Abstract
Background
Tenascin‐C (TN‐C), an extracellular matrix glycoprotein, appears at several important steps of cardiac development in the embryo, but is sparse in the normal adult heart. TN‐C re‐expresses under pathological conditions including myocarditis, and is closely associated with tissue injury and inflammation in both experimental and clinical settings. However, the pathophysiological role of TN‐C in the development of myocarditis is not clear. We examined how TN‐C affects the initiation of experimental autoimmune myocarditis, immunologically.
Methods and Results
A model of experimental autoimmune myocarditis was established in BALB/c mice by immunization with murine α‐myosin heavy chains. We found that TN‐C knockout mice were protected from severe myocarditis compared to wild‐type mice. TN‐C induced synthesis of proinflammatory cytokines, including interleukin (IL)‐6, in dendritic cells via activation of a Toll‐like receptor 4, which led to T‐helper (Th)17 cell differentiation and exacerbated the myocardial inflammation. In the transfer experiment, dendritic cells loaded with cardiac myosin peptide acquired the functional capacity to induce myocarditis when stimulated with TN‐C; however, TN‐C‐stimulated dendritic cells generated from Toll‐like receptor 4 knockout mice did not induce myocarditis in recipients.
Conclusions
Our results demonstrated that TN‐C aggravates autoimmune myocarditis by driving the dendritic cell activation and Th17 differentiation via Toll‐like receptor 4. The blockade of Toll‐like receptor 4‐mediated signaling to inhibit the proinflammatory effects of TN‐C could be a promising therapeutic strategy against autoimmune myocarditis.
Keywords: dendritic cell, myocarditis, tenascin‐C, Th17, TLR4
Introduction
Myocarditis is induced by a variety of agents, including toxins, alcohols, parasites, bacteria, and viruses.1–2 Postinfectious autoimmune myocarditis often results in idiopathic dilated cardiomyopathy (DCM), which is sometimes a lethal disorder characterized by left ventricular (LV) enlargement and systolic dysfunction.1–2 Experimental autoimmune myocarditis (EAM) is a mouse model of such a kind of inflammation‐based cardiomyopathy.3–4 EAM is known as a CD4+ T‐cell‐mediated disease, and activation of self‐antigen‐loaded dendritic cells (DCs) is critical for the expansion of autoreactive CD4+ T cells.5 Upon encountering immunologic danger, the maturated and activated DCs act as an important commander regulating naïve CD4+ T cells by means of antigen presentation, costimulatory molecule engagement, and release of a cocktail of polarizing cytokines.6 DC activation is mediated by the recognition of pathogen‐associated molecular patterns and damage‐associated molecular patterns, which are sensed by pattern‐recognized receptors such as Toll‐like receptors (TLRs).6
Recently, accumulating evidence has highlighted the proinflammatory role of several extracellular matrix molecules including tenascin‐C (TN‐C), osteopontin, and galectin.7–10 Of these, TN‐C acts as a damage‐associated molecular pattern and directly activates inflammatory cells including lymphocytes, macrophages, synovial fibroblasts, and DCs.11–14 TN‐C is synthesized by various cell types in response to inflammatory cytokines and mechanical stress.12,15–16 TN‐C is not normally expressed in the adult heart but is specifically upregulated during clinical conditions accompanying inflammation,7,17 such as acute myocardial infarction,18 hypertensive cardiac fibrosis,19 myocarditis,15,20 and some cases of DCM.21 In particular, our previous analysis of the myocardium obtained from left ventriculoplasty specimens of patients with DCM showed that about half of the patients had significant active inflammation associated with TN‐C expression.21 Thus, it has been recognized that TN‐C is closely associated with inflammatory status in cardiovascular diseases. However, how TN‐C acts as a proinflammatory stimulus by mediating the immune system in myocarditis has not been fully understood.
Here, we show that TN‐C leads to TLR4‐mediated inflammatory responses in DCs and exacerbates myocardial inflammation. TN‐C knockout (TNKO) mice were protected from severe myocarditis in a model of cardiac myosin‐induced autoimmune myocarditis. Furthermore, we found that TN‐C induced synthesis of proinflammatory cytokines, including interleukin‐6 (IL‐6), in DCs via activation of TLR4, which led to Th17 cell differentiation. Finally, in the transfer experiment, DCs loaded with cardiac myosin peptide acquired the functional capacity to induce myocarditis when stimulated with TN‐C; however, TN‐C‐stimulated DCs generated from TLR4 knockout (TLR4KO) mice did not induce myocarditis in recipients.
Materials and Methods
Mice
TNKO mice, originally generated by Saga et al., were backcrossed with BALB/c mice for more than 12 generations.22 Male TNKO mice and wild‐type (WT) littermates (5 to 7 weeks of age) were used in the experiments. Other male BALB/c mice (same age) that were used to obtain bone marrow cells were purchased from CLEA Japan. TLR4KO mice were purchased from Oriental Yeast. All animal experiments were approved by the Institutional Animal Experiment Committee of the University of Tsukuba.
Induction of EAM
The mice were immunized with 100 μg of α‐myosin H‐chain peptide (MyHC‐α) (MyHC‐α614–629 [Ac‐RSLKLMATLFSTYASADR‐OH]; Toray Research Center) emulsified 1:1 in a PBS/complete Freund's adjuvant (1 mg/mL; H37Ra; Sigma‐Aldrich) on days 0 and 7, as described previously.23 At different time points (0, 2, 5, 8, 11, 14, 17, 21, 25, 29, and 33 days) after the first immunization, a total of 44 mice were anesthetized with pentobarbital (60 mg/kg i.p.) and their hearts were removed.
Myosin‐Specific Bone Marrow‐Derived Dendritic Cell (BMDC)‐Induced Myocarditis
BMDCs were generated as previously described.24 BMDCs were pulsed overnight with 10 μg/mL MyHC‐α peptide and stimulated for another 4 hours with 1 μg/mL lipopolysaccharide (LPS) (Sigma‐Aldrich) or 10 μg/mL TN‐C and 5 μg/mL anti‐CD40L (AbD Serotec).5 Recipient mice received 5×105 pulsed and activated BMDCs i.p. on days 0, 2, and 4 and were killed 10 days after the first injection.
Histopathological and Immunohistochemical Examination
The hearts were fixed in 4% paraformaldehyde in PBS and embedded in paraffin wax. For a histological analysis, 3‐μm‐thick sections were cut and stained with hematoxylin and eosin. To evaluate the expression of TN‐C, we performed immunohistochemistry as previously described.25 In brief, sections after antigen retrieval were incubated with polyclonal rabbit anti‐TN‐C antibodies,15,26 followed by treatment with horseradish peroxidase–conjugated goat anti‐rabbit antibody (MBL, Nagoya, Japan). The antibody reactions were visualized using diaminobenzidine chromogen and were counterstained with hematoxylin. Myocarditis severity was scored on hematoxylin and eosin–stained sections using grades from 0 to 4: 0, no inflammation; 1, >25% of the heart section involved; 2, 25% to 50%; 3, >50% to 75%; and 4, >75%, as described previously.23 Two independent researchers scored the slides separately in a blinded manner.
Assessment of LV Function
The assessment of the LV function was performed in naïve or EAM WT and TNKO mice (14 days after immunization). The mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg). The LV apex was exposed via a subdiaphragmatic incision. An apical stab was made with a 27‐gauge needle containing a fiber pressure sensor (FPI‐LS‐PT9; FISO Technologies, Inc, Québec, Canada),27 placed to span the long axis of the LV. Pressure measurements were obtained at steady state. All signals were analyzed with a signal conditioner (FPI‐LS‐10; FISO Technologies, Inc) and data acquisition system (LabTrax 4, World Precision Instruments, Sarasota, FL) and then stored on disks for off‐line analysis using software (LabScribe, iWorx Systems, Inc, Dover, NH). The following indices were assessed: heart rate, systolic and end‐diastolic LV pressures, and maximal and minimum rates of LV pressure development (±dP/dt).
Flow Cytometric Analyses and Intracellular Cytokine Staining
Heart inflammatory cells were isolated and processed as previously described.5,28 For the flow cytometric analysis of the surface markers and cytoplasmic cytokines, the cells were stained directly using fluorochrome‐conjugated mouse‐specific antibodies and analyzed with a FACSCalibur instrument (BD Biosciences). For the analysis of the intracellular cytokine production, the cells were stimulated with 50 ng/mL phorbol 12‐myristate 13‐acetate (Sigma‐Aldrich), 750 ng/mL ionomycin (Sigma‐Aldrich), and 10 μg/mL brefeldin A (eBioscience) for 5 hours. The antibodies were purchased from eBioscience and included CD4, CD45, Forkhead box protein (Foxp)3, interferon (IFN)‐γ, and IL‐17A.
Stimulation of DCs
BMDCs (5×105) generated as described above were cultured with TN‐C (10 μg/mL) for 48 hours. In some experiments, 0.1 μmol/L TAK242 (Millipore), a selective TLR4 signal transduction inhibitor, was added. Culture supernatants were subjected to measurements of cytokine or chemokine production. For the Western blotting analysis, we used DCs obtained from spleens of naïve WT and TNKO mice by anti‐CD11c microbeads (Miltenyi Biotec). To evaluate the TLR4 expression or NF‐ĸB signaling, the DCs were stimulated with TN‐C (10 μg/mL) or tumor necrosis factor‐α (20 ng/mL) for 15 minutes and were subjected to analysis.
In Vitro Th17 Induction
We used a CD4+CD62L+ T‐cell isolation kit (Miltenyi Biotec) and anti‐CD11c microbeads (Miltenyi Biotec) to isolate CD4+ naïve T cells and DCs from spleens, respectively. The CD4+ naïve T cells (1×106) were co‐cultured with DCs (5×104) and stimulated with 10 μg/mL TN‐C for 72 hours. In some experiments, DCs were pretreated with TAK242 (0.1 μmol/L) or anti‐IL‐6 antibody (10 μg/mL; R&D Systems). The supernatants were collected and the cytokines were measured.
Measurements of Cytokines and Chemokines
The hearts were homogenized in media containing 10% fetal bovine serum. The supernatants were collected after centrifugation and stored at −80°C. The concentrations of cytokines and chemokines in the heart homogenates, serum, or culture supernatants were measured by the use of Millipore multiplex immunoassay panels (Millipore). In some cases, the cytokine levels were confirmed with a Quantikine ELISA kit (R&D Systems). For measurements of the TN‐C concentration, the hearts were homogenized in a buffer containing 150 mmol/L NaCl, 25 mmol/L Tris (pH 7.4), 5 mmol/L EDTA, 10 mmol/L sodium pyrophosphate, 10 mmol/L NaF, 1 mmol/L Na3NO4, and complete miniprotease inhibitors (Roche). The proteins were extracted from the homogenized samples by adding Triton X‐100 (Sigma) to a final concentration of 1%. Suspensions of hearts mixed with 1% Triton X were vortexed and incubated on ice for 2 hours. Then, the suspensions were centrifuged at 22 200 g for 15 minutes, and the supernatants were collected. The concentration of the TN‐C was measured by a Tenascin‐C Large (FNIII‐B) Assay kit (IBL).
Serum Troponin Determinations
Blood was collected from mice at the time of sacrifice, and the serum levels of cardiac troponin I were measured with an ELISA kit (Mouse Cardiac Tn‐I, Ultra Sensitive; Life Diagnostics).
Western Blot Analysis
The total lysates from WT and TNKO DCs were immunoblotted and probed with primary antibodies against TLR4, NF‐ĸB, and phospho‐NF‐ĸB (Ser536) (Cell Signaling Technology). Horseradish peroxidase–conjugated secondary antibodies (Cell Signaling Technology) were used to identify the binding sites of the primary antibody.
RNA Extraction and Quantitative Real‐Time Reverse Transcription Polymerase Chain Reaction
All of the hearts removed for the reverse transcription polymerase chain reaction were snap frozen and stored at −80°C. For the preparation of the total RNA, the tissue was homogenized using a bead kit (MagNA Lyser Green Beads; Roche Diagnostics) according to the manufacturer's instructions. The total RNA was extracted using a MagNA Pure Compact Instrument (Roche Applied Science) together with a MagNA Pure Compact RNA Isolation Kit (Roche Applied Science) according to the manufacturer's instructions. cDNA was synthesized from 1 μg total RNA with an Omniscript RT kit (Qiagen). A quantitative‐reverse transcription polymerase chain reaction analysis was performed with the LightCycler 480 system (Roche Applied Science) with a Universal Probe Library (Roche Applied Science). The primers for the mouse Tnc were 5′‐CCCTCTCTCTGTTGAGGTCTTG‐3′ (sense) and 5′‐CCCAGCTGACCTCAGTCAC‐3′ (antisense). The primers for the mouse Hprt were 5′‐TCCTCCTCAGACCGCTTTT‐3′ (sense) and 5′‐CCTGGTTCATCATCGCTAATC‐3′ (antisense). Hprt RNA was used as an internal control.
Statistics
All data are expressed as means±SEM. The normality was tested with the Shapiro–Wilk test. The TN‐C mRNA and protein levels after the myocarditis induction were compared with the baseline levels using an unpaired 2‐tailed t test (Figure 1B). The heart‐to‐body‐weight ratios, serum troponin I concentrations, flow cytometric analyses data, hemodynamic parameters, and cytokines/chemokine levels were compared between 2 groups by an unpaired 2‐tailed t test (Figures 2C through 2H, 3, 4, 5B through 5D, 6, and 8C and 8D). A 1‐way analysis of variance was used to compare the levels of the TN‐C in multiple groups (Figure 5A). To compare the severity scores of myocarditis between 2 groups, the Mann–Whitney U test was used (Figures 2B, 8B, and Table). The Fisher Exact test was used to compare the prevalence of DC‐induced myocarditis between the control group and the other 4 groups, respectively (Table). A value of P<0.05 was considered to be statistically significant.
Figure 1.
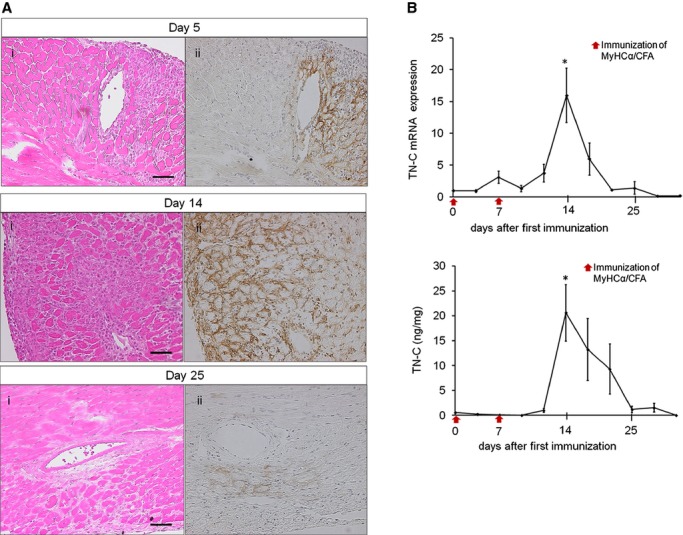
Tenascin‐C (TN‐C) expression in cardiac myosin‐induced autoimmune myocarditis. BALB/c mice were immunized twice, on days 0 and 7, with 100 μg of cardiac myosin epitope peptide (MyHC‐α). A, Representative histology of myocarditis on days 5, 14, and 25, respectively. Hematoxylin and eosin staining (i) and immunostaining for TN‐C (ii). Scale bars=50 μm. B, The expression of TN‐C in hearts obtained from immunized mice at the indicated time points. Immunization on days 0 and 7 are indicated with red arrows. TN‐C mRNA expression was evaluated by quantitative reverse transcription–polymerase chain reaction. The results are reported as the fold change in the gene expression relative to the expression on day 0. The TN‐C protein levels were measured by an ELISA. n=4 per group at each time point. Error bars represent the mean±SEM. *P<0.05 vs day 0. CFA indicates complete Freund's adjuvant.
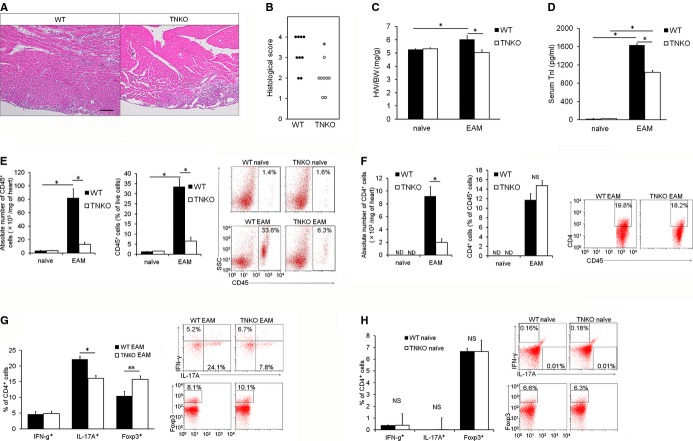
Figure 2.
Tenascin‐C (TN‐C) deficiency inhibits inflammation in the heart. Wild‐type (WT) and TN‐C knockout (TNKO) mice were immunized with cardiac myosin peptide on days 0 and 7. A, Representative hematoxylin and eosin–stained sections of hearts on day 14 from WT and TNKO mice. Scale bar=100 μm. B, Severity of myocarditis in the heart sections. C, Heart‐to‐body‐weight ratios (HW/BW) in WT and TNKO mice before and after experimental autoimmune myocarditis (EAM) induction (day 14). D, Circulating troponin I (TnI) concentration of WT and TNKO mice before and after EAM induction (day 14). E through G, Inflammatory cells infiltrating the heart were isolated and analyzed by flow cytometry of WT and TNKO mice before and after EAM induction (on day 14). E, Absolute number of CD45+ cells, frequency of CD45+ cells within live cells, and representative plots are shown. F, Absolute number of CD4+ cells, frequency of CD4+ cells within CD45+ cells, and representative plots are shown. Representative plots (gated on CD4+ T cells) and the frequency of both the interferon (IFN)‐γ+ (Th1), interleukin (IL)‐17+ (Th17), and Foxp3+ (regulatory T‐cell) cells among all CD4+ cells (G) are shown. n=5 to 8 per group (B through G). H, Splenocytes in naïve WT and TNKO mice were isolated and analyzed by a flow cytometric analysis. Representative plots (gated on CD4+ T cells) and the frequency of the IFN‐γ+ (Th1), IL‐17+ (Th17), and Foxp3+ cells among all CD4+ cells is shown. n=4 per group. The bar graphs show the group mean±SEM. The results of 1 of 2 representative experiments are shown. *P<0.01, **P<0.05. Foxp indicates Forkhead box protein; ND, not detected; SSC, side scatter.
Figure 3.
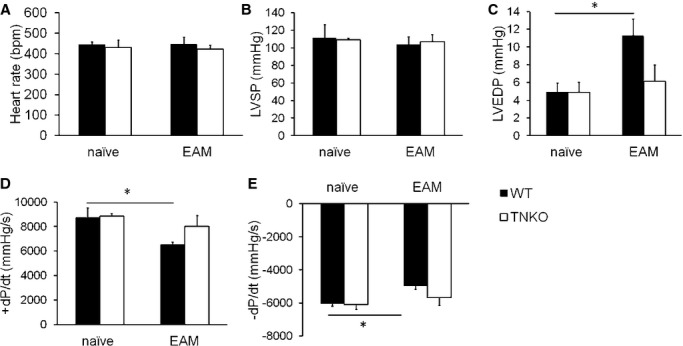
Effects of tenascin‐C (TN‐C) deficiency on the hemodynamic parameters in experimental autoimmune myocarditis (EAM) mice. A, Heart rate; (B) Left ventricular (LV) systolic pressure (LVSP); (C) LV end‐diastolic pressure (LVEDP); (D) Maximal rate of the increase in the LV pressure (+dP/dt); and (E), Maximal rate of the decrease in the LV pressure (−dP/dt). Naïve or EAM wild‐type (WT) and TN‐C knockout (TNKO) mice (day 14) were analyzed. n=5 to 7 per group. Bar graphs show the group mean±SEM. *P<0.05.
Figure 4.
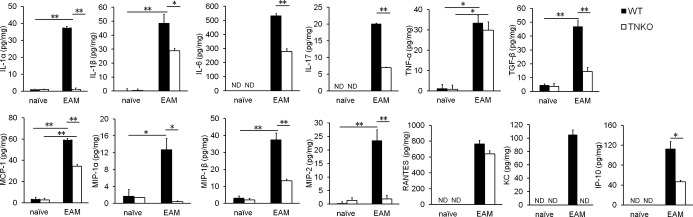
Tenascin‐C (TN‐C) deficiency affected the cytokine milieu in the heart. Cytokine and chemokine secretion in homogenized hearts obtained from naïve and experimental autoimmune myocarditis (EAM) (on day 14) wild‐type (WT) and TN‐C knockout (TNKO) mice was assessed by an ELISA. n=4 to 5 per group. The bar graphs show the group mean±SEM. The results of 1 of 2 representative experiments are shown. *P<0.05, **P<0.01. IL indicates interleukin; IP, IFN‐γ‐induced protein; KC, keratinocyte chemoattractant; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; ND, not detected; RANTES, regulated on activation, normal T‐cell expressed and secreted; TGF, transforming growth factor; TNF, tumor necrosis factor.
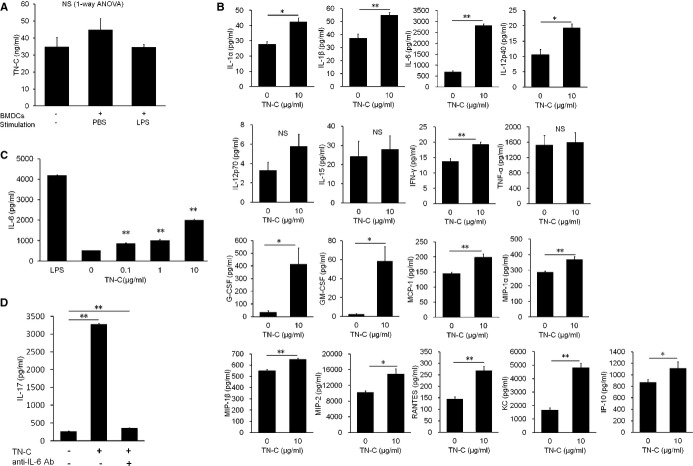
Figure 5.
Tenascin‐C (TN‐C) stimulated production of proinflammatory cytokines and chemokines by bone marrow (BM)–derived dendritic cells (DCs) and differentiated naïve CD4+ cells into Th17 cells. A, DCs generated from BM (BMDCs) were cultured in the presence or absence of lipopolysaccharide (LPS) 1 μg/mL for 72 hours. TN‐C secretions from BMDCs and the TN‐C concentration in the medium were measured by an ELISA. B, BMDCs were cultured in the presence of 10 μg/mL of TN‐C for 48 hours, and the supernatants were subjected to multiplex immunoassay panels for the production of proinflammatory cytokines, chemokines, and growth factors. C, BMDCs were cultured in the presence of the indicated dose of TN‐C for 48 hours. TN‐C‐dose‐dependent IL‐6 secretions from BMDCs were measured by an ELISA. D, CD62high naïve CD4+ T cells were cultured with DCs, which were obtained from the spleen, in the presence of anti‐CD3 mAb (1 μg/mL), TGF‐β (2 ng/mL), and TN‐C (10 μg/mL) for 72 hours. In some wells, anti‐IL‐6 Ab (10 μg/mL) was added. The secretion of IL‐17 in the supernatants was analyzed by an ELISA. The values are expressed as means±SEM of triplicate culture wells. The results of 1 of 2 representative experiments are shown. *P<0.05, **P<0.01 (compared to no TN‐C stimulation). GM‐CSF indicates granulocyte/macrophage colony‐stimulating factor; IFN, interferon; IL, interleukin; IP, IFN‐γ‐induced protein; KC, keratinocyte chemoattractant; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; NS, not significant; RANTES, regulated on activation, normal T‐cell expressed and secreted; TGF, transforming growth factor; TNF, tumor necrosis factor.
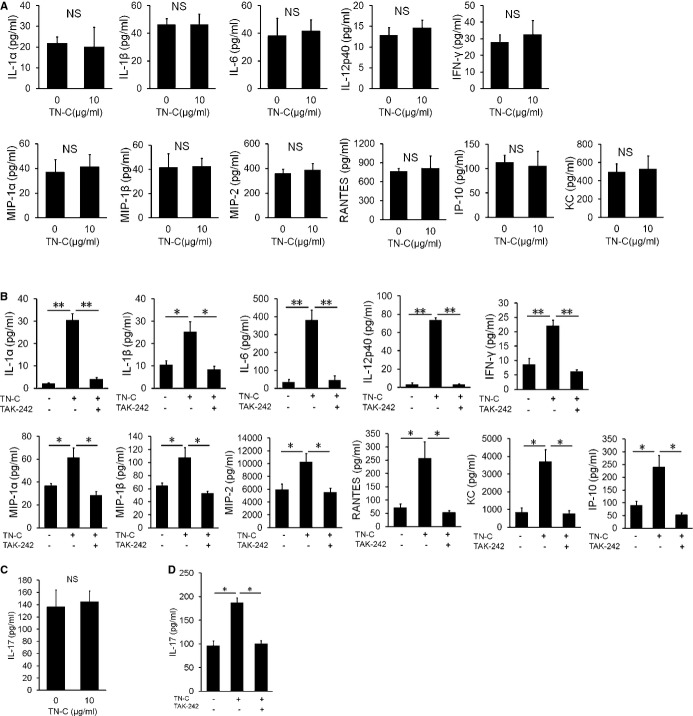
Figure 6.
Blocking of toll‐like receptor (TLR) 4‐mediated tenascin‐C (TN‐C) signaling reduced the IL‐6 secretion and Th17 generation. A, Bone marrow‐derived dendritic cells (BMDCs) generated from TLR4 knockout mice were cultured in the presence of 10 μg/mL of TN‐C for 48 hours. The supernatants were subjected to an ELISA analysis for the production of proinflammatory cytokines and chemokines. B, BMDCs generated from wild‐type mice were cultured in the presence of 10 μg/mL of TN‐C for 48 hours. In some wells, TLR4 inhibitor TAK242 (0.1 μmol/L) was added. The supernatants were subjected to an ELISA analysis for the production of proinflammatory cytokines and chemokines. C, CD62high naïve CD4+ T cells were cocultured with BMDCs generated from TLR4 knockout mice in the presence of anti‐CD3 mAb (1 μg/mL), TGF‐β (2 ng/mL), and full TN‐C (10 μg/mL) for 72 hours. The secretion of IL‐17 in the supernatants was analyzed by an ELISA. D, CD62high naïve CD4+ T cells were co‐cultured with DCs, which were obtained from the spleen, in the presence of anti‐CD3 mAb (1 μg/mL), TGF‐β (2 ng/mL), and TN‐C (10 μg/mL) for 72 hours. In some wells, TLR4 inhibitor TAK242 (0.1 μmol/L) was added. The secretion of IL‐17 in the supernatants was analyzed by an ELISA. The values are expressed as means±SEM of triplicate culture wells. The results of 1 of 2 representative experiments are shown. *P<0.05, **P<0.01. IFN indicates interferon; IL, interleukin; IP, IFN‐γ‐induced protein; KC, keratinocyte chemoattractant; MIP, macrophage inflammatory protein; NS, not significant; RANTES, regulated on activation, normal T‐cell expressed and secreted; TGF, transforming growth factor.
Table 1.
Prevalence and Severity of MyHC‐α‐Loaded DC‐Induced Myocarditis in WT and TN‐C KO Mice
| Donors | Recipients | Activation | Prevalence (Day 10) | Median Severity Grade at Day 10 (Individual Data) |
|---|---|---|---|---|
| WT | WT | PBS | 0/7 | 0 |
| WT | WT | LPS | 7/7* | 2 (2, 2, 2, 2, 3, 3, 3)† |
| WT | WT | TN‐C | 7/7* | 2 (1, 2, 2, 2, 3, 3, 3)† |
| TNKO | TNKO | PBS | 0/7 | 0 |
| TNKO | TNKO | TN‐C | 7/7* | 2 (1, 1, 2, 2, 2, 3, 3)† |
DC indicates dendritic cell; LPS, lipopolysaccharide; MyHC‐α, α‐myosin H‐chain peptide; TN‐C, tenascin‐C; TNKO, TN‐C knockout; WT, wild‐type.
*P<0.001 (Fisher's exact test), †P<0.01 (Mann‐Whitney U test) vs WT‐DCs (with PBS)‐induced myocarditis.
Results
Expression of TN‐C in the EAM Hearts
First, we examined the expression of TN‐C in WT mice with EAM that was induced by immunization with cardiac myosin. Around 5 to 6 days after the first immunization, small clusters of infiltrating inflammatory cells appeared, and TN‐C became detectable (Figure 1A). The TN‐C expression peaked at day 14, and the molecule was localized to the interstitial spaces in areas where inflammatory cell infiltration was evident (Figure 1A). The myocardial inflammation and TN‐C expression gradually subsided and disappeared around 25 days after immunization (Figure 1A). A quantitative‐reverse transcription polymerase chain reaction analysis and ELISA showed that TN‐C was expressed in parallel with the histological findings (Figure 1B).
TNKO Mice Are Protected From Progression of EAM
To determine whether TN‐C contributed to the progression of myocarditis, we compared the severity of myocarditis in WT and TNKO mice. On day 14 after immunization, when the TN‐C expression had peaked, a histopathological examination revealed larger areas occupied by heart‐infiltrating cells in the myocardium of WT mice compared with TNKO mice (Figure 2A). TNKO mice had a significantly lower myocarditis severity score than did the WT mice (Figure 2B). The heart‐to‐body‐weight ratio in the EAM‐TNKO mice had significantly decreased compared with that in the EAM‐WT mice (Figure 2C), as did the level of circulating cardiac troponin I, a clinical marker of cardiomyocyte damage,29 but these parameters were comparable between the WT and TNKO mice at baseline (Figure 2D). A flow cytometric analysis of the heart infiltrates revealed an attenuation of the inflammatory cells (CD45+ leukocytes) in TNKO mice compared with that in WT mice (Figure 2E). No difference was found in the percentage of CD4+ T cells among the CD45+ cells between both groups (Figure 2F). Intracellular staining of the CD4+ cells revealed that the proportion of IL‐17A+ cells (Th17) was higher and that of Foxp3+ cells (regulatory T cells) was lower in WT than TNKO mice, whereas the proportion of IFN‐γ+ cells (Th1) was similar between WT and TNKO mice (Figure 2G). The CD4+ T‐cell proportions in splenocytes at day 0 did not differ between WT and TNKO mice (Figure 2H), indicating that the deficiency of TN‐C did not influence CD4+ T‐cell differentiation in the non‐EAM condition. To further evaluate the effects of TN‐C deficiency on the severity of EAM, we examined hemodynamic parameters using a pressure sensor. In the naïve mice, there was no statistical difference in the heart rate, LV systolic pressure, LV end‐diastolic pressure, or ±dP/dt between the WT and TNKO mice (Figure 3A through 3E). The LV end‐diastolic pressure increased and ±dP/dt decreased more in the WT EAM mice than in the WT naïve mice, consistent with severe myocarditis (Figure 3B through 3E). In comparison, TNKO mice showed no significant deterioration in these parameters even after the induction of EAM (Figure 3B through 3E). Taken together, the presence of TN‐C in the heart during the acute phase of EAM might contribute to the infiltration of inflammatory cells including Th17 into the heart and to the progression of myocarditis and LV dysfunction.
TN‐C Promotes Proinflammatory Cytokine and Chemokine Synthesis in the Heart
To gain new insights into the mechanism of protection against myocarditis in TNKO mice, we examined whether a TN‐C deficiency affected the cytokine and chemokine milieu in the heart. In naive WT and TNKO hearts, the expression of proinflammatory cytokines and chemokines was quite small to none (Figure 4). On day 14 following immunization, the heart homogenates from TNKO mice had significantly reduced levels of the proinflammatory cytokines IL‐1α, IL‐1β, IL‐6, IL‐17, tumor necrosis factor‐α, and transforming growth factor‐β (Figure 4). Also, the lack of TN‐C had significantly reduced the levels of the following chemokines: macrophage inflammatory protein (MIP)‐1α (CCL3), MIP‐1β (CCL4), MIP‐2 (CXCL2), monocyte chemoattractant protein‐1 (MCP‐1), keratinocyte chemoattractant (KC) (CXCL1), and IFN‐γ‐induced protein (IP)‐10 (CXCL10) (Figure 4). Thus, protection from autoimmune myocarditis in TNKO mice is associated with abrogation of proinflammatory molecules and chemokines in the heart.
TN‐C Mediates DC Activation and Th17 Cell Development
EAM is a CD4+ T‐cell‐mediated disease,3–4 and DCs are the major antigen‐presenting cells (and key players in the priming of appropriate CD4+ T‐cell responses. To assess whether TN‐C is secreted by DCs, we generated BMDCs in vitro by culturing bone marrow cells obtained from WT mice with granulocyte/macrophage colony‐stimulating factor. We found that BMDCs activated with LPS produced only a small amount of TN‐C, as did nonstimulated BMDCs (Figure 5A). Therefore, we cultured WT BMDCs in the presence of TN‐C and investigated the effects of TN‐C on BMDCs in proinflammatory cytokine and chemokine production. When BMDCs were stimulated with TN‐C, they produced greater amounts of the proinflammatory cytokines IL‐1α, IL‐1β, IL‐6, IL‐12p40, and IFN‐γ than did those without TN‐C (Figure 5B). TN‐C stimulation also promoted BMDCs to produce the chemokines MCP‐1, MIP‐1α, MIP‐1β, MIP‐2, IP‐10, regulated upon activation, normal T‐cell expressed and secreted (RANTES), and KC and growth factors G‐CSF and granulocyte/macrophage colony‐stimulating factor (Figure 5B). These data indicated that TN‐C promotes the production of many types of chemokines and inflammatory cytokines from DCs, which participate in the proliferation, accumulation, and activation of immune cells such as monocytes, macrophages, and lymphocytes. Moreover, TN‐C induced the production of IL‐6, a key cytokine in Th17 development,30 by BMDCs in a dose‐dependent manner (Figure 5C). On the basis of this significant effect of TN‐C on IL‐6 secretion by DCs, we next attempted to confirm that TN‐C‐stimulated BMDCs induce Th17 development. We cocultured TN‐C‐stimulated DCs and naïve CD4+ T cells in the presence of anti‐CD3 monoclonal antibody and transforming growth factor‐β and analyzed IL‐17 production in the culture supernatant. As expected, we found that TN‐C‐stimulated DCs promoted IL‐17 production from CD4+ T cells (Figure 5D). Moreover, we confirmed the neutralization of IL‐6 by using a specific antibody that inhibited the effects of TN‐C‐stimulated DCs on the promotion of IL‐17 production from CD4+ T cells (Figure 5D). These results indicated that TN‐C encouraged the generation of Th17 via the secretion of IL‐6 from DCs.
TN‐C Activates DCs via TLR4
Multiple cell‐surface receptors are known to bind to the TN‐C molecule.31 Among them, TLR4 has been shown to be a receptor that transmits important signals to modulate inflammatory response.13 To determine whether TN‐C activates DCs via TLR4, we developed BMDCs from TLR4KO mice (TLR4KO‐BMDC) and examined whether TN‐C induced the cytokine and chemokine secretion. TLR4KO‐BMDCs failed to produce the proinflammatory cytokines IL‐1α, IL‐1β, IL‐6, IL‐12p40, and IFN‐γ even under stimulation with adequate amounts of TN‐C (Figure 6A). TN‐C did not increase the chemokine production, including that of MIP‐1α, MIP‐1β, MIP‐2, RANTES, KC, and IP‐10 from TLR4KO‐BMDCs (Figure 6A). Furthermore, TLR4 inhibitor TAK242 attenuated TN‐C‐induced production of IL‐1α, IL‐1β, IL‐6, IL‐12p40, IFN‐γ, MIP‐1α, MIP‐1β, MIP‐2, RANTES, KC, and IP‐10 (Figure 6B). These data indicated that TN‐C‐activated DCs produce proinflammatory cytokine/chemokine mainly via a TLR4‐mediated signaling cascade. Next, we cocultured naïve CD4+ T cells and TN‐C‐stimulated TLR4KO‐BMDCs. Despite the presence of a sufficient amount of TN‐C, the IL‐17 secretion from CD4+ T cells did not increase (Figure 6C). Furthermore, we cocultured naïve CD4+ T cells and TN‐C‐stimulated DCs from WT mice with or without TAK242. Pretreatment with TAK242 also resulted in an attenuation of the IL‐17 secretion from CD4+ T cells (Figure 6D). Collectively, DCs activated by the TLR4‐mediated signaling mainly contributed to the activation of DCs by TN‐C, which promoted Th17 differentiation.
TN‐C‐Stimulated DCs Gain a Myocarditis‐Inducing Capacity via TLR4
To collect direct evidence that TN‐C provides DCs with the ability to induce autoimmune myocarditis, we used another myocarditis model induced by the transfer of cardiac myosin peptide‐loaded BMDCs. In this model, DC‐mediated autoimmune myocarditis only occurs when DCs are activated through Toll‐like receptors,5 and it is a useful model to dissect the role of TN‐C in the promotion of disease‐inducing DCs. Injection of nonstimulated immature DCs did not absolutely generate EAM, whereas injection of TN‐C‐stimulated WT DCs, and LPS‐stimulated DCs induced significant myocarditis at a high prevalence (Table). In addition, nonactivated TNKO‐BMDCs could not induce EAM, but exogenous TN‐C stimulation of TNKO‐BMDCs recovered their ability to induce EAM (Table).
To determine whether TLR4 expression differed between WT and TNKO DCs, we analyzed DCs obtained from the spleen of naïve WT and TNKO mice. In the Western blot analysis, TNKO DCs showed a level of TLR4 expression comparable to that for WT DCs at baseline or in response to TN‐C stimulation (Figure 7A). MyD88 and IRAK‐1 play crucial roles as adaptor molecules in the signal transduction of TLR4, and the expression of these proteins leads to the activation of NF‐κB.32 To evaluate whether this signaling was altered in the TNKO mice, we examined the difference of NF‐κB activation in response to TN‐C or tumor necrosis factor‐α stimulation between WT and TNKO DCs. TNKO DCs showed no defect in the phosphorylation of NF‐κBp65, a major component of NF‐κB at serine 536, in response to TN‐C or tumor necrosis factor‐α stimulation in comparison to WT DCs (Figure 7B). These data confirmed that the protection of TNKO mice from myocarditis did not depend on their insufficient TLR4‐ or NF‐κB‐signaling pathways.
Figure 7.

Tenascin‐C (TN‐C) deficiency does not affect Toll‐like receptor (TLR) 4 expression and NF‐ĸB signaling. A, Western blot of TLR4 expression in naïve wild‐type (WT) and TN‐C knockout (TNKO) DCs left untreated or 15 minutes after stimulation with TN‐C (10 μg/mL). B, Western blot of phosphorylation of NF‐ĸB p65 at Ser 536 and NF‐ĸB p65 in naïve WT and TNKO DCs 15 minutes after stimulation with TN‐C (10 μg/mL) or TNF‐α (20 ng/mL). DCs indicates dendritic cells; TNF, tumor necrosis factor.
As described above, TLR4‐mediated signaling mainly contributed to the activation of DCs by TN‐C. Therefore, to directly evaluate the role of the TN‐C‐TLR4 pathway in DCs on disease induction, we stimulated MyHC‐α‐loaded‐BMDCs generated from TLR4KO mice with TN‐C and transferred them into recipient mice. As expected, TN‐C‐stimulated BMDCs from TLR4KO mice failed to induce myocarditis into the recipients (Figure 8A and 8B) with a decreased heart‐to‐body weight ratio (Figure 8C) and IL‐6 production in the heart. (Figure 8D). These results indicated that TN‐C activates DCs through TLR4 and exacerbates myocarditis.
Figure 8.
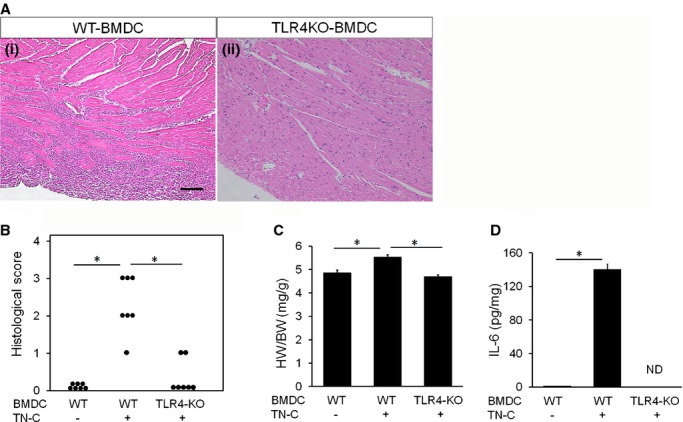
Transfer of myosin‐specific bone marrow–derived dendritic cells (BMDCs) generated from Toll‐like receptor 4 knockout (TLR4‐KO) mice reduced the myocardial inflammation. BMDCs generated from wild‐type (WT) or TLR4KO mice were pulsed overnight with 10 μg/mL MyHC‐α peptide and stimulated for another 4 hours with 10 μg/mL TN‐C and 5 μg/mL anti‐CD40L. Recipient mice received 5×105 pulsed and activated WT‐BMDCs or TLR4KO‐BMDCs i.p. on days 0, 2, and 4 and were killed 10 days after the first injection. A, Representative histology (hematoxylin and eosin staining) of the myocarditis on day 10 after the DC transfer. Left image, WT‐BMDCs transferred myocarditis, right image, TLR4KO‐BMDCs transferred myocarditis. Scale bars=100 μm. B, Severity of myocarditis on day 10 after DC transfer. C, Heart‐to‐body‐weight ratios (HW/BW) on day 10 after DC transfer. D, IL‐6 secretion in the homogenized hearts 10 days after BMDC transfer was assessed by an ELISA. The bar graphs show the group mean±SEM. n=7 per group. *P<0.01. IL indicates interleukin; MyHC, myosin H‐chain peptide; ND, not detected; TN‐C, tenascin‐C.
Discussion
In the cardiovascular system, TN‐C is expressed during embryonic development and plays important roles with regard to the differentiation of cardiomyocytes and angiogenesis.7,17 TN‐C is sparsely detected in normal adults but is upregulated under pathological conditions accompanying tissue injury and inflammation.7,17 Because of its specific expression style, TN‐C has been used to date as a biomarker33 or a target for nuclear imaging in the diagnosis20 of myocarditis. With this study, we clearly demonstrated the proinflammatory role of TN‐C in the initiation of autoimmune myocarditis. To gain new insights into the immunological influence of TN‐C on autoimmune myocarditis, we used models of EAM induced in 2 different ways. The first experiment, using MyHC‐α/complete Freund's adjuvant immunization‐induced EAM, revealed that the presence of TN‐C accelerated myocardial inflammation in vivo. The second experiment, using myosin‐specific DC‐mediated EAM, provided direct evidence that TN‐C is essential for DCs to acquire a sufficient ability to induce autoimmune myocarditis.
DCs are professional antigen‐presenting cells and key players in the priming of appropriate antigen‐specific T‐cell responses against foreign antigens or sometimes self‐tissue.6,34 We directly revealed that TN‐C aggravates inflammation through interaction with DCs in EAM, and this finding was similar to that of a previous experiment using an arthritis model.12 One of the important functions of DCs during inflammation is the production of various pro‐inflammatory cytokines.6 Actually, we found that TN‐C‐stimulated DCs promoted more production of proinflammatory cytokines than nonstimulated DCs did (Figure 5B), which was similar to previous studies that investigated the effects of TN‐C or other extracellular matrix proteins (e.g., osteopontin and galectin‐1) on DCs.12,35–39 Our in vivo study also showed that larger amounts of the pro‐inflammatory cytokines IL‐1α, IL‐1β, and IL‐6 were contained in the heart homogenates of EAM WT mice than in EAM TNKO mice (Figure 4). As a result, TNKO mice showed less MyHC‐α/complete Freund's adjuvant–induced myocarditis and stable hemodynamic parameters compared to WT mice (Figures 2 and 3). Taken together, TN‐C contributed to the progression of EAM through the production of the cytokines IL‐1, IL‐6, and IL‐12p40, which are critically necessary in the pathogenesis of autoimmune heart disease.40–42 As with the cytokines, TN‐C also accelerated the production of chemokines including MCP‐1, MIP‐1α, MIP‐1β, MIP‐2, RANTES, KC, and IP‐10 by DCs (Figure 5B), and they were also increased more in the hearts of WT EAM mice compared to TNKO EAM mice (Figure 4). In previous experimental myocarditis studies, each of MCP‐1,43 MIP‐1α,43 MIP‐1β,44 MIP‐2,45 RANTES,44 KC,46 and IP‐1047 has been identified as a chemotactic factor effecting the infiltration of various inflammatory cells into inflamed tissue. At the site of injury and inflammation, TN‐C can provide a scaffold for immune cell adhesion and migration.10 In fact, our flow cytometric analysis of infiltrating cells into inflamed WT hearts revealed that peak TN‐C expression was associated with an increase in infiltrating leukocytes at around 14 days after the first immunization (Figures 1 and 2E through 2G).
We also revealed that TN‐C was important for myosin‐loaded DCs to acquire the ability to induce EAM. This DC‐mediated myocarditis has been established as a model of EAM progression into DCM and heart failure even after resolution of acute inflammatory infiltrates.5 Consistent with a previous report, our results showed that LPS‐stimulated DCs generated significant myocarditis, whereas nonactivated DCs did not (Table).5 We also showed that stimulation of DCs with TN‐C instead of LPS successfully induced EAM at a high prevalence similar to that with LPS stimulation (Table). DC‐mediated autoimmunity and heart disease occur only when DCs are activated through Toll‐like receptors.5 Thus, these results suggest that TN‐C has an ability comparable to that of LPS for providing DCs with the capacity to induce myocarditis via TLR4 activation. Furthermore, immature BMDCs from TNKO mice did not initiate myocarditis in recipient TNKO mice, but the presence of exogenous TN‐C provided TNKO‐BMDCs with the full ability to induce EAM (Table). This indicated that the presence of exogenous TN‐C is important for acquiring DC activation to induce myocarditis. In clinical settings, myocardial tissues obtained from DCM patients sometimes show active myocarditis without evidence of an active viral invasion into their hearts.48 Taken together, it is possible that continuous activation of DCs is inducible by extracellular environments regulated in the presence of inflammation even after the active infection and microbe elements in the inflamed heart are diminished.
We showed that activated BMDCs synthesized TN‐C at a very low level (Figure 5A), although other published data demonstrated that DCs obtained from peripheral blood or immunized draining lymph nodes can produce TN‐C.12,49 The differences in the maturation and activation between artificially generated BMDCs and in vivo–derived DCs might have affected the production of TN‐C. In the inflamed heart, interstitial fibroblasts at the site of injury are the major source of TN‐C.26,50 TN‐C molecules secreted by interstitial cells in the extracellular spaces could modulate immune cell activity in a paracrine fashion.
TN‐C influenced the generation of Th17 cells infiltrating into inflamed heart (Figure 2G). Moreover, naïve CD4+ T cells cocultured with TN‐C‐activated DCs were more differentiated into Th17 cells than were those without TN‐C stimulation (Figure 5D). Recently, a subset of IL‐17‐producing Th17 cells has been described and shown to have a crucial role in the induction of autoimmune tissue injury.1 Actually, EAM is worsened by the transplantation of Th17 cells, and treatment by IL‐17‐blocking antibody or active vaccination against IL‐17 attenuates the severity of EAM.28,51 We also found that the frequency of Foxp3+ regulatory T cells was reduced more in the myocardium of WT mice than TNKO mice (Figure 2G). Foxp3+ regulatory T cells inhibit autoimmunity and protect against tissue injury.52 The current consensus is that IL‐6 induces Th17 differentiation together with transforming growth factor‐β30 and is essential for the initiation of EAM through Th17 differentiation.53–54 Conversely, transforming growth factor‐β‐mediated conversion of naïve CD4+ cells into Foxp3+ regulatory T cells is strongly inhibited by IL‐6.30 In vitro, TN‐C‐stimulated DCs produced a high amount of IL‐6 (Figure 5B and 5C), and then a blockade of IL‐6 inhibits the TN‐C‐mediated Th17 polarization (Figure 5D). Taken together, TN‐C influences the generation of pathogenic Th17 cells through its ability to promote IL‐6 synthesis from DCs and forms an important link from innate to adaptive immunity. Moreover, IL‐1 and granulocyte/macrophage colony‐stimulating factor are known as factors promoting the generation and maintenance of Th17 cells.55–56 Our results indicating that TN‐C promoted IL‐1 and granulocyte/macrophage colony‐stimulating factor production by DCs (Figure 5B) might also explain the Th17 expansion in WT mice. Consistent with our data, there is evidence that TN‐C plays a role in promoting T‐cell activation14 and polarization.12,57
Finally, we confirmed that the interaction of TN‐C and DCs depended on TLR4‐mediated signaling. Originally, TLR4 was shown to play a critical role in the recognition of LPS and subsequent signal transduction.32 However, recent studies have indicated that TLR4 also plays a critical role in inflammatory responses to endogenous triggers.58 Moreover, it was reported that self‐antigen‐loaded DCs activated via TLR4 stimulation by endogenous ligands generated by tissue damage is sufficient for the initiation of an autoimmune response in genetically susceptible individuals.59 Thus, the TLR4 signaling pathway is an important mediator of autoimmune reactions that cause inflammation‐induced injury in the myocardium. In the present study, the lack of TLR4‐mediated signaling canceled the effects of TN‐C on DCs to produce cytokines or chemokines and IL‐17 secretion from Th17 cells (Figure 6). TLR4 signaling is composed of 2 distinct pathways. One is a MyD88‐dependent pathway that is critical to the induction of inflammatory cytokines including IL‐1β, IL‐6, and IL‐12p40.32,60 The other is a Toll/IL‐1R domain‐containing adapter inducing IFN‐β (TRIF)‐dependent pathway that regulates the enhancement of DC maturation and induction of IP‐10.32,60 TAK242, a selective TLR4 signal transduction inhibitor, interferes with the interaction between TLR4 and the adaptor molecules of both pathways.61 Therefore, TAK242 could reduce IL‐1β, IL‐6, IL‐12p40, and IP‐10 secretion from DCs at the same level of TLR4 deficiency generated by the inhibition of both the MyD88‐ and TRIF‐dependent pathways (Figure 6A and 6B). Regarding the concern that a deficiency of TLR4 and its downstream signaling in TNKO mice may have an effect on the protection from myocarditis, we found no differences in TLR4 expression and NF‐ĸB activation between WT and TNKO mice (Figure 7). Lastly, we confirmed that a TLR4 deficiency canceled the effect of TN‐C in promoting DC‐mediated myocarditis and attenuated the inflammation in vivo (Figure 8). This result provides direct evidence that blocking the TLR4‐mediated pathway is effective in inhibiting TN‐C‐mediated myocardial inflammation.
A growing body of evidence suggests that TN‐C is highly expressed in various inflammatory lesions of the heart, such as the Coxsackie virus B3–induced viral myocarditis model, another mouse model of myocarditis,62–63 or the angiotensin II–induced hypertensive inflammation/fibrosis heart model,19,64 and it may modulate the immune system during tissue remodeling. Indeed, deletion of TN‐C reduces ventricular remodeling after myocardial infarction, suggesting that TN‐C may play an important role in the pathology of remodeling by modulating inflammation.65
In conclusion, TN‐C plays crucial roles in Th17 differentiation through the induction of IL‐6 from DCs as a critical event for the initiation of EAM. Our proposed mechanism of a TN‐C‐mediated progression of myocardial inflammation is illustrated in Figure 9. Our data provide the first evidence that TN‐C activation of DCs via the common receptor, TLR4, is critical for the expansion of EAM and provides new insight into a possible therapeutic target for autoimmune myocarditis.
Figure 9.
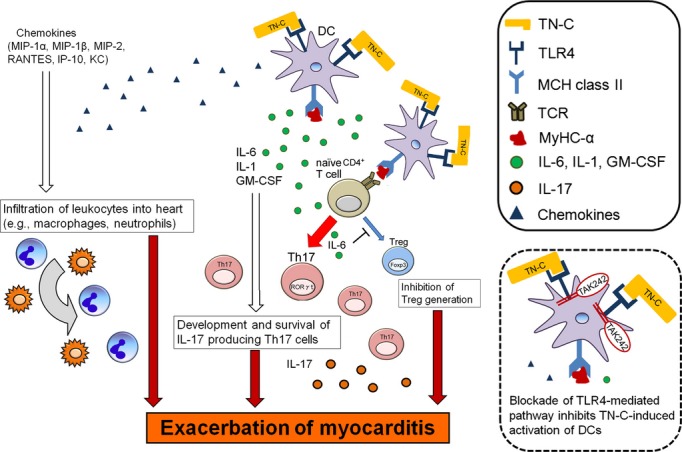
Schematic illustration showing how tenascin‐C (TN‐C)‐stimulated dendritic cells (DCs) induce myocarditis. TN‐C aggravates myocardial inflammation by stimulation of myosin‐loaded DCs via Toll‐like receptor (TLR)‐4‐mediated signaling. DCs stimulated by TN‐C produce cytokines and growth factors (IL‐6, IL‐1, and GM‐CSF) that contribute to the generation of cardiac myosin epitope peptide (MyHC)‐α‐specific Th17 cells. Chemokines secreted by TN‐C‐stimulated DCs help to accumulate inflammatory cells into the inflamed heart. GM‐CSF indicates granulocyte/macrophage colony‐stimulating factor; IL, interleukin; IP, IFN‐γ‐induced protein; KC, keratinocyte chemoattractant; MHC, major histocompatibility complex; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T‐cell expressed and secreted; TCR, T‐cell receptor.
Sources of Funding
This work was supported by Health Science Research grants from the Ministry of Health, Labor and Welfare of Japan and the Ministry of Education, Culture, Sports, Science and Technology of Japan to Tajiri (No. 25860581), and a research grant for intractable diseases from the Ministry of Health, Labor and Welfare of Japan and The Okasan‐Kato Foundation to Imanaka‐Yoshida.
Disclosures
None.
Acknowledgments
The authors thank Y. Tsujimura for critical discussions and M. Hara and M. Namikata for providing technical assistance.
References
- 1.Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008; 99:95-114. [DOI] [PubMed] [Google Scholar]
- 2.Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Bohm M. Update on myocarditis. J Am Coll Cardiol. 2012; 59:779-792. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson U, Penninger JM. Autoimmune heart failure: new understandings of pathogenesis. Int J Biochem Cell Biol. 2005; 37:27-32. [DOI] [PubMed] [Google Scholar]
- 4.Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. J Autoimmun. 2001; 16:175-186. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM. Dendritic cell‐induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med. 2003; 9:1484-1490. [DOI] [PubMed] [Google Scholar]
- 6.Joffre O, Nolte MA, Spörri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009; 227:234-247. [DOI] [PubMed] [Google Scholar]
- 7.Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin‐C biology. Cell Mol Life Sci. 2011; 68:3175-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009; 3:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Yu Y, Koehn CD, Zhang Z, Su K. Galectins in the pathogenesis of rheumatoid arthritis. J Clin Cell Immunol. 2013; 4:1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiquet‐Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. Tenascins in stem cell niches. Matrix Biol. 2014. 10.1016/j.matbio.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 11.El‐Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka‐Yoshida K. Deficiency of tenascin‐C attenuates liver fibrosis in immune‐mediated chronic hepatitis in mice. J Pathol. 2007; 211:86-94. [DOI] [PubMed] [Google Scholar]
- 12.Kanayama M, Morimoto J, Matsui Y, Ikesue M, Danzaki K, Kurotaki D, Ito K, Yoshida T, Uede T. α9β1 integrin‐mediated signaling serves as an intrinsic regulator of pathogenic Th17 cell generation. J Immunol. 2011; 187:5851-5864. [DOI] [PubMed] [Google Scholar]
- 13.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin‐C is an endogenous activator of Toll‐like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009; 15:774-780. [DOI] [PubMed] [Google Scholar]
- 14.Nakahara H, Gabazza EC, Fujimoto H, Nishii Y, D'Alessandro‐Gabazza CN, Bruno NE, Takagi T, Hayashi T, Maruyama J, Maruyama K, Imanaka‐Yoshida K, Suzuki K, Yoshida T, Adachi Y, Taguchi O. Deficiency of tenascin C attenuates allergen‐induced bronchial asthma in the mouse. Eur J Immunol. 2006; 36:3334-3345. [DOI] [PubMed] [Google Scholar]
- 15.Imanaka‐Yoshida K, Hiroe M, Yasutomi Y, Toyozaki T, Tsuchiya T, Noda N, Maki T, Nishikawa T, Sakakura T, Yoshida T. Tenascin‐C is a useful marker for disease activity in myocarditis. J Pathol. 2002; 197:388-394. [DOI] [PubMed] [Google Scholar]
- 16.Kanayama M, Kurotaki D, Morimoto J, Asano T, Matsui Y, Nakayama Y, Saito Y, Ito K, Kimura C, Iwasaki N, Suzuki K, Harada T, Li HM, Uehara J, Miyazaki T, Minami A, Kon S, Uede T. α9 integrin and its ligands constitute critical joint microenvironments for development of autoimmune arthritis. J Immunol. 2009; 182:8015-8025. [DOI] [PubMed] [Google Scholar]
- 17.Imanaka‐Yoshida K. Tenascin‐C in cardiovascular tissue remodeling: from development to inflammation and repair. Circ J. 2012; 76:2513-2520. [DOI] [PubMed] [Google Scholar]
- 18.Sato A, Aonuma K, Imanaka‐Yoshida K, Yoshida T, Isobe M, Kawase D, Kinoshita N, Yazaki Y, Hiroe M. Serum tenascin‐C might be a novel predictor of left ventricular remodeling and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2006; 47:2319-2325. [DOI] [PubMed] [Google Scholar]
- 19.Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, Hiroe M, Imanaka‐Yoshida K. Eplerenone attenuates myocardial fibrosis in the angiotensin II‐induced hypertensive mouse: involvement of tenascin‐C induced by aldosterone‐mediated inflammation. J Cardiovasc Pharmacol. 2007; 49:261-268. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Toyozaki T, Odaka K, Uehara T, Arano Y, Hasegawa H, Yoshida K, Imanaka‐Yoshida K, Yoshida T, Hiroe M, Tadokoro H, Irie T, Tanada S, Komuro I. Detection of experimental autoimmune myocarditis in rats by 111 in monoclonal antibody specific for tenascin‐C. Circulation. 2002; 106:1397-1402. [DOI] [PubMed] [Google Scholar]
- 21.Tsukada B, Terasaki F, Shimomura H, Otsuka K, Otsuka K, Katashima T, Fujita S, Imanaka‐Yoshida K, Yoshida T, Hiroe M, Kitaura Y. High prevalence of chronic myocarditis in dilated cardiomyopathy referred for left ventriculoplasty: expression of tenascin C as a possible marker for inflammation. Hum Pathol. 2009; 40:1015-1022. [DOI] [PubMed] [Google Scholar]
- 22.Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992; 6:1821-1831. [DOI] [PubMed] [Google Scholar]
- 23.Tajiri K, Imanaka‐Yoshida K, Matsubara A, Tsujimura Y, Hiroe M, Naka T, Shimojo N, Sakai S, Aonuma K, Yasutomi Y. Suppressor of cytokine signaling 1 DNA administration inhibits inflammatory and pathogenic responses in autoimmune myocarditis. J Immunol. 2012; 189:2043-2053. [DOI] [PubMed] [Google Scholar]
- 24.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999; 223:77-92. [DOI] [PubMed] [Google Scholar]
- 25.Kalembey I, Yoshida T, Iriyama K, Sakakura T. Analysis of tenascin mRNA expression in the murine mammary gland from embryogenesis to carcinogenesis: an in situ hybridization study. Int J Dev Biol. 1997; 41:569-573. [PubMed] [Google Scholar]
- 26.Imanaka‐Yoshida K, Hiroe M, Nishikawa T, Ishiyama S, Shimojo T, Ohta Y, Sakakura T, Yoshida T. Tenascin‐C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab Invest. 2001; 81:1015-1024. [DOI] [PubMed] [Google Scholar]
- 27.Pinet É. Fabry‐Pérot fiber‐optic sensors for physical parameters measurement in challenging conditions. J Sens. 2009; 2009:1-9. [Google Scholar]
- 28.Valaperti A, Marty RR, Kania G, Germano D, Mauermann N, Dirnhofer S, Leimenstoll B, Blyszczuk P, Dong C, Mueller C, Hunziker L, Eriksson U. Cd11b+ monocytes abrogate Th17 CD4+ T cell‐mediated experimental autoimmune myocarditis. J Immunol. 2008; 180:2686-2695. [DOI] [PubMed] [Google Scholar]
- 29.Twerenbold R, Jaffe A, Reichlin T, Reiter M, Mueller C. High‐sensitive troponin T measurements: what do we gain and what are the challenges? Eur Heart J. 2012; 33:579-586. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006; 441:235-238. [DOI] [PubMed] [Google Scholar]
- 31.Orend G. Potential oncogenic action of tenascin‐C in tumorigenesis. Int J Biochem Cell Biol. 2005; 37:1066-1083. [DOI] [PubMed] [Google Scholar]
- 32.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88‐independent pathway and results in activation of IFN‐regulatory factor 3 and the expression of a subset of lipopolysaccharide‐inducible genes. J Immunol. 2001; 167:5887-5894. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto N, Onishi K, Sato A, Terasaki F, Tsukada B, Nozato T, Yamada T, Imanaka‐Yoshida K, Yoshida T, Ito M, Hiroe M. Incremental prognostic values of serum tenascin‐C levels with blood B‐type natriuretic peptide testing at discharge in patients with dilated cardiomyopathy and decompensated heart failure. J Card Fail. 2009; 15:898-905. [DOI] [PubMed] [Google Scholar]
- 34.Waldner H. The role of innate immune responses in autoimmune disease development. Autoimmun Rev. 2009; 8:400-404. [DOI] [PubMed] [Google Scholar]
- 35.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, Martin SF, Simon JC, Weiss JM. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1‐polarizing phenotype. Blood. 2005; 106:946-955. [DOI] [PubMed] [Google Scholar]
- 36.Schulz G, Renkl AC, Seier A, Liaw L, Weiss JM. Regulated osteopontin expression by dendritic cells decisively affects their migratory capacity. J Invest Dermatol. 2008; 128:2541-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulcher JA, Chang MH, Wang S, Almazan T, Hashimi ST, Eriksson AU, Wen X, Pang M, Baum LG, Singh RR, Lee B. Galectin‐1 co‐clusters CD43/CD45 on dendritic cells and induces cell activation and migration through Syk and protein kinase c signaling. J Biol Chem. 2009; 284:26860-26870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fulcher JA, Hashimi ST, Levroney EL, Pang M, Gurney KB, Baum LG, Lee B. Galectin‐1‐matured human monocyte‐derived dendritic cells have enhanced migration through extracellular matrix. J Immunol. 2006; 177:216-226. [DOI] [PubMed] [Google Scholar]
- 39.Levroney EL, Aguilar HC, Fulcher JA, Kohatsu L, Pace KE, Pang M, Gurney KB, Baum LG, Lee B. Novel innate immune functions for galectin‐1: galectin‐1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J Immunol. 2005; 175:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual role of the IL‐12/IFN‐gamma axis in the development of autoimmune myocarditis: induction by IL‐12 and protection by IFN‐gamma. J Immunol. 2001; 167:5464-5469. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson U, Kurrer MO, Sonderegger I, Iezzi G, Tafuri A, Hunziker L, Suzuki S, Bachmaier K, Bingisser RM, Penninger JM, Kopf M. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J Exp Med. 2003; 197:323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eriksson U, Kurrer MO, Schmitz N, Marsch SC, Fontana A, Eugster HP, Kopf M. Interleukin‐6‐deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003; 107:320-325. [DOI] [PubMed] [Google Scholar]
- 43.Göser S, Ottl R, Brodner A, Dengler TJ, Torzewski J, Egashira K, Rose NR, Katus HA, Kaya Z. Critical role for monocyte chemoattractant protein‐1 and macrophage inflammatory protein‐1alpha in induction of experimental autoimmune myocarditis and effective anti‐monocyte chemoattractant protein‐1 gene therapy. Circulation. 2005; 112:3400-3407. [DOI] [PubMed] [Google Scholar]
- 44.Leib C, Göser S, Luthje D, Öttl R, Tretter T, Lasitschka F, Zittrich S, Pfitzer G, Katus HA, Kaya Z. Role of the cholinergic antiinflammatory pathway in murine autoimmune myocarditis. Circ Res. 2011; 109:130-140. [DOI] [PubMed] [Google Scholar]
- 45.Kishimoto C, Kawamata H, Sakai S, Shinohara H, Ochiai H. Enhanced production of macrophage inflammatory protein 2 (MIP‐2) by in vitro and in vivo infections with encephalomyocarditis virus and modulation of myocarditis with an antibody against MIP‐2. J Virol. 2001; 75:1294-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritzman AM, Hughes‐Hanks JM, Blaho VA, Wax LE, Mitchell WJ, Brown CR. The chemokine receptor CXCR2 ligand KC (CXCL1) mediates neutrophil recruitment and is critical for development of experimental Lyme arthritis and carditis. Infect Immun. 2010; 78:4593-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue Y, Gui J, Ai W, Xu W, Xiong S. Direct gene transfer with IP‐10 mutant ameliorates mouse CVB3‐induced myocarditis by blunting Th1 immune responses. PLoS One. 2011; 6:e18186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmermann O, Kochs M, Zwaka TP, Kaya Z, Lepper PM, Bienek‐Ziolkowski M, Hoher M, Hombach V, Torzewski J. Myocardial biopsy based classification and treatment in patients with dilated cardiomyopathy. Int J Cardiol. 2005; 104:92-100. [DOI] [PubMed] [Google Scholar]
- 49.Goh FG, Piccinini AM, Krausgruber T, Udalova IA, Midwood KS. Transcriptional regulation of the endogenous danger signal tenascin‐C: a novel autocrine loop in inflammation. J Immunol. 2010; 184:2655-2662. [DOI] [PubMed] [Google Scholar]
- 50.Morimoto S, Imanaka‐Yoshida K, Hiramitsu S, Kato S, Ohtsuki M, Uemura A, Kato Y, Nishikawa T, Toyozaki T, Hishida H, Yoshida T, Hiroe M. Diagnostic utility of tenascin‐C for evaluation of the activity of human acute myocarditis. J Pathol. 2005; 205:460-467. [DOI] [PubMed] [Google Scholar]
- 51.Sonderegger I, Röhn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, Bachmann MF, Kopf M. Neutralization of IL‐17 by active vaccination inhibits IL‐23‐dependent autoimmune myocarditis. Eur J Immunol. 2006; 36:2849-2856. [DOI] [PubMed] [Google Scholar]
- 52.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self‐tolerance and negative control of immune responses. Annu Rev Immunol. 2004; 22:531-562. [DOI] [PubMed] [Google Scholar]
- 53.Cruz‐Adalia A, Jiménez‐Borreguero LJ, Ramírez‐Huesca M, Chico‐Calero I, Barreiro O, López‐Conesa E, Fresno M, Sánchez‐Madrid F, Martín P. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation. 2010; 122:1396-1404. [DOI] [PubMed] [Google Scholar]
- 54.Yamashita T, Iwakura T, Matsui K, Kawaguchi H, Obana M, Hayama A, Maeda M, Izumi Y, Komuro I, Ohsugi Y, Fujimoto M, Naka T, Kishimoto T, Nakayama H, Fujio Y. IL‐6‐mediated Th17 differentiation through RORγt is essential for the initiation of experimental autoimmune myocarditis. Cardiovasc Res. 2011; 91:640-648. [DOI] [PubMed] [Google Scholar]
- 55.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin‐1 signaling. Immunity. 2009; 30:576-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. GM‐CSF mediates autoimmunity by enhancing IL‐6‐dependent Th17 cell development and survival. J Exp Med. 2008; 205:2281-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruhmann M, Piccinini AM, Kong PL, Midwood KS. Endogenous activation of adaptive immunity: tenascin‐C drives interleukin‐17 synthesis in murine arthritic joint disease. Arthritis Rheum. 2012; 64:2179-2190. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013. 10.1007/s12016‐013‐8402‐y [DOI] [PubMed] [Google Scholar]
- 59.Tsan MF, Gao B. Endogenous ligands of Toll‐like receptors. J Leukoc Biol. 2004; 76:514-519. [DOI] [PubMed] [Google Scholar]
- 60.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin‐induced maturation of MyD88‐deficient dendritic cells. J Immunol. 2001; 166:5688-5694. [DOI] [PubMed] [Google Scholar]
- 61.Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK‐242 (resatorvid), a small‐molecule inhibitor of Toll‐like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011; 79:34-41. [DOI] [PubMed] [Google Scholar]
- 62.Ruppert V, Meyer T, Pankuweit S, Jonsdottir T, Maisch B. Activation of STAT1 transcription factor precedes up‐regulation of coxsackievirus‐adenovirus receptor during viral myocarditis. Cardiovasc Pathol. 2008; 17:81-92. [DOI] [PubMed] [Google Scholar]
- 63.Leipner C, Grün K, Müller A, Buchdunger E, Borsi L, Kosmehl H, Berndt A, Janik T, Uecker A, Kiehntopf M, Böhmer FD. Imatinib mesylate attenuates fibrosis in coxsackievirus B3‐induced chronic myocarditis. Cardiovasc Res. 2008; 79:118-126. [DOI] [PubMed] [Google Scholar]
- 64.Fujita S, Shimojo N, Terasaki F, Otsuka K, Hosotani N, Kohda Y, Tanaka T, Nishioka T, Yoshida T, Hiroe M, Kitaura Y, Ishizaka N, Imanaka‐Yoshida K. Atrial natriuretic peptide exerts protective action against angiotensin II‐induced cardiac remodeling by attenuating inflammation via endothelin‐1/endothelin receptor A cascade. Heart Vessels. 2013; 28:646-657. [DOI] [PubMed] [Google Scholar]
- 65.Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka‐Yoshida K. Tenascin‐C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol. 2010; 298:H1072-H1078. [DOI] [PubMed] [Google Scholar]