Abstract
Background
Atrial fibrillation (AF) is associated with increased risk of hospitalization. Little is known about the impact of AF on utilization of noninpatient health care or about sex or race differences in AF‐related utilization. We examined rates of inpatient and outpatient utilization by AF status in the Atherosclerosis Risk in Communities study.
Methods and Results
Participants with incident AF enrolled in fee‐for‐service Medicare for at least 12 continuous months between 1991 and 2009 (n=932) were matched on age, sex, race and field center with up to 3 participants without AF (n=2729). Healthcare utilization was ascertained from Medicare claims and classified by primary International Classification of Diseases, ninth revision code. The average annual numbers of days hospitalized were 13.2 (95% CI 11.6 to 15.0) and 2.8 (95% CI 2.5 to 3.1) for those with and without AF, respectively. The corresponding numbers of annual outpatient claims were 53.3 (95% CI 50.5 to 56.3) and 22.9 (95% CI 22.1 to 23.8) for those with and without AF, respectively. Most utilization among AF patients was attributable to non‐AF conditions. The adjusted rate ratio for annual days hospitalized for other cardiovascular disease–related reasons was 4.58 (95% CI: 3.41 to 6.16) for those with AF versus those without AF. The association between AF and healthcare utilization was similar among men and women and among white and black participants.
Conclusions
Participants with AF had considerably greater healthcare utilization, and the difference in utilization for other cardiovascular disease–related reasons was substantial. In addition to rate or rhythm treatment, AF management should focus on the accompanying cardiovascular comorbidities.
Keywords: atrial fibrillation, epidemiology, health care, health disparities
Introduction
In the United States, the most recent annual national data reported 479 000 hospitalizations with atrial fibrillation (AF) as the primary diagnosis.1 Hospitalizations with AF as the primary diagnosis increased by 34% from 1996 to 2001.2 The outpatient burden is also high, with 5 million physician office visits, 276 000 emergency department visits, and 234 000 hospital outpatient visits attributed to AF in the United States in 2001.3 AF patients have many comorbidities: More than half of AF diagnoses can be explained by having at least 1 nonoptimal risk factor.4 Furthermore, AF is a cause of stroke5–6 and is strongly associated with other cardiovascular morbidity, including heart failure (HF)7–8 and acute myocardial infarction,9–10 and mortality.11 Healthcare utilization among AF patients is significant from both economic and clinical perspectives; however, it is unknown whether this utilization is related directly to AF or to comorbidities, which are common among AF patients.
Administrative claims data indicate that, compared with age‐ and sex‐matched beneficiaries without AF, those with AF had twice as many hospitalizations during the 12‐month period following initial AF diagnosis.12 A more granular analysis reported that the primary reasons for first hospitalization following AF diagnosis were AF (26.4%), HF (21.7%), coronary or peripheral arterial causes (21.6%), and thromboembolic events (10.5%).13 In addition to hospitalizations, there is evidence of overall increased utilization among patients with AF compared with matched controls.14
The overall clinical burden of AF is substantial, and sex and race disparities in access to and quality of health care among AF patients have been documented15–18; however, it is unknown whether the association between AF and healthcare utilization is similar among men and women and among white and black patients.
Given the substantial and increasing burden of AF on healthcare utilization, the limited knowledge about inpatient and noninpatient utilization, and the lack of sex‐ and race‐specific data, we sought to improve understanding of how AF patients utilize health care and to provide data that can be used to allocate adequate resources for the care of AF patients. Specifically, we compared healthcare utilization (inpatient or outpatient) and primary reason (AF related, other cardiovascular disease [CVD] related, and non‐CVD related) for seeking medical care among Atherosclerosis Risk in Communities (ARIC) study participants with AF and those without AF. We also described differences in utilization by sex and race.
Methods
Data Sources
The ARIC study is a population‐based prospective study of CVD in a cohort of 15 792 predominantly black and white participants aged 45 to 64 years at enrollment in 1987–1989.19 Participants were sampled from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; the northwestern suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Additional participant contact occurred through 4 follow‐up clinic visits and through annual telephone contact to obtain information regarding all hospitalizations and vital status, details of which have been reported previously.20
The ARIC study has an interagency agreement with the Centers for Medicare and Medicaid Services (CMS) to obtain Medicare data for ARIC cohort participants. Participants are matched on social security number, sex, and date of birth. The finder file included 15 738 ARIC participants, of which 14 530 (92.3%) were matched successfully and linked to CMS Medicare claims. Data for participants who matched successfully were linked to inpatient, outpatient, and carrier files. The Medicare Provider Analysis and Review (MedPAR) file contains claims for inpatient services covered under Medicare Part A. The outpatient files contain claims for services covered under Medicare Part B, including institutional claims (outpatient file) for outpatient services and noninstitutional physician claims (carrier file). CMS claims for inpatient and outpatient services have been available for research since 1991.
Study Sample
For this analysis, ARIC participants enrolled in fee‐for‐service (FFS) Medicare, both Parts A and B, for at least 12 continuous months between January 1, 1991, and December 31, 2009, were eligible for inclusion; for participants with multiple FFS enrollment periods (n=647), only the first was included (Figure 1). Medicare FFS enrollment was necessary because Medicare Advantage insurance plans are not required to submit claims for beneficiaries. In addition, those enrolled in only Part A (FFS) do not have claims data for Part B services (eg, outpatient and physician visits). Participants whose race was neither white nor black and nonwhites from the Minneapolis and Washington County field centers were excluded because of small numbers. Both active ARIC cohort follow‐up and surveillance of CMS data were used to identify and exclude all participants with prevalent AF. As such, based on initial ARIC study examination, participants with missing or unreadable ECG and those with prevalent AF on the baseline ECG were excluded. We were interested in incident AF; therefore, using all available information from ARIC and CMS, we excluded participants diagnosed with AF prior to January 1, 1992 (CMS data were available for research beginning January 1, 1991), participants with AF diagnosed prior to FFS enrollment or during the first year of FFS enrollment, and participants who stopped participating in ARIC follow‐up. Participants enrolled in Medicare because of disability or certain covered medical conditions were not included in the study unless they met study eligibility criteria after becoming age eligible (aged ≥65 years). Participants who died on the date of AF diagnosis were excluded.
Figure 1.
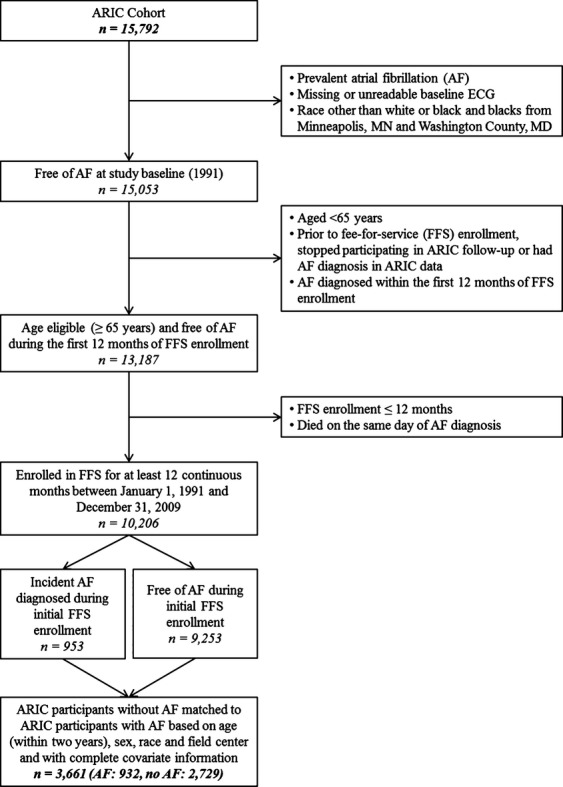
Derivation of study sample. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; FFS, fee‐for‐service.
Participants with incident AF diagnosed based on Medicare data during their initial FFS enrollment period were matched with up to 3 ARIC participants without AF based on age (within 2 years), sex, race, and field center; matching was used to account for strong confounders and was performed with the SAS macro gmatch, developed at the Mayo Clinic.21 Three matches were found for 93% of participants with AF. Of the 3737 participants, 3661 (932 with AF and 2729 without AF) had complete covariate information and composed our final sample. Each field center's institutional review board approved the study, and all participants provided informed consent.
Definition of Atrial Fibrillation
Incident AF cases were ascertained through MedPAR and outpatient CMS claims. Incident AF was defined as an AF discharge diagnosis, with International Classification of Diseases, ninth revision (ICD‐9) code 427.3x, in any position, on a single short‐stay inpatient (MedPAR) claim or on 2 outpatient claims within 7 to 365 days. A minimum of 2 outpatient claims at least 7 days apart were required to reduce the likelihood of including “rule out” diagnoses and to improve the algorithm specificity.22–23 The AF incidence date was defined as the discharge date for a MedPAR short‐stay claim or the date of the second qualifying outpatient claim, whichever occurred earliest. AF following cardiac operative procedures occurs frequently.24 Accordingly, AF diagnosis occurring simultaneously with cardiac revascularization (ICD‐9 code 36.x) or other cardiac surgery involving heart valves or septa (ICD‐9 code 35.x) during the index hospitalization without a subsequent AF diagnosis was not included.
Definition of Healthcare Utilization
Healthcare utilization was ascertained from short‐stay inpatient (MedPAR files) and outpatient (outpatient and carrier files) Medicare claims. Each claim was classified based on the primary discharge diagnosis code as AF related (ICD‐9 code 427.3x), other CVD related (ICD‐9 codes 390.x to 459.x) excluding AF, and non‐CVD related (all other valid ICD‐9 codes). Claims with an invalid or missing primary diagnosis code were classified based on the first‐listed usable diagnosis code. Length of hospitalization was taken into account for inpatient healthcare utilization by calculating length of stay. Multiple claims for the same date of service with identical diagnosis codes were considered one claim.
Assessment of Covariates
During the baseline ARIC study examination, standardized methods were used to collect data on age, race, sex, educational achievement, cigarette smoking, ethanol consumption, height, weight, blood pressure, antihypertensive medication use, diabetes mellitus, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, creatinine‐based estimated glomerular filtration rate, forced expiratory volume in 1 second, previous myocardial infarction, HF, or coronary heart disease.19 An ECG Cornell voltage >28 mm in men or >22 mm in women was considered evidence of left ventricular hypertrophy.25 Data on these covariates were updated during additional ARIC study examinations. Creatinine‐based estimated glomerular filtration rate was measured at visits 1, 2, and 4, whereas forced expiratory volume in 1 second was considered only at visit 1. For this analysis, the behavioral and clinical characteristics were updated to reflect the information from the closest study examination preceding AF diagnosis (for those with incident AF) or date of matching (for those without incident AF).
Statistical Analysis
Person‐years of follow‐up were calculated as the date of incident AF diagnosis or the matching date until the date of disenrollment in FFS Medicare, death, or December 31, 2009, whichever occurred earliest. The annualized rate of inpatient (MedPAR) utilization was calculated by dividing the total number of days hospitalized by the corresponding person‐years of follow‐up, which can be interpreted as the average annual number of days hospitalized per person. The annualized rate of outpatient utilization was calculated by dividing the total number of unique claims per date of service by the corresponding person‐years of follow‐up. Negative binomial regression models were used to calculate rates and rate ratios of inpatient and outpatient utilization, comparing those with and without AF; models include an offset of log follow‐up time to account for differential follow‐up. Covariate data were updated to reflect the closest ARIC examination preceding AF diagnosis or the matched reference date for those without AF. Participants with missing covariate data were excluded (n=32). Sex‐ and race‐specific rates of healthcare utilization (inpatient and outpatient) were also calculated. The rate of utilization, classified based on the primary diagnosis code as AF, other CVD, or non‐CVD related, was calculated with the same approach as described above for inpatient and outpatient utilization. Covariates with a statistically significant univariable association were retained in multivariable models. A descriptive analysis, restricted to hospitalizations for other CVD–related reasons and stratified by AF status, was performed to identify the primary reasons for and rates of hospitalization.
Prespecified 2‐way multiplicative interactions of healthcare utilization (inpatient [MedPAR] and outpatient considered separately) with sex and race were examined. A sensitivity analysis to assess secular trends in the association of AF with healthcare utilization was conducted by including year (defined as year of AF diagnosis for those with incident AF or year of matching for those without incident AF) modeled in quartiles (1992–1999, 2000–2003, 2004–2006, and 2007–2009) and an interaction term between year and AF status in a negative binomial regression model. A sensitivity analysis, restricted to matched AF and non‐AF participants with similar propensity scores, was performed. A logistic regression model was used to determine the probability of AF based on known risk factors for AF development. Suitable propensity score matches were identified within previously matched AF and non‐AF participants and defined as a caliper width ≤0.02, which corresponds to 25% of the standard deviation, as recommended by Rosenbaum and Rubin.26 All statistical analyses were performed with SAS version 9.2.
Results
Of the original 15 792 ARIC participants, our final analytic sample included 3661 participants (932 with AF and 2729 without AF) who were enrolled in FFS Medicare for at least 12 continuous months between January 1, 1991, and December 31, 2009. Characteristics of the study sample, stratified by AF status and updated to reflect the closest ARIC study examination values preceding AF diagnosis or matching, are shown in Table 1. The mean age at AF diagnosis or matching was 73.3 years (SD 4.7 years). Women composed ≈45% and black participants composed nearly 15% of the study sample. Participants with AF were more likely to be current smokers and to have higher body mass indices, hypertension, diabetes mellitus, left ventricular hypertrophy, HF, and coronary heart disease and lower creatinine‐based estimated glomerular filtration rates and forced expiratory volume in 1 second.
Table 1.
Characteristics of Participants by AF Status Based on Closest Atherosclerosis Risk in Communities Study Examination Preceding AF Diagnosis or Matching
| AF (n=932) | No AF (n=2729) | P Value | |
|---|---|---|---|
| Age at matching, y | 73.5±4.8 | 73.3±4.6 | |
| Women | 44.3 | 45.3 | |
| Black | 13.2 | 14.1 | |
| High school graduate | 75.0 | 77.1 | 0.20 |
| Current smoker | 19.0 | 13.5 | <0.0001 |
| Current drinker | 49.4 | 51.7 | 0.21 |
| Body mass index, kg/m2 | 29.3±5.7 | 28.3±5.2 | <0.0001 |
| Hypertension | 60.0 | 48.8 | <0.0001 |
| Antihypertensive medication | 47.8 | 35.8 | <0.0001 |
| Diabetes mellitus | 21.0 | 16.3 | 0.001 |
| Total cholesterol, mg/dL | 199.8±37.3 | 203.8±37.5 | 0.004 |
| LDL‐c, mg/dL | 124.0±34.6 | 126.6±34.3 | 0.05 |
| HDL‐c, mg/dL | 44.5±18.1 | 46.0±18.6 | 0.03 |
| Triglycerides, mg/dL | 147.6±83.5 | 145.0±84.5 | 0.41 |
| eGFRcreat, mL/min per 1.73 m2 | 83.6±16.7 | 84.9±15.4 | 0.03 |
| FEV1, L | 2.8±0.8 | 2.9±0.7 | 0.04 |
| Left ventricular hypertrophy | 3.9 | 2.1 | 0.002 |
| Heart failure | 10.5 | 3.7 | <0.0001 |
| Coronary heart disease | 19.0 | 9.4 | <0.0001 |
Categorical variables presented as percentages; continuous variables presented as mean±SD. P values comparing the distributions of age, sex, and race are not presented because these variables were used to match participants without AF to those with AF, ensuring balance in their distribution. AF indicates atrial fibrillation; eGFRcreat, creatinine‐based estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 second; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol.
During a mean follow‐up of 4.1 years, there were 2604 hospitalizations among the 932 participants with AF; the median length of stay was 5 days (interquartile range: 3 to 9 days). Among the 2729 without AF, there were 2965 hospitalizations during a mean follow‐up of 4.2 years; the median length of stay was 5 days (interquartile range: 3 to 8 days). The unadjusted mean days hospitalized per year were 13.2 (95% CI 11.6 to 15.0) and 2.8 (95% CI 2.5 to 3.1) for participants with and without AF, respectively (Table 2). After accounting for matching criteria, the number of days hospitalized per year was 4.85 (95% CI 4.03 to 5.84) times higher among participants with AF than in those who remained free of AF. After adjustment for potential confounders, the rate of days in the hospital was 3.94 (95% CI 3.29 to 4.73) times greater among those with AF. Healthcare utilization in the outpatient setting was higher among participants with AF compared with those without AF (Table 2). The median number of claims during follow‐up was 122.5 (interquartile range: 47 to 232.5) for those with AF and 50 (interquartile range: 17 to 115) for those without AF, based on unique claims per date of service. The unadjusted annual rate of outpatient utilization was 53.3 (95% CI 50.5 to 56.3) and 22.9 (95% CI 22.1 to 23.8) for those with and without AF, respectively. After accounting for matching criteria and other potential confounders, the rate ratio for outpatient utilization remained significantly greater among those with AF compared with those without AF (rate ratio 2.14, 95% CI 2.00 to 2.29).
Table 2.
Association of AF With Inpatient (MedPAR) and Outpatient Healthcare Utilization Among Atherosclerosis Risk in Communities Study Participants
| AF (n=932) | No AF (n=2729) | |
|---|---|---|
| Follow‐up, years (mean±SD) | 4.1±3.6 | 4.2±3.6 |
| Inpatient (MedPAR) utilization, days | ||
| Per person, median (IQR)* | 10 (0 to 30) | 0 (0 to 7) |
| Unadjusted rate* | 13.2 (11.6 to 15. 0) | 2.8 (2.5 to 3.1) |
| Unadjusted rate ratio | 4.84 (4.02 to 5.82) | Reference |
| Rate ratio adjusted for matching criteria* | 4.85 (4.03 to 5.84) | Reference |
| Fully adjusted rate ratio* | 3.94 (3.29 to 4.73) | Reference |
| Outpatient utilization* | ||
| Per person, median (IQR)* | 122.5 (47 to 232.5) | 50 (17 to 115) |
| Unadjusted rate* | 53.3 (50.5 to 56.3) | 22.9 (22.1 to 23.8) |
| Unadjusted rate ratio | 2.34 (2.18 to 2.50) | Reference |
| Rate ratio adjusted for matching criteria* | 2.33 (2.17 to 2.49) | Reference |
| Fully adjusted rate ratio* | 2.14 (2.00 to 2.29) | Reference |
AF indicates atrial fibrillation; IQR, interquartile range; MedPAR, Medicare Provider Analysis and Review.
During follow‐up.
Rate per year.
Adjusted for matching criteria: age (±2 years), sex, race, and field center.
Adjusted for matching criteria and for high school graduate, current smoking, current drinking, body mass index, hypertension, antihypertensive medication, diabetes mellitus, creatinine‐based estimated glomerular filtration rate, forced expiratory volume in 1 second, and prior heart failure and coronary heart disease.
Outpatient utilization defined as unique claims per date of service.
The interaction of sex with AF was not significant for inpatient (MedPAR) (P=0.12) or outpatient (P=0.39) utilization. The unadjusted annual rate (mean days hospitalized) of inpatient (MedPAR) healthcare utilization was highest for women with AF at 15.7 (95% CI 12.9 to 19.1) and lowest for women without AF at 2.5 (95% CI 2.2 to 3.0) (Table 3).
Table 3.
Association of AF With Inpatient (MedPAR) and Outpatient Healthcare Utilization Among Atherosclerosis Risk in Communities Participants Stratified by Sex
| Men | Women | |||
|---|---|---|---|---|
| AF (n=519) | No AF (n=1494) | AF (n=413) | No AF (n=1235) | |
| Follow‐up, years (mean±SD) | 4.2±3.7 | 4.0±3.5 | 4.0±3.5 | 4.4±3.7 |
| Inpatient (MedPAR) utilization, days | ||||
| Per person, median (IQR)* | 9 (0 to 28) | 0 (0 to 7) | 10 (0 to 32) | 0 (0 to 7) |
| Unadjusted rate* | 11.3 (9.5 to 13.4) | 3.1 (2.7 to 3.5) | 15.7 (12.9 to 19.1) | 2.5 (2.2 to 3.0) |
| Unadjusted rate ratio | 3.80 (2.97 to 4.85) | Reference | 6.43 (4.87 to 8.50) | Reference |
| Rate ratio adjusted for matching criteria* | 3.54 (2.77 to 4.52) | Reference | 6.72 (5.06 to 8.93) | Reference |
| Fully adjusted rate ratio* | 3.42 (2.69 to 4.36) | Reference | 4.77 (3.59 to 6.35) | Reference |
| Outpatient utilization* | ||||
| Per person, median (IQR)* | 127 (49 to 245) | 48 (17 to 113) | 111 (40 to 221) | 52 (15 to 116) |
| Unadjusted rate* | 53.1 (49.7 to 56.6) | 23.9 (22.7 to 25.1) | 53.6 (49.0 to 58.7) | 21.8 (20.6 to 23.0) |
| Unadjusted rate ratio | 2.24 (2.05 to 2.45) | Reference | 2.46 (2.21 to 2.74) | Reference |
| Rate ratio adjusted for matching criteria* | 2.20 (2.01 to 2.40) | Reference | 2.46 (2.21 to 2.74) | Reference |
| Fully adjusted rate ratio* | 2.06 (1.88 to 2.25) | Reference | 2.26 (2.03 to 2.51) | Reference |
AF indicates atrial fibrillation; IQR, interquartile range; MedPAR, Medicare Provider Analysis and Review.
During follow‐up.
Rate per year.
Adjusted for matching criteria: age (within 2 years), sex, race, and field center.
Adjusted for matching criteria and for high school graduate, current smoking, current drinking, body mass index, hypertension, antihypertensive medication, diabetes mellitus, creatinine‐based estimated glomerular filtration rate, forced expiratory volume in 1 second, and prior heart failure and coronary heart disease.
Outpatient utilization defined as unique claims per date of service.
Healthcare utilization following AF diagnosis did not differ significantly between white and black participants for inpatient (MedPAR) (P‐interaction=0.65) or outpatient (P‐interaction=0.13) utilization. Black participants with AF had the highest unadjusted annual rate of days hospitalized (rate 17.8, 95% CI 12.0 to 26.3) and outpatient utilization (rate 53.9, 95% CI 45.5 to 63.9) (Table 4).
Table 4.
Association of AF With Inpatient (MedPAR) and Outpatient Healthcare Utilization Among Atherosclerosis Risk in Communities Participants Stratified by Race
| White | Black | |||
|---|---|---|---|---|
| AF (n=809) | No AF (n=2344) | AF (n=123) | No AF (n=385) | |
| Follow‐up, years (mean±SD) | 4.2±3.6 | 4.3±3.6 | 3.6±3.2 | 4.0±3.6 |
| Inpatient (MedPAR) utilization, days | ||||
| Per person, median (IQR)* | 10 (0 to 29) | 0 (0 to 7) | 8 (0 to 37) | 0 (0 to 5) |
| Unadjusted rate* | 12.5 (10.9 to 14.3) | 2.8 (2.5 to 3.1) | 17.8 (12.0 to 26.3) | 3.2 (2.3 to 4.5) |
| Unadjusted rate ratio | 4.70 (3.87 to 5.70) | Reference | 5.64 (3.15 to 10.09) | Reference |
| Rate ratio adjusted for matching criteria* | 4.70 (3.87 to 5.71) | Reference | 5.69 (3.17 to 10.23) | Reference |
| Fully adjusted rate ratio* | 3.94 (3.25 to 4.77) | Reference | 4.69 (2.59 to 8.50) | Reference |
| Outpatient utilization* | ||||
| Per person, median (IQR)* | 127 (49 to 236) | 52.5 (18 to 118) | 94 (33 to 176) | 37 (8 to 97) |
| Unadjusted rate* | 53.2 (50.3 to 56.3) | 23.2 (22.3 to 24.1) | 53.9 (45.5 to 63.9) | 21.3 (18.8 to 24.0) |
| Unadjusted rate ratio | 2.30 (2.14 to 2.47) | Reference | 2.55 (2.02 to 3.22) | Reference |
| Rate ratio adjusted for matching criteria* | 2.29 (2.13 to 2.46) | Reference | 2.57 (2.03 to 3.25) | Reference |
| Fully adjusted rate ratio* | 2.11 (1.96 to 2.26) | Reference | 2.37 (1.88 to 2.98) | Reference |
AF indicates atrial fibrillation; IQR, interquartile range; MedPAR, Medicare Provider Analysis and Review.
During follow‐up.
Rate per year.
Adjusted for matching criteria: age (within 2 years), sex, race, and field center.
Adjusted for matching criteria and for high school graduate, current smoking, current drinking, body mass index, hypertension, antihypertensive medication, diabetes mellitus, creatinine‐based estimated glomerular filtration rate, forced expiratory volume in 1 second, and prior heart failure and coronary heart disease.
Outpatient utilization defined as unique claims per date of service.
Among participants with AF, the unadjusted annual rate of days hospitalized with AF as the primary diagnosis was 0.5 (95% CI 0.3 to 0.7). The annual rate of days hospitalized for other CVD‐related reasons was 4.2 (95% CI 3.5 to 5.2) among those with AF and 0.8 (95% CI 0.6 to 0.9) among those without AF (Table 5). After adjustment for matching criteria and other potential confounders, the adjusted rate ratio for days hospitalized per year for other CVD–related reasons was 4.58 (95% CI 3.41 to 6.16) for those with and those without AF. The magnitude of the difference was smaller for non‐CVD–related hospitalized days (adjusted rate ratio 3.52, 95% CI 2.87 to 4.31). The unadjusted annual rate of days hospitalized for non‐CVD–related reasons was 8.2 (95% CI 7.1 to 9.5) for those with AF and 2.0 (95% CI 1.8 to 2.3) for those without AF. A descriptive analysis revealed that HF was the leading cause of non‐AF CVD‐related hospitalizations for those with AF (51.6 per 1000 person‐years). Among those without AF, the rate of non‐AF CVD‐related hospitalization was greatest for cerebrovascular disease (17.4 per 1000 person‐years) (Table 6). Outpatient utilization followed a similar pattern; the magnitude of the difference between those with AF and those without AF was greatest for other CVD–related reasons (adjusted rate ratio 2.46, 95% CI 2.24 to 2.70).
Table 5.
Primary Reason for Inpatient (MedPAR) and Outpatient Healthcare Utilization Among Atherosclerosis Risk in Community Study Participants Stratified by AF Status
| AF (n=932) | No AF (n=2729) | Adjusted Rate Ratio* | |
|---|---|---|---|
| Inpatient (MedPAR) utilization, days | |||
| Unadjusted rates* | |||
| AF related | 0.5 (0.3 to 0.7) | — | — |
| Other CVD related | 4.2 (3.5 to 5.2) | 0.8 (0.6 to 0.9) | 4.58 (3.41 to 6.16) |
| Non‐CVD related | 8.2 (7.1 to 9.5) | 2.0 (1.8 to 2.3) | 3.52 (2.87 to 4.31) |
| Outpatient utilization* | |||
| Unadjusted rates* | |||
| AF related | 4.8 (4.3 to 5.4) | 0.01 (0.01 to 0.02) | — |
| Other CVD related | 9.0 (8.4 to 9.7) | 3.3 (3.1 to 3.5) | 2.46 (2.24 to 2.70) |
| Non‐CVD related | 38.8 (36.7 to 41.1) | 19.4 (18.7 to 20.2) | 1.85 (1.72 to 1.99) |
AF indicates atrial fibrillation; CVD, cardiovascular disease; MedPAR, Medicare Provider Analysis and Review.
Adjusted for matching criteria and for high school graduate, current smoking, current drinking, body mass index, hypertension, antihypertensive medication, diabetes mellitus, creatinine‐based estimated glomerular filtration rate, forced expiratory volume in 1 second, and prior heart failure and coronary heart disease.
Rates are days per year for inpatient (MedPAR) data and number of unique claims per date of service per year for outpatient claims.
Outpatient utilization defined as unique claims per date of service.
Table 6.
Descriptive Analysis of the Primary Diagnosis Codes for Non‐AF CVD‐Related Hospitalizations Stratified by AF Status
| AF (n=932) | No AF (n=2729) | |||
|---|---|---|---|---|
| Number | Rate* | Number | Rate | |
| Total person‐years of follow‐up | 3818 | 11 516 | ||
| Total non‐AF CVD‐related hospitalizations | 804 | 210.6 | 723 | 62.8 |
| Hypertensive disease (401 to 405) | 40 | 10.5 | 27 | 2.3 |
| Myocardial infarction (410) | 74 | 19.4 | 81 | 7.0 |
| Coronary atherosclerosis, native vessel (414.01) | 97 | 25.4 | 117 | 10.2 |
| Heart failure (428) | 197 | 51.6 | 81 | 7.0 |
| Cerebrovascular disease (430 to 438) | 141 | 36.9 | 200 | 17.4 |
AF indicates atrial fibrillation; CVD, cardiovascular disease.
Unadjusted rates per 1000 person‐years.
A sensitivity analysis of secular trends in the association of AF and healthcare utilization revealed statistically significant effect modification of the association by year of matching for inpatient (MedPAR) (P=0.03) and outpatient (P=0.01) utilization. The fully adjusted rate ratios for those with and those without AF increased over the study period (Figure 2). In 1992–1999, the multivariable adjusted rate ratio for inpatient (MedPAR) utilization was 2.73 (95% CI 1.96 to 2.09); in 2007–2009, it was 7.00 (95% CI 4.04 to 12.12). The trend in outpatient utilization was similar but with a less sizable increase. The multivariable adjusted rate ratios in 1992–1999 and 2007–2009 were 1.82 (95% CI 1.59 to 2.09) and 2.53 (95% CI 2.22 to 2.89), respectively. The observed trend remained essentially the same after including an age×period term in the model.
Figure 2.
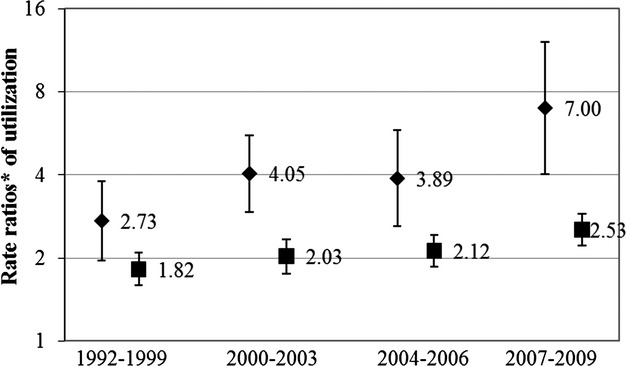
Multivariable adjusted rate ratios of inpatient (MedPAR) and outpatient healthcare utilization by year of matching comparing participants with and without atrial fibrillation. Diamonds represent rate ratios for inpatient (MedPAR) utilization. Squares represent rate ratios for outpatient utilization. *Rate ratios adjusted for matching criteria—age (±2 years), sex, race, and field center—and for high school graduate, current smoking, current drinking, body mass index, hypertension, antihypertensive medication, diabetes mellitus, estimated glomerular filtration rate, forced expiratory volume in 1 second, and prior heart failure and coronary heart disease. MedPAR indicates Medicare Provider Analysis and Review.
In a sensitivity analysis restricted to matched AF and non‐AF participants with similar propensity scores, the primary results were corroborated. Among the 354 ARIC participants with AF matched with 354 participants without AF, characteristics at the time of matching were similar (Table 7). The unadjusted days per year in the hospital were 8.7 (95% CI 7.1 to 10.7) and 2.3 (95% CI 1.7 to 3.1) for participants with and without AF, respectively (rate ratio 3.87, 95% CI 2.77 to 5.42) (Table 8). In the outpatient setting, the unadjusted annual rate of utilization was 47.1 (95% CI 43.3 to 51.3) and 21.8 (95% CI 19.5 to 24.3) for those with and without AF, respectively (rate ratio 2.18, 95% CI 1.89 to 2.50). Based on the primary diagnosis code in the propensity score–matched model, for those with AF compared with those without AF, the rate ratio for days hospitalized per year for other CVD–related reasons was 3.82 (95% CI 2.21 to 6.59) and for non‐CVD–related reasons was 4.19 (95% CI 2.86 to 6.12) (Table 9). The propensity score–matched rate ratio for other CVD–related reasons in the outpatient setting was 2.55 (95% CI 2.13 to 3.05) for those with and those without AF; for non‐CVD–related reasons, the propensity score–matched rate ratio was 1.89 (95% CI 1.64 to 2.18). Similar secular trends in healthcare utilization were observed. The impact of AF on inpatient (MedPAR) utilization increased over time (P=0.004), but there was no effect modification of the association between AF and outpatient utilization (P=0.16) (data not shown).
Table 7.
Characteristics of Propensity Score Matched Participants by AF Status Based on Closest Atherosclerosis Risk in Communities Study Examination Preceding AF Diagnosis or Matching
| AF (n=354) | No AF (n=354) | P Value | |
|---|---|---|---|
| Propensity score | 0.23±0.06 | 0.23±0.06 | 0.78 |
| Age at matching, y | 73.6±4.8 | 73.6±4.7 | |
| Women | 48.9 | 48.9 | |
| Black | 11.6 | 11.6 | |
| High school graduate | 78.8 | 81.1 | 0.45 |
| Current smoker | 9.9 | 13.6 | 0.13 |
| Current drinker | 50 | 52.8 | 0.45 |
| Body mass index, kg/m2 | 28.3±4.9 | 28.3±4.9 | 0.85 |
| Hypertension | 46.9 | 44.6 | 0.55 |
| Antihypertensive medication | 32.8 | 31.1 | 0.63 |
| Diabetes mellitus | 15.3 | 13.3 | 0.45 |
| Total cholesterol, mg/dL | 200.3±34.3 | 201.7±36.9 | 0.6 |
| LDL‐c, mg/dL | 124.1±31.5 | 125.5±33.1 | 0.57 |
| HDL‐c, mg/dL | 46.0±19.6 | 46.4±18.8 | 0.83 |
| Triglycerides, mg/dL | 136.0±72.5 | 138.2±74.2 | 0.69 |
| eGFRcreat, mL/min per 1.73 m2 | 83.9±15.4 | 84.6±14.0 | 0.52 |
| FEV1, L | 2.8±0.8 | 2.8±0.7 | 0.26 |
| Left ventricular hypertrophy | 2.5 | 1.1 | 0.16 |
| Heart failure | 1.7 | 0.9 | 0.31 |
| Coronary heart disease | 5.7 | 4.8 | 0.61 |
Categorical variables presented as percentage; continuous variables presented as mean±SD. P values comparing the distributions of age, sex, and race are not presented because these variables were used to match participants without AF to those with AF, ensuring balance in their distribution. AF indicates atrial fibrillation; eGFRcreat, creatinine‐based estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 second; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol.
Table 8.
Association of AF With Inpatient (MedPAR) and Outpatient Healthcare Utilization Among Propensity Score–Matched Atherosclerosis Risk in Communities Study Participants
| AF (n=354) | No AF (n=354) | Rate Ratio | |
|---|---|---|---|
| Follow‐up, years (mean±SD) | 4.3±3.7 | 4.1±3.6 | |
| Inpatient (MedPAR) utilization, days | |||
| Per person, median (IQR)* | 7 (0 to 25) | 0 (0 to 6) | 3.87(2.77 to 5.42) |
| Rate* | 8.7 (7.1 to 10.7) | 2.3 (1.7 to 3.1) | |
| Outpatient utilization* | |||
| Per person, median (IQR)* | 128 (47 to 226) | 43 (12 to 108) | 2.18 (1.89 to 2.50) |
| Rate* | 47.1 (43.3 to 51.3) | 21.8 (19.5 to 24.3) |
AF indicates atrial fibrillation; IQR, interquartile range; MedPAR, Medicare Provider Analysis and Review.
During follow‐up.
Rate per year.
Outpatient utilization defined as unique claims per date of service.
Table 9.
Primary Reason for Inpatient (MedPAR) and Outpatient Healthcare Utilization Among Propensity Score–Matched Atherosclerosis Risk in Communities Study Participants Stratified by AF Status
| AF (n=354) | No AF (n=354) | Rate Ratio | |
|---|---|---|---|
| Inpatient (MedPAR) utilization, days | |||
| Rates* | |||
| AF related | 0.2 (0.1 to 0.3) | — | — |
| Other CVD related | 2.0 (1.4 to 2.8) | 0.7 (0.4 to 1.1) | 3.82 (2.21 to 6.59) |
| Non‐CVD related | 6.5 (5.1 to 8.3) | 1.6 (1.2 to 2.2) | 4.19 (2.86 to 6.12) |
| Outpatient utilization* | |||
| Rates* | |||
| AF related | 4.7 (3.9 to 5.5) | 0.01 (0.00 to 0.03) | — |
| Other CVD related | 6.8 (6.0 to 7.5) | 2.9 (2.5 to 3.4) | 2.55 (2.13 to 3.05) |
| Non‐CVD related | 35.5 (32.3 to 38.9) | 18.8 (16.8 to 21.1) | 1.89 (1.64 to 2.18) |
AF indicates atrial fibrillation; CVD, cardiovascular disease; MedPAR, Medicare Provider Analysis and Review.
Rates are days per year for inpatient (MedPAR) data and number of unique claims per date of service per year for outpatient claims.
Outpatient utilization defined as unique claims per date of service.
Discussion
In this sample of AF patients and matched controls from a community‐based prospective study, rates of both inpatient (MedPAR) and outpatient healthcare utilization were substantially higher among participants with AF compared with those matched with cases and without AF. In both the inpatient and outpatient settings, healthcare utilization was greatest for non‐CVD–related reasons for those with and those without AF; however, the magnitude of the difference in utilization between those with and without AF was greatest for other CVD–related reasons. Our findings underscore the high prevalence of cardiovascular comorbidities, particularly HF, triggering healthcare utilization among AF patients. At the time of AF diagnosis or matching, HF was almost 3 times more prevalent in AF patients compared with matched participants. The prevalence of other comorbidities, including hypertension, diabetes, coronary heart disease, and low creatinine‐based estimated glomerular filtration rate, were significantly higher among AF patients compared with the non‐AF matched controls. Although AF has long been thought of as an electrical conduction problem, the high prevalence of cardiovascular comorbidities and the higher rate of healthcare utilization among those with AF, especially for other CVD–related reasons, provide evidence that AF should not be considered as just an electrical problem but rather as a marker of underlying vascular disease and overall cardiovascular risk. A Danish nationwide study reported that, within each age group, hospitalization rates for CVD and non‐CVD admissions were higher among those with AF compared with those without AF.27 Furthermore, a 30‐year follow‐up of Olmsted County, Minnesota, residents diagnosed with lone AF, considered to be purely an electrical conduction problem, revealed that those with AF had a slightly elevated risk of developing HF or a cerebrovascular event compared with the age‐ and sex‐matched Minnesota population.28 Moreover, in a contemporary anticoagulated AF population, 90% of deaths were related to reasons other than stroke,29 corroborating the impact of cardiovascular comorbidities on mortality reported from the AFFIRM trial30 and the Atrial Fibrillation and Congestive Heart Failure (AF‐CHF) trial.31 In the present study, the associations between AF and inpatient (MedPAR) and outpatient healthcare utilization remained significant but were attenuated after adjusting for comorbidities. The overall adjusted rate ratio for days hospitalized because of other CVD–related reasons was 4.58 (95% CI 3.41 to 6.16) among those with AF compared with those without AF; the corresponding adjusted rate ratio for outpatient utilization was 2.46 (95% CI 2.24 to 2.70). These findings suggest that it is not just the presence of AF that causes healthcare utilization but also the company it keeps—hypertension, diabetes mellitus, HF, and coronary heart disease—contributing, in part, to higher rates of healthcare utilization among AF patients. Our results underscore the importance of adequate management and control of these comorbidities. Despite the attenuation with adjustment for comorbidities, healthcare utilization remained substantially greater among those with AF, indicating considerable additional medical demand among this subset of the population.
Despite published disparities in AF treatment between men and women32–34 and between white and black patients,17–18,35 our study did not identify a differential impact of AF by sex or race on inpatient (MedPAR) or outpatient utilization; however, the numbers of women and black participants with AF were small and were from a small number of geographic areas, so it is possible that our study was underpowered to detect a difference.
There was evidence of a secular trend in the association of AF with both inpatient (MedPAR) and outpatient utilization; the impact of AF on healthcare utilization increased during the study period. Due to the relatively narrow age range—ARIC participants were recruited in 1987–1989, when they were aged 45 to 64 years—it is difficult to separate secular trends from the increased risk of disease with age. It is possible that the results from the sensitivity analysis reflect comparatively more frailty among those with AF compared with those without AF in the later period of the follow‐up. Increased awareness and treatment of AF over time or greater emphasis on quality metrics could explain the trend of increased impact of AF on healthcare utilization. The present study did not have data to address these potential explanations.
The matched propensity score results reinforced the primary findings. In this sensitivity analysis, the comorbidity burden was similar at the time of matching among those with AF compared with those without AF. Still, the rates of inpatient (MedPAR) and outpatient utilization were higher among participants with AF compared with those free of AF, suggesting that the differences in utilization were not due to differences in comorbidities at the time of matching. This study has several limitations. The CMS Medicare files for ARIC analysis did not contain line items; therefore, multiple claims for the same date of service with identical diagnosis codes could not be included in this study. Without the line items, it is impossible to know whether the claims are for multiple services (laboratory, x‐ray, physician) related to the same condition or for the same claim submitted multiple times. By excluding claims with identical diagnostic codes for the same date of service, our estimates are conservative and, if anything, underestimate healthcare utilization. In addition, our study had to be restricted to FFS Medicare enrollment windows because Medicare Advantage plans are not required to submit claims on their beneficiaries. Although exclusion of Medicare Advantage enrollment windows limits the generalizability of study findings, the results are applicable to the FFS population. A weakness of the ARIC study is that it includes white and black participants from only 3 and 2 communities, respectively, and might not be generalizable to all white and black patients in the United States. This study is innovative in its use of a community‐based prospective study to identify the study sample; however, it is crucial to consider these threats to generalizability and not mistakenly project the findings too broadly. Moreover, because of small numbers of diagnosed AF, the power to detect significant effect modification by sex or race was low; among women and black participants, 413 and 123 participants were diagnosed with AF, respectively. Consequently, we described healthcare utilization by subgroups but cannot make conclusive statements about similarities or differences by sex or race. Nevertheless, this study is the first to provide sex‐ and race‐specific data on inpatient and outpatient utilization.
This study also has several strengths. Claims data contain little information on clinical characteristics because their primary purpose is reimbursement. In this study, data collected as part of the ARIC study examinations were linked to Medicare data; consequently, information unavailable in claims data, such as detailed and validated demographic, behavioral, and comorbid conditions measured using standardized methodology, were present and included in analyses. Second, prior research on AF did not study outpatient healthcare utilization.12–13 Furthermore, previous studies were conducted in a predominately white population13 or did not consider differences by sex or race.12–13 In response to the noted gaps in the literature, we described overall healthcare utilization among participants with and without AF and sex‐ and race‐specific utilization for both inpatient (MedPAR) and outpatient services.
In conclusion, this study provides evidence of the burden of AF healthcare utilization. The results highlight that AF is not just an electrical problem and that treatment guidelines should incorporate assessment of overall cardiovascular risk and provide recommendations on comprehensive management of the patient. Participants with AF had greater underlying vascular disease and spent significantly more days hospitalized and seeking outpatient care than similar participants without AF. The magnitude of the difference in utilization between those with and without AF was greatest for other CVD–related reasons and emphasizes the need to treat the underlying vascular disease in addition to rhythm or rate management among those with AF.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. This study additionally was supported by NHLBI grants RC1‐HL‐099452 and T32‐HL‐07779 (Bengtson) and the American Heart Association grant 09SDG2280087.
Disclosures
None.
Acknowledgments
We thank the staff and participants of the ARIC study for their important contributions.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013; 127:e6-e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khairallah F, Ezzedine R, Ganz LI, London B, Saba S. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004; 94:500-504. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006; 9:348-356. [DOI] [PubMed] [Google Scholar]
- 4.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011; 123:1501-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Seward JB, Bailey KR, Iwasaka T, Tsang TS. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000: report of a community‐based study. Stroke. 2005; 36:2362-2366. [DOI] [PubMed] [Google Scholar]
- 6.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983-988. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community‐based study over two decades. Eur Heart J. 2006; 27:936-941. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003; 107:2920-2925. [DOI] [PubMed] [Google Scholar]
- 9.Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011; 123:2094-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta‐analysis. Circulation. 2011; 123:1587-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98:946-952. [DOI] [PubMed] [Google Scholar]
- 12.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011; 4:313-320. [DOI] [PubMed] [Google Scholar]
- 13.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Seward JB, Tsang TS. Changing trends of hospital utilization in patients after their first episode of atrial fibrillation. Am J Cardiol. 2008; 102:568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boggon R, Lip GY, Gallagher AM, van Staa TP. Resource utilization and outcomes in patients with atrial fibrillation: a case control study. Appl Health Econ Health Policy. 2012; 10:249-259. [DOI] [PubMed] [Google Scholar]
- 15.Niska R, Han B. Anticoagulation for patients with atrial fibrillation in ambulatory care settings. J Am Board Fam Med. 2009; 22:299-306. [DOI] [PubMed] [Google Scholar]
- 16.LaPointe NM, Sun JL, Kaplan S. In‐hospital management of patients with atrial flutter. Am Heart J. 2010; 159:370-376. [DOI] [PubMed] [Google Scholar]
- 17.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial disparities in awareness and treatment of atrial fibrillation: the REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010; 41:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in medicare managed care. JAMA. 2002; 287:1288-1294. [DOI] [PubMed] [Google Scholar]
- 19. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989; 129:687-702. [PubMed] [Google Scholar]
- 20.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African–Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergstralh E, Kosanke J. SAS Macros: gmatch; 2004. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/gmatch.sas Accessed April 26, 2013.
- 22.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012; 5:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welch HG, Sharp SM, Gottlieb DJ, Skinner JS, Wennberg JE. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011; 305:1113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997; 336:1429-1434. [DOI] [PubMed] [Google Scholar]
- 25.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex‐specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987; 75:565-572. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985; 39:33-38. [Google Scholar]
- 27.Christiansen CB, Olesen JB, Gislason G, Lock‐Hansen M, Torp‐Pedersen C. Cardiovascular and non‐cardiovascular hospital admissions associated with atrial fibrillation: a Danish nationwide, retrospective cohort study. BMJ Open. 2013; 3:e001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ. Long‐term progression and outcomes with aging in patients with lone atrial fibrillation: a 30‐year follow‐up study. Circulation. 2007; 115:3050-3056. [DOI] [PubMed] [Google Scholar]
- 29.Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom JW, Themeles E, Ezekowitz MD, Wallentin L, Yusuf S. Causes of death and influencing factors in patients with atrial fibrillation: a competing risk analysis from the randomized evaluation of long‐term anticoagulant therapy study. Circulation. 2013; 128:2192-2201. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg JS, Sadaniantz A, Kron J, Krahn A, Denny DM, Daubert J, Campbell WB, Havranek E, Murray K, Olshansky B, O'Neill G, Sami M, Schmidt S, Storm R, Zabalgoitia M, Miller J, Chandler M, Nasco EM, Greene HL. Analysis of cause‐specific mortality in the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004; 109:1973-1980. [DOI] [PubMed] [Google Scholar]
- 31.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008; 358:2667-2677. [DOI] [PubMed] [Google Scholar]
- 32.Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000; 31:822-827. [DOI] [PubMed] [Google Scholar]
- 33.Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New‐onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001; 103:2365-2370. [DOI] [PubMed] [Google Scholar]
- 34.Sudlow M, Thomson R, Thwaites B, Rodgers H, Kenny RA. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998; 352:1167-1171. [DOI] [PubMed] [Google Scholar]
- 35.Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta‐analysis. BMC Fam Pract. 2012; 13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


