CT angiogram of the head and neck to rapidly rule out hemorrhage and assess the cerebrovasculature, followed by magnetic resonance imaging (MRI) DWI for optimal determination of core infarct volume.14–15
Pilot QI Project
This pilot project was supported by the Clinical Process Improvement Program (CPIP) at our hospital. The aim of CPIP is to address the challenges of clinical care in a complex healthcare system through process analysis, rapid cycle improvement, and management of multidisciplinary teams. It utilized methodology developed at Intermountain Healthcare, a national leader in QI, for measuring, understanding, and managing variation in clinical work flow.16
A multidisciplinary team comprised of clinical experts in stroke care from our hospital was assembled to oversee this effort. The project was conducted in 4 phases: retrospective data collection and analysis; identification of process delays; implementation of NI process improvement; and measurement of impact on time to treatment.
Phase 1
With approval from the institutional review board (IRB), the retrospective analysis examined door‐to‐puncture times for consecutive acute ischemic stroke patients presenting to the ED from March 2007 to October 2011. Main inclusion criteria were evidence of an anterior circulation proximal artery occlusion on noninvasive imaging and emergently being taken for IAT. To characterize the pre‐existing workflow, specific time points in the clinical process were collected: (in chronological order) patient presentation to the ED; start of CT scan acquisition; start of MRI scan acquisition; patient arrival into the NI suite; and groin puncture. CT and MRI time points were obtained from an electronic time stamp found on the first sequence acquired within each scan. The following nonoverlapping process interval times were calculated in minutes: ED‐to‐CT; CT‐to‐MRI; picture‐to‐suite; and suite‐to‐puncture. “Picture” refers to the start time of the scan (CT or MRI) performed immediately preceding the patient being taken to the NI suite for IAT. In addition, key global metrics were calculated and reported: door‐to‐suite; door‐to‐puncture; and picture‐to‐puncture.10,17
Phase 2
To identify significant sources of delay within our workflow, we compared median times for each nonoverlapping process interval. We also compared the sizes of the interquartile ranges (IQRs), as a measure of variability and the potential for improvement with standardization. We identified the interval with the longest delay and greatest variation as the target of our initiative. In order to identify specific areas for practice improvement, process mapping was performed to detail every step required for patient evaluation and triage. Using the lean management model for clinical work flow optimization,18 an impact‐versus‐effort matrix was created (Figure S1) in order to identify the process improvement measure that would yield the most substantial time savings while being easy to implement.
Phase 3
Before the official launch of the new process, the proposed changes were presented to team members and feedback obtained. The process was assessed in several pilot cases (not included in analysis) to ensure the staff understood their roles, and modifications were made, as necessary, during QI case reviews. To facilitate widespread adoption and standardization of the new workflow, reference cards for the new IAT process were distributed to all team members, and the new process map was posted in relevant acute stroke workflow areas of our hospital. The process improvement phase of the project (described in the Results) was officially instituted in November 2011.
Phase 4
To assess the effectiveness of the new IAT process in reducing door‐to‐puncture times, a prospective QI database was established and included consecutive acute stroke patients presenting to the ED for whom the NI team was alerted. For the purposes of this analysis, data collection was closed in August 2013. With approval from our IRB, we examined 2 IAT cohorts: pre‐ and postimplementation of the QI measure. We compared time intervals for each phase of evaluation from door‐to‐groin puncture, as well as the broader time metrics.
Statistical Analysis
Time intervals are reported as median and IQR. The Friedman's test was performed to compare the nonoverlapping time intervals within the sequential period; subsequently, the Wilcoxon's signed‐rank test was performed for pair‐wise comparisons. The Mann‐Whitney's U test was performed to compare the respective time intervals before and after process improvement. Multivariable linear regression was performed to identify predictors of door‐to‐puncture time. Proportions achieving a door‐to‐puncture time of 120 minutes or less were compared between the cohorts using the chi‐square test. Similarly, this was done to compare the proportions of modified Rankin Scale (mRS) 0 to 2 outcomes. Statistical significance was taken at 2‐tailed P<0.05. Analysis was performed using MedCalc software (v.11.6.1.0; MedCalc Software, Ostend, Belgium).
Results
Retrospective Analysis and Identification of Major Delays
Examination of the nonoverlapping, component process intervals for the pre‐QI cohort (n=93) demonstrated that the picture‐to‐suite interval had the longest duration and the most variability. The median duration of this interval was 62 minutes (IQR, 40‐82); the remainder were on the order of 30 minutes or less (P<0.0001 for all pair‐wise comparisons; Figure 1). Moreover, the magnitude of the IQR was 42 minutes, approximately twice as large as for the other intervals. To maximize impact on overall door‐to‐puncture time, subsequent efforts were targeted to reducing the picture‐to‐suite time.
Figure 1.
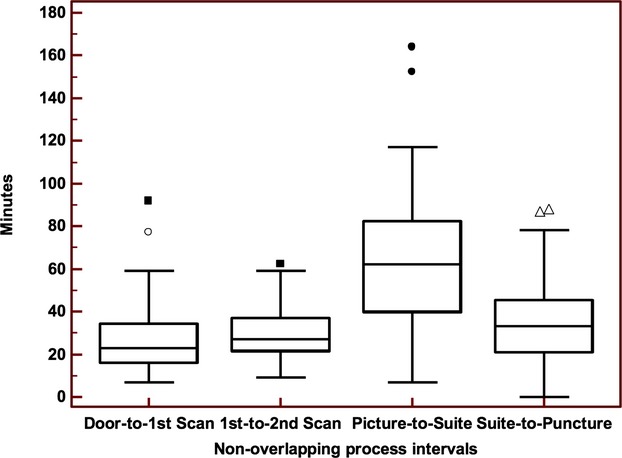
Identification of delays in pre‐QI cohort (sequential IAT process). Box‐whisker plots demonstrate median (line within box) and interquartile ranges (top and bottom edge of box) of the nonoverlapping process intervals. There was a significant difference among the intervals (overall P < 0.0001; Friedman's test). In pair‐wise comparison, the picture‐suite interval was significantly longer than the others (all P < 0.0001). IAT indicates intra‐arterial therapy; QI, quality improvement.
In the subset of patients within the bottom quartile of door‐to‐puncture times, data on sources of delay were gathered from paper charts, electronic medical records, and acute stroke management logs in order to identify measures to reduce picture‐to‐suite times (Figure S2). A multidisciplinary team representing neurology, emergency medicine, neurointervention, and anesthesia convened to review the NI workflow. It was deemed that a major source of delay was the late activation of key team members. First, NI fellow notification routinely took place only after discovery of a proximal artery occlusion, preventing early coordination of care. Second, the NI technologist, nurse, and anesthesia team were mobilized after the decision was made by the stroke and NI attendings to proceed with treatment. This decision typically occurred upon completion of all necessary imaging studies. This sequential activation of the multidisciplinary teams necessitated patients to wait in the ED after completion of imaging studies, to allow sufficient time for decision making, procedure consent, and setup of the neuroangiography suite (Figure 2A).
Figure 2.
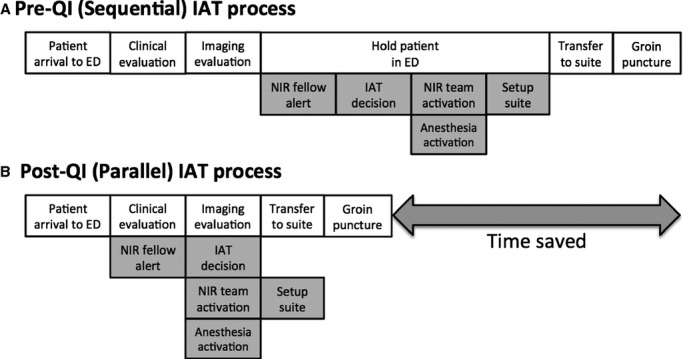
Comparison of IAT processes. Schematic highlighting delays to IAT for the sequential (A) and parallel (B) workflows. ED indicates emergency department; IAT, intra‐arterial therapy; NIR, nuerointerventional radiology; QI, quality improvement.
Implementation of QI Measure
Using lean methodology, an early NI activation system was established as a high‐impact, low‐effort measure to reduce the time from picture‐to‐suite (Figure S1). The aim of this early activation protocol was to reduce time through parallel (rather than sequential) workflow of the various team members. As a first step, the NI fellow would be alerted before imaging acquisition based on clinical criteria alone (NIHSS score ≥8 and LSW ≤7 hours at time of evaluation by stroke team; Figure 2B). The remainder of the multidisciplinary team would then be activated by the NI fellow after confirmation of proximal artery occlusion at the time of vessel imaging. The goal was to ready the NI suite and assemble all necessary team members in parallel with the completion of imaging and treatment decision making. With this approach, patients deemed suitable for IAT could be promptly transferred from the ED to the NI suite, thus reducing the picture‐to‐suite time interval. A door‐to‐IAT process map with details on team member roles (Figure S3) was distributed and posted in relevant work areas to promote adherence to the QI measure.
Impact of Process Improvement
There were 144 total patients in this analysis: 93 patients in the sequential process (pre‐QI) cohort and 51 in the parallel process (post‐QI) cohort. In the combined population, mean age was 69±15 years, median NIHSS score was 16 (IQR, 14 to 19), and 72 (50%) were female. Sixty‐six (46%) patients presented during work hours (ie, weekday from 6 am to 6 pm). There was no significant difference in these variables between the 2 groups.
Comparing the nonoverlapping, component process intervals between the 2 groups, the only statistically significant difference was observed in the picture‐to‐suite duration (Figure 3). Median duration was 62 (IQR, 40 to 82) versus 29 minutes (IQR, 21 to 41) for the pre‐ versus post‐QI process, respectively (P<0.0001). This improvement translated into time savings of similar magnitude for the global metrics of door‐to‐puncture (143 [IQR, 112 to 170] vs. 107 minutes [IQR, 87 to 124]), door‐to‐suite (109 [IQR, 86 to 131] vs, 73 minutes [IQR, 59 to 90]), and picture‐to‐puncture times (97 [IQR, 73 to 116] vs. 60 minutes [IQR, 48 to 75]; all P<0.0001; Figure 3). With respect to the guideline recommendation for door‐to‐puncture time of 120 minutes or less, only 29% of the pre‐QI cohort achieved this benchmark, compared to 73% of the post‐QI cohort (P<0.0001).
Figure 3.
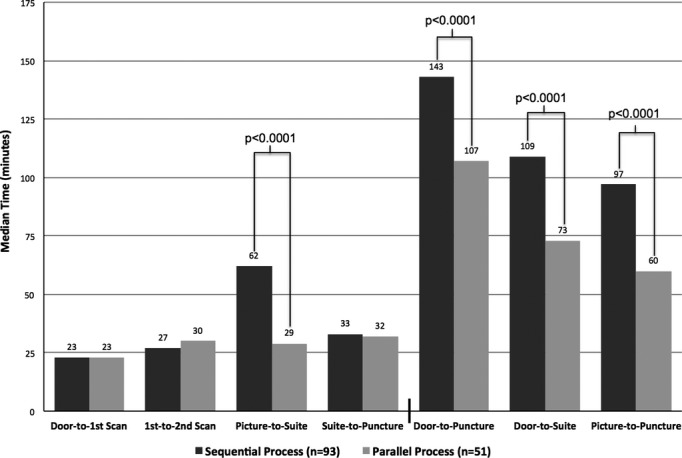
Impact of process improvement. Significant P values are shown for comparisons between the sequential and parallel processes (Mann‐Whitney's U test). Median times are listed above the bars.
Not all patients had both CT and MRI scans performed. More patients in the post‐QI cohort underwent a single imaging modality (45% vs. 27%; P=0.04). Because this might affect the time differences between the 2 cohorts, a similar analysis was performed for only those patients who underwent both imaging modalities (Figure 4). This yielded similar findings where significant improvements were again observed in the post‐QI cohort for the picture‐to‐suite interval (61 [IQR, 36 to 77] vs. 24 minutes [IQR, 19 to 39]; P<0.0001) and for the global time metrics of door‐to‐puncture (144 [IQR, 120 to 165] vs. 116 minutes [IQR, 101 to 128]; P<0.0001), door‐to‐suite (111 [IQR, 89 to 130] vs. 80 minutes [IQR, 72 to 95]; P<0.0001), and picture‐to‐puncture intervals (92 [IQR, 65 to 113] vs. 53 minutes [IQR, 47 to 78]; P<0.0001).
Figure 4.
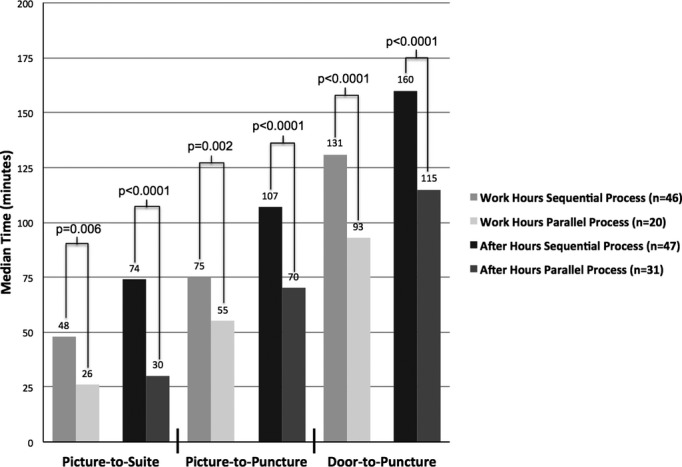
Comparison of IAT processes based on time of day. Significant P values are shown comparing sequential and parallel processes based on time of presentation (Mann‐Whitney's U test). Median times are listed above the bars. IAT indicates intra‐arterial therapy.
Adjusting for age and NIHSS score, use of the early alert protocol (ie, parallel process) and presentation during work hours were independent predictors of shorter door‐to‐puncture times (both multivariate P<0.0001). There was no interaction between these factors. Parallel workflow resulted in significant time improvements regardless of whether patients presented during work or after hours. With respect to long‐term clinical outcome, the rates of 90‐day modified Rankin score 0 to 2 were 27% (25 of 91) in the sequential process cohort and 36% (16 of 45) in the parallel process cohort (P=0.44). The distribution of mRS scores for both cohorts is shown in Figure 5.
Figure 5.
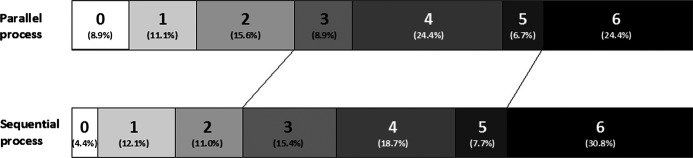
Distribution of modified Rankin Scale scores. Distributions of the Rankin scores are displayed for both the parallel and sequential process groups. The proportions are listed in parentheses.
Because the stroke fellow alerted the NI fellow based on clinical suspicion for proximal artery occlusion (ie, before any imaging), we captured the instances when no proximal occlusion was present on imaging. The rate of false‐positive alerts was 23% (37 of 162).
Discussion
Delays to reperfusion therapy result in worse outcomes after acute ischemic stroke.4,9,19 This has led to an American Stroke Association initiative which aims to improve door‐to‐needle times for IV tPA through the establishment of best practice strategies.6 Similar QI efforts in other countries have produced dramatic results with reported door‐to‐needle times as low as 20 minutes.20 Unfortunately, there are much less data on the magnitude of time delays and their causative factors for IAT, thus limiting efforts to improve treatment delivery.11,21 The companion studies by Sun et al. and our group provide foundational data for this important, yet neglected, healthcare need.
A recent guideline recommendation for CSCs established a goal time from patient arrival to start of interventional treatment of 2 hours or less.10 Until now, it has been unclear what the duration of this interval is in real‐world practice. The retrospective results of the Rapid Reperfusion Registry demonstrate that, even at stroke centers that are highly experienced with IAT, the 2‐hour goal is achieved in only approximately half of patients.22 Confirming the importance of this time metric, patient outcomes were worse with longer door‐to‐puncture times.
Building on these results, our pilot QI initiative establishes the feasibility of achieving a major reduction in door‐to‐puncture time using a simple process change that can be readily duplicated at other centers. Specifically, we instituted a protocol for early activation of the NI team so that resource mobilization (ie, technologist travel, anesthesia setup, and suite preparation) would occur in parallel with patient evaluation and treatment decision making. This resulted in shortening of door‐to‐puncture times by a median of 36 minutes. Putting this in context, a post‐hoc analysis of the Interventional Management of Stroke (IMS) 3 trial demonstrated that, for every 30‐minute delay from symptom onset to reperfusion, there was a 10% relative reduction in the chance of functional independence at 90 days.23 Moreover, in the present study, the proportion of patients that satisfied the guideline recommendation of 120 minutes for door‐to‐puncture time more than doubled with the new protocol (72% vs. 29%).
There are likely several factors responsible for the negative results of the recent randomized, controlled trials that compared IAT against best medical management, including IV tPA.9–10 Of particular concern are the time delays that were observed in these studies. In IMS 3, the mean time from patient arrival to groin puncture was approximately 150 minutes.24 In the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial, the mean time from imaging to groin puncture alone was 124 minutes.25 Based on data from the retrospective Rapid Reperfusion Registry, these times are much too long—the worst clinical outcomes were noted with door‐to‐puncture times of 136 minutes or greater.22 In contrast, our door‐to‐puncture times using the new process were substantially shorter (mean±SD, 106±22 minutes). To remedy this problem, ongoing randomized trials of IAT have incorporated maximum allowable time limits for treatment delivery. However, establishing a time goal is just 1 step in the quality improvement process and is unlikely to produce durable systemic change by itself. Rather, we believe that the multistep approach described here provides an effective template for similar initiatives that seek to reduce times and standardize treatment delivery for acute stroke intervention.
An important lesson from our experience is that a formal process for documenting time delays is essential. As a first step, it reveals the magnitude of the problem and thus provides necessary feedback data for evaluation of QI efforts. Although there were several perceived sources of delay in our IAT process before the start of this initiative (Figure S1), the median door‐to‐puncture time of 143 minutes that we measured in the retrospective cohort was much larger than anticipated. This finding, along with the recent trial experience, suggests that similar delays are occurring at the vast majority of stroke centers that offer IAT.11
Furthermore, data collection should be anchored by key time points during patient triage and treatment, including patient arrival to the hospital, start of neuroimaging, NI suite arrival, and groin puncture. Only then can delays from the individual component phases of patient evaluation be quantified. This granularity is critical because the causes of delay and their potential solutions are likely specific to the phase in question. To identify the interval that accounts for the most significant delays, which should be the focus of QI efforts, it is important to not only look at the absolute time duration, but also the degree of variability (ie, SD or IQR). High case‐to‐case variability reflects the lack of a standardized approach and thus the potential for time savings through a formal QI process.
In our retrospective analysis, the picture‐to‐suite phase represented both the longest and the most variable interval, owing to the fact that treatment decision making and team mobilization were occurring sequentially during this time. As a result, the patient was routinely brought back to the ED to wait while the team was assembled and the suite was prepared. By arranging these time‐intensive steps in parallel, we were able to achieve a significant time savings with modest effort and little change to the other workflow processes in place. Importantly, this approach is likely to be broadly effective across institutions. In a recent survey of IAT practice patterns, only approximately one third of respondents reported an established protocol for alerting the NI team (ie, at a specific point in patient evaluation).26 Consequently, it is highly probable that significant variability and delay within the picture‐to‐suite interval exist at the majority of centers.
The delivery of IAT requires the coordination of a multidisciplinary team that includes nursing, emergency medicine, stroke neurology, diagnostic neuroradiology, neurointervention, and anesthesia. Therefore, any QI effort must have the support and participation of these groups, whose multidisciplinary expertise will provide important insights during process mapping, brainstorming, and implementation of the QI initiative. With regard to implementation, we used 2 straightforward strategies to promote protocol adherence by the diverse team members. First, a simple checklist was distributed to the acute stroke neurology team listing the clinical criteria for early activation of the NI fellow. Second, a process diagram was created and posted in clinical areas to provide clear roles and responsibilities for the various teams during each phase of evaluation from door‐to‐NI suite (Figure S3). The improvement in protocol adherence is evidenced by the >50% reduction in the size of the IQR of the picture‐to‐suite phase (Figure S4). Collectively, these efforts demonstrate the feasibility of process improvement in a complex multidisciplinary environment.
In addition to the use of parallel workflow, patient presentation during work hours was an independent predictor of shorter door‐to‐puncture times. This is not surprising given that all necessary personnel are present in the hospital during work hours, obviating the time required for the technologist, the neurointerventionist, and the stroke neurologist to travel to the hospital during after hours. Importantly, early notification of the NI team and parallel workflow significantly improved door‐to‐puncture times for both work‐ and after‐hour presentations. In order to bridge the gap between work‐ and after‐hour times to IAT, prehospital notification by first responders will likely be necessary in order to assemble the team before patient arrival. This has been successful in reducing door‐to‐balloon times in acute myocardial infarction (AMI).27–28 However, even with the use of prehospital stroke scales, such as the Los Angeles Motor Score,29 a tolerable rate of false team activation must be established because a significant number of patients will be excluded from treatment based on imaging demonstrating hemorrhage, the absence of vessel occlusion, or the presence of a large infarct. In this study, activation of the NI fellow using clinical criteria (ie, NIHSS score ≥8 and LSW ≤7 hours) led to a 23% false‐positive rate for proximal artery occlusion alone, consistent with a previous study that reported a false‐positive rate of 19% for NIHSS score ≥10.30
Finally, it is important to stress that there is still much left to do in order to reduce treatment delays to an acceptable level. Indeed, the existing guideline recommendation of 120 minutes from door to treatment is too long when one considers the relatively rapid rate of infarct growth, on average, and the dramatic effect of infarct volume on clinical outcome. In order to achieve optimal outcomes in ischemic stroke for patients presenting directly to a CSC, door‐to‐puncture times will ultimately need to be shorter than the 60‐minute benchmark for treatment of AMI, notwithstanding the substantial challenges in realizing this.
Our study has several limitations. First, we were likely underpowered for demonstrating the impact of our QI initiative on clinical outcomes. There was a 9% absolute increase (33% relative increase) in the rate of good outcomes after process improvement, which was not statistically significant. Although the ultimate goal of QI is better patient outcomes, the aim of this pilot QI project was to identify in‐hospital delays in the delivery of IAT and assess the feasibility of implementing process changes to reduce time to treatment. Additionally, during the course of this initiative, stent retrievers were introduced into our NI practice and may serve as a major confounder for evaluating the clinical effect of our QI efforts as a result of their higher revascularization rates, as demonstrated in recent randomized trials. Second, delays occurring after patient arrival to the NI suite and preceding puncture or revascularization were not the focus of this QI pilot and are under active investigation by our group. Finally, it is unclear whether parallel activation protocols can be successfully implemented outside major academic medical centers, which have large multidisciplinary teams with clinical residents and fellows providing 24‐7 in‐house coverage. In smaller hospitals, limited personnel or resources may make this approach challenging. Fortunately, most centers that offer IAT are larger, tertiary care centers. In the prospective phase of the Rapid Reperfusion Registry, we plan to test whether our single‐center success can be translated across multiple centers to reduce time to IAT and improve clinical outcomes.
Conclusion
In‐hospital factors are a major contributor to overall treatment delay for IAT. Early activation of the NI team and parallel workflow during decision making and resource mobilization can produce substantial time savings. In this single‐center pilot QI project, median door‐to‐puncture time was reduced by 36 minutes or 25%. Utilizing clinical criteria to predict proximal artery occlusion resulted in an acceptable false‐positive rate of 23%.
Supplementary Material
Supplement 1. LEAN Model for Process Change Implementation
Supplement 2. Sources of Delays in Sequential IAT Process
Supplement 3. Team Member Roles in Parallel IAT Workflow
Supplement 4. Reduction in Case-to-Case Variability with a Standardized IAT Process
Sources of Funding
The CPIP at our hospital provided strategic guidance and administrative funding during the course of this project.
Disclosures
None.
References
- 1.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue‐type plasminogen activator use for ischemic stroke in the united states: a doubling of treatment rates over the course of 5 years. Stroke. 2011; 42:1952-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999; 282:2003-2011. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Tomsick TA. Reimbursement for thrombectomy devices in patients who are ineligible for intravenous tissue‐type plasminogen activator. Stroke. 2013; 44:1215-1216. [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, ECASS, ATLANTIS, NINDS and EPITHET rt‐PA Study Group Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010; 375:1695-1703. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, Schwamm LHGWTG‐Stroke Steering Committee and Investigators. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010; 41:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED, Sacco RL, Schwamm LH. Improving door‐to‐needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association's target: Stroke initiative. Stroke. 2011; 42:2983-2989. [DOI] [PubMed] [Google Scholar]
- 7.Patel MD, Rose KM, O'Brien EC, Rosamond WD. Prehospital notification by emergency medical services reduces delays in stroke evaluation: findings from the North Carolina stroke care collaborative. Stroke. 2011; 42:2263-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikulík R, Kadlecová P, Czlonkowska A, Kobayashi A, Brozman M, Svigelj V, Csiba L, Fekete K, Kõrv J, Demarin V, Vilionskis A, Jatuzis D, Krespi Y, Ahmed NSafe Implementation of Treatments in Stroke‐East Registry (SITS‐EAST) Investigators. Factors influencing in‐hospital delay in treatment with intravenous thrombolysis. Stroke. 2012; 43:1578-1583. [DOI] [PubMed] [Google Scholar]
- 9.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time‐dependent. Neurology. 2009; 73:1066-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leifer D, Bravata DM, Connors JJ, III, Hinchey JA, Jauch EC, Johnston SC, Latchaw R, Likosky W, Ogilvy C, Qureshi AI, Summers D, Sung GY, Williams LS, Zorowitz RAmerican Heart Association Special Writing Group of the Stroke Council; Atherosclerotic Peripheral Vascular Disease Working Group; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Nursing. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow‐up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42:849-877. [DOI] [PubMed] [Google Scholar]
- 11.Eesa M, Menon BK, Hill MD, Demchuk A, Goyal M. Achieving faster recanalization times by ia thrombolysis in acute ischemic stroke: where should we direct our efforts? Interv Neuroradiol. 2011; 17:228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rost NS, Smith EE, Nogueira RG, Fitzpatrick KM, Yoo AJ, Hirsch JA, Schwamm LH. Implementation of a patient selection protocol for intra‐arterial therapy increases treatment rates in patients with acute ischemic stroke. J Neurointerv Surg. 2013; 5suppl 1:i44-i47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009; 72:2104-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González RG, Copen WA, Schaefer PW, Lev MH, Pomerantz SR, Rapalino O, Chen JW, Hunter GJ, Romero JM, Buchbinder BR, Larvie M, Hirsch JA, Gupta R. The Massachusetts General Hospital acute stroke imaging algorithm: an experience and evidence based approach. J Neurointerv Surg. 2013; 5suppl 1:i7-i12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI‐based selection for intra‐arterial stroke therapy: value of pretreatment diffusion‐weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009; 40:2046-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011; 30:1185-1191. [DOI] [PubMed] [Google Scholar]
- 17.Sun CH, Nogueira RG, Glenn BA, Connelly K, Zimmermann S, Anda K, Camp D, Frankel MR, Belagaje SR, Anderson AM, Isakov AP, Gupta R. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013; 127:1139-1148. [DOI] [PubMed] [Google Scholar]
- 18.Gomez MA, II, Hirsch JA, Stingley P, Byers E, Sheridan RM. Applying the lean management philosophy to neurointerventional radiology. J Neurointerv Surg. 2010; 2:83-86. [DOI] [PubMed] [Google Scholar]
- 19.Mazighi M, Chaudhry SA, Ribo M, Khatri P, Skoloudik D, Mokin M, Labreuche J, Meseguer E, Yeatts SD, Siddiqui AH, Broderick J, Molina CA, Qureshi AI, Amarenco P. Impact of onset‐to‐reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013; 127:1980-1985. [DOI] [PubMed] [Google Scholar]
- 20.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in‐hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012; 79:306-313. [DOI] [PubMed] [Google Scholar]
- 21.Nedeltchev K, Arnold M, Brekenfeld C, Isenegger J, Remonda L, Schroth G, Mattle HP. Pre‐ and in‐hospital delays from stroke onset to intra‐arterial thrombolysis. Stroke. 2003; 34:1230-1234. [DOI] [PubMed] [Google Scholar]
- 22.Sun CH, Ribo M, Goyal M, Yoo AJ, Jovin TG, Cronin CA, Zaidat OO, Nogueira RG, Nguyen T, Hussain S, Menon BK, Mehta BP, Jindal G, Horev A, Norbash A, Leslie‐Mazwi TM, Gupta R. Door‐to‐puncture: a practical metric for capturing and enhancing system processes associated with endovascular stroke care, preliminary results from the rapid reperfusion registry. J Am Heart Assoc. 2014; 3:e000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri P; IMS III Investigators. Time to angiographic reperfusion is highly associated with good clinical outcome in the IMS III trial. International Stroke Conference, 2013.
- 24.Broderick JP; IMS III. Overall results and major subgroup comparisons. International Stroke Conference, 2013.
- 25.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH, Yoo AJ, Marshall RS, Meyers PM, Yavagal DR, Wintermark M, Guzy J, Starkman S, Saver JLMR RESCUE Investigators. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013; 368:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta B, Leslie‐Mazwi TM, Chandra RV, Chaudhry ZA, Rabinov JD, Hirsch JA, Schwamm LH, Rost NS, Yoo AJ. Assessing variability in neurointerventional practice patterns for acute ischemic stroke. J Neurointerv Surg. 2013; 5suppl 1:i52-i57. [DOI] [PubMed] [Google Scholar]
- 27.Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM. Strategies for reducing the door‐to‐balloon time in acute myocardial infarction. N Engl J Med. 2006; 355:2308-2320. [DOI] [PubMed] [Google Scholar]
- 28.Bagai A, Al‐Khalidi HR, Muñoz D, Monk L, Roettig ML, Corbett CC, Garvey JL, Wilson BH, Granger CB, Jollis JG. Bypassing the emergency department and time to reperfusion in patients with prehospital ST‐segment‐elevation: findings from the reperfusion in acute myocardial infarction in Carolina Emergency Departments Project. Circ Cardiovasc Interv. 2013; 6:399-406. [DOI] [PubMed] [Google Scholar]
- 29.Llanes JN, Kidwell CS, Starkman S, Leary MC, Eckstein M, Saver JL. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004; 8:46-50. [DOI] [PubMed] [Google Scholar]
- 30.Maas MB, Furie KL, Lev MH, Ay H, Singhal AB, Greer DM, Harris GJ, Halpern E, Koroshetz WJ, Smith WS. National Institutes of Health Stroke Scale score is poorly predictive of proximal occlusion in acute cerebral ischemia. Stroke. 2009; 40:2988-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. LEAN Model for Process Change Implementation
Supplement 2. Sources of Delays in Sequential IAT Process
Supplement 3. Team Member Roles in Parallel IAT Workflow
Supplement 4. Reduction in Case-to-Case Variability with a Standardized IAT Process


