Abstract
Background
The Zwolle Risk Score (ZRS) identifies ST‐elevation myocardial infarction (STEMI) patients treated with primary percutaneous coronary intervention (PPCI) eligible for early discharge. We aimed to investigate whether baseline N‐terminal pro–brain natriuretic peptide (NT‐proBNP) is also able to identify these patients and could improve future risk strategies.
Methods and Results
PPCI patients included in the Ongoing Tirofiban in Myocardial Infarction Evaluation (On‐TIME) II study were candidates (N=861). We analyzed whether ZRS and baseline NT‐proBNP predicted 30‐day mortality and assessed the occurrence of major adverse cardiac events (MACEs) and major bleeding. Receiver operating characteristic curve analysis was used to assess discriminative accuracy for ZRS, NT‐pro‐BNP, and their combination. After multiple imputation, 845 patients were included. Both ZRS >3 (hazard ratio [HR]=9.42; P<0.001) and log NT‐pro‐BNP (HR=2.61; P<0.001) values were associated with 30‐day mortality. On multivariate analysis, both the ZRS (HR=1.41; 95% confidence interval [CI]=1.27 to 1.56; P<0.001) and log NT‐proBNP (HR=2.09; 95% CI=1.59 to 2.74; P<0.001) independently predicted death at 30 days. The area under the curve for 30‐day mortality for combined ZRS/NT‐proBNP was 0.94 (95% CI=0.90 to 0.99), with optimal predictive values of a ZRS ≥2 and a NT‐proBNP value of ≥200 pg/mL. Using these cut‐off values, 64% of the study population could be identified as very low risk with zero mortality at 30 days follow‐up and low occurrence of MACEs and major bleeding between 48 hours and 10 days (1.3% and 0.6%, respectively).
Conclusion
Baseline NT‐proBNP identifies a large group of low‐risk patients who may be eligible for early (48‐ to 72‐hour) discharge, whereas optimal predictive accuracy is reached by the combination of both baseline NT‐proBNP and ZRS.
Keywords: discharge, mortality, NT‐proBNP, PCI, risk stratification, ST‐elevation myocardial infarction, Zwolle Risk Score
Introduction
In low‐risk patients with ST‐elevation myocardial infarction (STEMI), treated with primary percutaneous coronary intervention (PPCI), early discharge has been proven to be safe and feasible with good cost‐effectiveness.1–6
In order to identify PPCI patients eligible for early discharge, the Zwolle Risk Score (ZRS) has been developed. This validated score, assessed by a point system, was able to identify a large group of patients at low mortality risk who could be discharged between 48 and 72 hours after PPCI.7
For several years, the biomarkers BNP and N‐terminal pro–brain natriuretic peptide (NT‐proBNP) have been frequently used, especially in the diagnosis and prognosis of heart failure (HF). Interestingly, these biomarkers also predict major adverse cardiac events (MACEs) and mortality both early and late after ST elevation and acute non‐STEMI.8–14
We studied whether baseline NT‐proBNP levels could identify PPCI patients at low risk who may be eligible for early discharge above and beyond the ZRS.
Methods
Patients
Our population consists of patients with diagnosis of STEMI admitted for PPCI who were included in the Ongoing Tirofiban in Myocardial Infarction Evaluation (On‐TIME) II trial,15 a prospective, multicenter, placebo‐controlled, randomized, clinical trial. The rationale, design, and primary results of On‐TIME II have been previously described.15–16 The trial is registered under No. ISRCTN06195297.
Briefly, enrollment was from June 2006 to November 2007. Eligible patients were 21 to 85 years of age with symptoms of acute myocardial infarction (MI) of more than 30 minutes, but less than 24 hours, and ST‐segment elevation of more than 1 mV in 2 adjacent ECG leads.
Exclusion criteria were severe renal dysfunction (glomerular filtration rate (GFR) <30 mL/min or serum creatinine >200 mmol/L) (>2.5 mg/dL), therapy‐resistant cardiogenic shock (systolic blood pressure [SBP] ≤80 mm Hg for >30 minutes), persistent severe hypertension (HTN; SBP >180 mm Hg or diastolic blood pressure >110 mm Hg), or a contraindication to anticoagulation or increased risk of bleeding. Also, patients with a left bundle branch block, pregnant and/or breastfeeding women, and patients with a life expectancy of less than 1 year were excluded. From each patient, a written informed consent for participation in both the On‐TIME II study and future data analysis was obtained. The study protocol was approved by all local ethics committees (institutional review boards) involved.
Procedures
Treatment
All patients were planned to undergo PPCI and were initially treated according to the On‐TIME II study protocol and randomly assigned to (prehospital) treatment with tirofiban (25 μg/kg bolus and 0.15 μg kg−1 min−1 maintenance infusion for 18 hours) or placebo. PPCI was performed with standard techniques if the coronary anatomy was suitable for angioplasty. Additional treatment with stents and devices was to the discretion of the treating cardiologist. All patients were treated with optimal drug therapy, including angiotensin‐converting enzyme inhibitors, β‐blockers, aspirin, and a statin. Final discharge and admission duration was to the discretion of the treating cardiologist, irrespective of NT‐proBNP values or ZRS.
Measurements
NT‐proBNP plasma levels were measured (Roche, Central Hematology Laboratory, University of Heidelberg, Heidelberg, Germany) in each patient on admission, after sheath insertion before PPCI was performed. For each patient, the ZRS was calculated. This score, assessed by a point system, has shown to be able to identify a large group of patients at very low risk who may safely be discharged early after PPCI.7 Very‐low‐risk patients are those with a score ≤3 (mortality rate of 0.1% at 2 days and 0.2% between 2 and 10 days; Figure 1).
Figure 1.
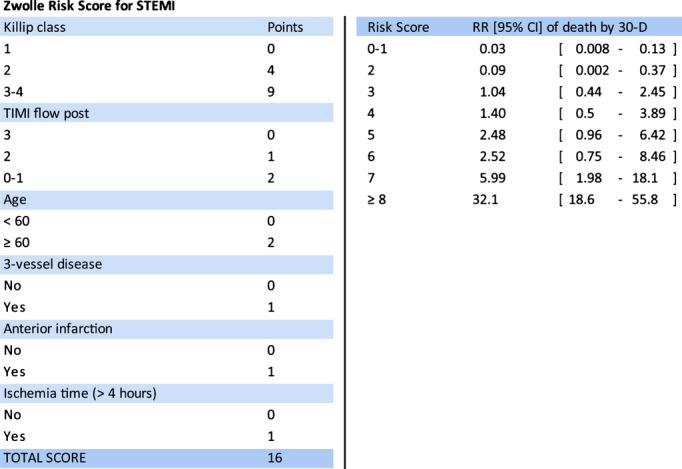
Zwolle Risk Score (A) and relative risk (RR) of 30‐day mortality for each score (B). CI indicates confidence interval; STEMI, ST‐elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Endpoints
The primary endpoint in this study was death from any cause at 30 days. Secondary endpoints were nonfatal MACEs and major bleeding until 10 days after admission as well as potentially lethal events that would interfere with the patients' eligibility for early (between 48 and 72 hours) and safe discharge. MACE was defined as recurrent myocardial infarction (RMI), urgent target vessel revascularization, and malignant cardiac arrhythmias (ventricular tachycardia [VT] or ventricular fibrillation [VF]) occurring later than 24 hours after PPCI.
RMI was defined as a new increase of creatine kinase (CK)/myocardial band that was 3 or more times the upper limit of normal, present in 2 different blood samples and accompanied by chest pain or ECG changes. VT (sustained or nonsustained) was defined as a sequence of 3 or more ventricular beats, with a frequency >100 beats per minute and was reported at the discretion of the physician.
Major bleeding was defined as either intracranial bleeding or overt bleeding with a decrease in hemoglobin ≥5 g/dL (≥3.1 mmol/L) or a decrease in hematocrit ≥15%.
Statistical Analysis
Patients were divided into percentiles of NT‐proBNP levels at the time of admission and according to their ZRS. We compared the predictive accuracy of the ZRS, NT‐proBNP, and combined NT‐proBNP/ZRS using receiver operating characteristic (ROC) curves. A total of 74 patients had missing values on ZRS and 36 on NT‐proBNP at baseline (3 patients had missing values on both). Assuming data were missing at random, a multiple missing value imputation procedure was applied in which the values of the NT‐proBNP and the values of the elements of the ZRS were imputed in case they were missing (PROC MI; SAS version 9.2; SAS Institute Inc., Cary, NC). The ZRS score was calculated from the individually imputed scores.
The following predictor variables were used for the multiple imputation: randomization to tirofiban or placebo; Killip class; gender; age; a history of diabetes; smoking; anterior infarction location; Thrombolysis in Myocardial Infarction (TIMI) post procedure; ischemic time; heart rate; cardiac troponin baseline; cardiac troponin 18 to 24 hours; cardiac troponin 72 to 96 hours; NT‐proBNP baseline; NT‐proBNP 18 to 24 hours; NT‐proBNP 72 to 96 hours; peak CK; cumulative ST deviation 30 to 180 minutes post‐PPCI; death after 30 days; vessel disease (single‐vessel disease, 2‐vessel disease, 3‐vessel disease, or left main disease); duration of hospital stay; and GFR.
We generated 10 data sets, and the results of all statistical analyses were pooled across the 10 data sets using the corresponding pooling procedure in SPSS or SAS. We calculated the SD of imputed variables in the baseline table by multiplying the estimated SE by the square root of N.
The correlation between NT‐proBNP and ZRS was calculated using Spearman's correlation coefficient. Multivariate Cox's regression analysis was used to estimate the influence of NT‐proBNP and ZRS on 30‐day mortality. We adjusted for randomization (tirofiban or placebo), gender, age, renal function (GFR, mL/min), and body mass index (BMI) in a backward Cox's regression with a P value of 0.05 for entry or exit of the variables in the model. All statistical analyses were performed using SPSS (version 19; SPSS, Inc., Chicago, IL). A 2‐sided P value of ≤0.05 was considered statistically significant. To perform an analysis using ZRS and NT‐proBNP together, we calculated weighted scores for each as follows: (β1×ZRS)+(β2×ln NT‐proBNP), where β1 and β2 denote β coefficients for the ZRS and log NT‐proBNP obtained from the multivariate Cox's regression model. Because NT‐proBNP was not normally distributed, we used logarithmically transformed values. We used ROC curves to define optimal cut‐off values in estimating mortality outcomes for ZRS, NT‐proBNP, and the combination of both. We calculated the pooled areas under the curve (AUC) and 95% confidence intervals (CIs) by averaging over the 10 AUCs from the imputed data sets using Rubin's rule.17 AUCs were compared in SAS using PROC Logistic, and the results were pooled across imputations using Rubin's rule in order to obtain Wald statistics and corresponding P values. In Rubin's rule, the overall estimate is the average of the individual estimates, and the overall SE is a function of the within‐imputation variance and the between‐imputation variance. The cut‐off scores were obtained from the mean scores across the 10 data sets, which gave the highest specificity in combination with 100% sensitivity (e.g., zero mortality).
In order to calibrate the prediction models, we used logistic regression models. The raw scores of ZRS and NT‐proBNP separately, ZRS and NT‐proBNP together, and the linear combination of ZRS and NT‐proBNP, respectively, were entered into a logistic regression model with 30‐day mortality as the dependent variable. This was done for each of the imputed data sets using the pooling procedure in SPSS. Because we have 10 imputed data sets, this gives 10 P values per analysis. If a P value is small, this is indicative of a lack of fit of the model. All statistical analyses were performed using SPSS (version 19) or SAS (version 9.2).
Results
During the study period, 984 patients were admitted with the diagnosis of STEMI. A total of 861 patients underwent PPCI. Baseline characteristics of the whole study population have been reported previously.15 Thirty‐day follow‐up was present in 845 patients, of whom 738 had a valid value for both the ZRS and baseline NT‐proBNP. Based on analysis with imputation of missing data, the ZRS was calculated from baseline characteristics at presentation and ranged from 0 to 15 points (mean±SD of 2.34±2.14 points). Of the 845 patients, 679 (80%) were in the very‐low‐ZRS group (previously defined as ZRS ≤3). The baseline NT‐proBNP values in the total cohort ranged from 9 to 33 927 pg/mL (mean±SD of 599±1883), with a median of 138 pg/mL. Median time from onset of ischemic symptoms to PPCI was 167 minutes (mean±SD of 238±252 minutes). The 25th and 75th percentile values of NT‐proBNP were 60 and 355 pg/mL, respectively. In Table 1, baseline characteristics according to the NT‐proBNP percentiles are given for patients below and above the 60th percentile of NT‐proBNP values (NT‐proBNP value=198). We chose this cut‐off point because all fatal cases had an NT‐proBNP value above the 60th percentile. In comparison to the lower percentiles, patients above the 60th percentile were older and more often had diabetes, HTN, a history of previous angina, and a history of previous MI. In addition, patients with an NT‐proBNP above the 60th percentile had a higher SBP, higher Killip class, higher hemoglobin level, higher levels of troponin, and a higher ZRS.
Table 1.
Baseline Characteristics of the Included Patients in the Imputed Data Set (n=845) According to the 60th Percentile of Baseline NT‐proBNP
| Variable | 0 to 60th Percentile (N=507) | 60th to 100th Percentile (N=338) | P Value |
|---|---|---|---|
| Age (y), mean±SD | 58.66±10.54 | 67.02±11.13 | <0.001 |
| Male gender | 427/507 (84.2%) | 215/338 (63.6%) | <0.001 |
| Current smoking | 272/506 (53.8%) | 137/336 (40.8%) | <0.001 |
| Diabetes mellitus | 37/506 (7.3%) | 53/338 (15.7%) | <0.001 |
| Hypertension | 129/507 (25.4%) | 153/338 (45.3%) | <0.001 |
| Hypercholesterolemia | 121/506 (23.9%) | 101/338 (29.9%) | 0.054 |
| Family history | 211/503 (41.9%) | 129/335 (38.5%) | 0.320 |
| Previous angina | 42/505 (8.3%) | 62/336 (18.5%) | <0.001 |
| Previous MI | 30/506 (5.9%) | 39/337 (11.6%) | 0.003 |
| Previous PTCA | 35/507 (6.9%) | 35/338 (10.4%) | 0.075 |
| Previous CABG | 7/507 (1.4%) | 5/338 (1.5%) | >0.99 |
| Previous CVA | 7/507 (1.4%) | 8/338 (2.4%) | 0.288 |
| Systolic blood pressure, mean±SD | 128.03±22.15 | 134.70±26.52 | <0.001 |
| Diastolic blood pressure, mean±SD | 76.34±14.19 | 76.92±16.22 | 0.588 |
| Killip class >I | 15/507 (3.0%) | 26/338 (7.7%) | 0.002 |
| Hemoglobin (mmol/L), mean±SD | 9.31±4.12 | 9.76±10.13 | 0.001 |
| Troponin (μg/L), mean±SD | 0.17±0.63 | 0.68±1.77 | <0.001 |
| Zwolle Risk Score, mean±SD | 1.76±1.57 | 3.21±2.53 | <0.001 |
All values are denoted as mean±SD or absolute numbers and their percentages of the total group, where appropriate. CABG indicates coronary artery bypass grafting; CVA, cerebrovascular accident; MI, myocardial infarction; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; PTCA, percutaneous transluminal coronary angioplasty.
At 30 days of follow‐up, 21 patients had died. Mortality in patients with ZRS <6 was significantly lower, in comparison with those with ZRS ≥6 (1.1% vs. 28.6%; P<0.001). Among the patients with ZRS ≤3 (previously defined as a very‐low‐risk group), 30‐day mortality was 1.0% (0.1% at 2 days and 0.6% between 2 and 10 days). In patients with NT‐proBNP levels below the 90th percentile, mortality was significantly lower, in comparison with NT‐proBNP levels above the 90th percentile (0.7% vs. 19.0%; P<0.001). In the 21 mortality cases, median NT‐proBNP was 2171 pg/mL (ranging from 213 to 33 927 pg/mL; interquartile range, 1512 to 3620). We found a significant correlation between ZRS and NT‐proBNP (Spearman's correlation of 0.419; P<0.001). In Figure 2, 30‐day mortality by quartiles of NT‐proBNP and the ZRS is depicted. In uni‐ and multivariate analysis, both baseline NT‐proBNP and ZRS were strongly predictive for 30‐day mortality. After adjustment for renal function, BMI (kg/m2), gender, age, and randomization to tirofiban or placebo, the main results of the multivariate analysis did not differ from the univariate results.
Figure 2.
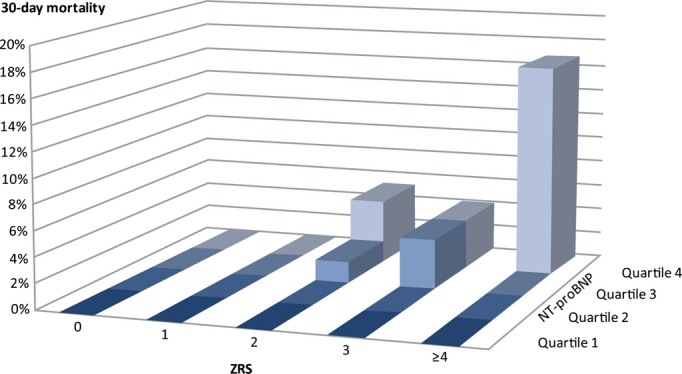
Thirty‐day mortality (%) by quartiles of NT‐proBNP and the Zwolle Risk Score in ST‐elevation myocardial infarction patients after primary percutaneous intervention. NT‐proBNP indicates N‐terminal pro–brain natriuretic peptide.
Predictive Accuracy for 30‐Day Mortality of ZRS, Baseline NT‐proBNP, and Combined Use
We compared predictive accuracy for 30‐day mortality of the ZRS, baseline NT‐proBNP, and combined use of baseline NT‐proBNP/ZRS by means of ROC curves (Figure 3). The weighted linear combination of baseline NT‐proBNP and ZRS, with the weights obtained from the multivariate Cox's regression model, demonstrated the best discriminatory accuracy in predicting 30‐day mortality, with an AUC of 0.94 (95% CI=0.90 to 0.99). Interestingly, the AUC for baseline NT‐proBNP was larger than the AUC for the ZRS (0.91 [95% CI=0.86 to 0.96] and 0.87 [95% CI=0.79 to 0.95], respectively, although the difference was not significant; P=0.34). The AUC for the weighted linear combination of ZRS/NT‐proBNP was higher, when compared with ZRS (0.94 versus 0.87 [95% CI=0.79 to 0.95]; P=0.023), and when compared with NT‐proBNP (0.94 versus 0.91 [95% CI=0.86 to 0.96]; P=0.028). The results of the calibration of the models were good: The chi‐square for the Hosmer and Lemeshow's goodness‐of‐fit test was not significant, with all P values greater than 10%.
Figure 3.
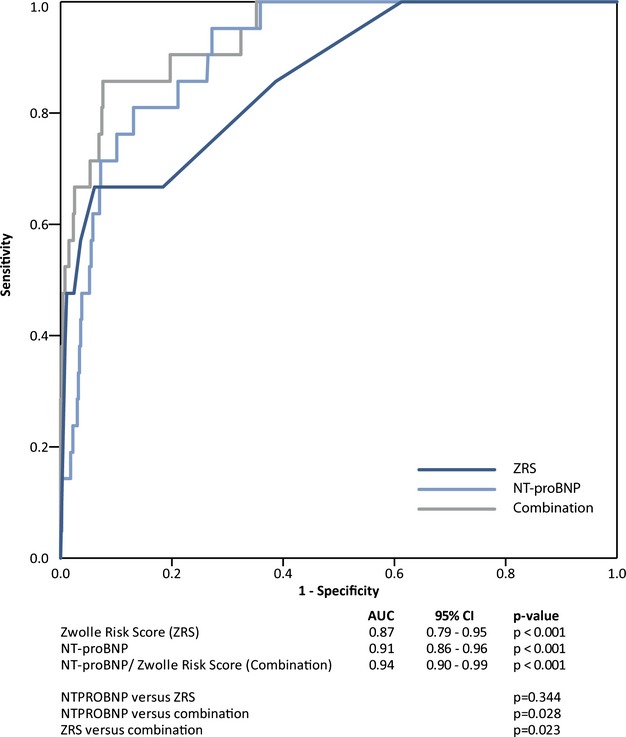
ROC curves of NT‐proBNP and the Zwolle Risk Score (ZRS) in assessing 30‐day mortality after primary percutaneous intervention in ST‐elevation myocardial infarction patients. AUC indicates area under the curve; CI, confidence interval; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; ROC, receiver operating characteristic.
When we excluded the 110 patients with imputed values, the results were comparable to the results of the imputed data set. The AUC for ZRS was 0.84 (95% CI=0.75 to 0.94), as compared to AUC=0.87 (95% CI=0.79 to 0.95) in the imputed data set. The AUC for NT‐proBNP was 0.92 (95% CI=0.87 to 0.97), as compared to AUC=0.91 (95% CI=0.86 to 0.96) in the imputed data set, and AUC for the combination of ZRS and NT‐proBNP was 0.94 (95% CI=0.89 to 0.99), as compared to AUC=0.94 (95% CI=0.94 to 0.99) in the imputed data set.
Subsequently, we determined optimal predictive values for ZRS and NT‐proBNP and the serial combination of the 2 tests, based on 100% sensitivity (and thus 100% survival), with the aim to identify a large group low‐risk patients suitable for very early discharge. In Table 2, cut‐off values with optimal predictive accuracy are given. It is shown that NT‐proBNP <200 pg/mL was able to classify 60% of the study population as very low risk, with zero mortality. In a serial test combination of both ZRS and NT‐proBNP, this was as high as 68%.
Table 2.
Cut‐off Values for ZRS, NT‐proBNP, and Combination of ZRS/NT‐proBNP
| Cut‐off Value | Sensitivity | Specificity (95% CI) | |
|---|---|---|---|
| ZRS | <2 | 1.00 | 0.39 (0.35 to 0.42) |
| NT‐proBNP | <200 pg/mL | 1.00 | 0.62 (0.58 to 0.65) |
| Combination (serial) | ZRS <2 or NT‐proBNP <200 pg/mL |
1.00 | 0.70 (0.67 to 0.73) |
P values between specificities. ZRS–NT‐proBNP: χ2=113.65, df=2, P<0.001; ZRS–combination: χ2=256, df=2, P<0.001; NT‐proBNP–combination: χ2=65, df=2, P<0.001. CI indicates confidence interval; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; ZRS, Zwolle Risk Score.
Based on this serial test combination, we created a decision rule implying that low‐risk patients, eligible for early discharge, are those with ZRS <2 or baseline NT‐proBNP <200 pg/mL.
Feasibility and Safety of Early Discharge in Low‐Risk Patients According to the Decision Rule
MACEs within 2 days after admission occurred significantly less frequently in low‐risk patients than in the higher‐risk group (32 [5.6%] of 542 vs. 29 [10.7%] of 271; P=0.007).
Major bleeding occurred in 8 versus 6 patients, respectively (1.4% vs. 2.2%; P=0.39). Of the 32 low‐risk patients experiencing MACEs within 2 days after PPCI, 13 (40.6%) had RMI and/or target vessel revascularization, and 19 (59.4%) had VT resulting in VF in 1 patient. Patients experiencing MACEs, major bleeding, or early malignant ventricular arrhythmia, totaling 37 patients, would be excluded for potential early discharge. Thus, 538 patients would have been eligible for very early discharge at 48 hours after PPCI. This accounts for 64% of the study population. Between 48 hours and 10 days after PPCI, 1.7% experienced an adverse event. MACEs occurred in 7 (1.3%) patients, and major bleeding occurred in 3 (0.6%). In 1 patient, both events occurred. Therefore, 9 (1.7%) readmissions within 10 days after PPCI would have been expected.
Of the patients with MACEs, 2 developed a malignant cardiac arrhythmia requiring cardioversion or defibrillation (1 patient at day 5 and the other patient at day 8 after PPCI). Because these events would have been potentially lethal in an outpatient setting, we looked at these patients in detail and noticed severe noncardiac comorbidity before VT/VF occurred in 1 patient. The other patient, who was in a clinical good condition, had unexpected VF without signs of RMI or HF, despite low ZRS1 and baseline NT‐proBNP (20 pg/mL) and a left ventricular ejection fraction >35%. This patient was discharged after placement of an implantable cardioverter defibrillator.
MACEs or major bleeding later than 10 days after PPCI occurred in 5 (0.9%) of the low‐risk patients, but these events probably could not have been prevented by initially longer hospitalization. In conclusion, based on the occurrence of MACEs and major bleeding, 538 patients (64% of the study population) could have been discharged 48 hours after PPCI, without mortality, and with low frequency of MACEs (1.3% of low‐risk patients eligible for very early discharge) and/or major bleeding (0.6% of low‐risk patients eligible for very early discharge; see Figure 4).
Figure 4.
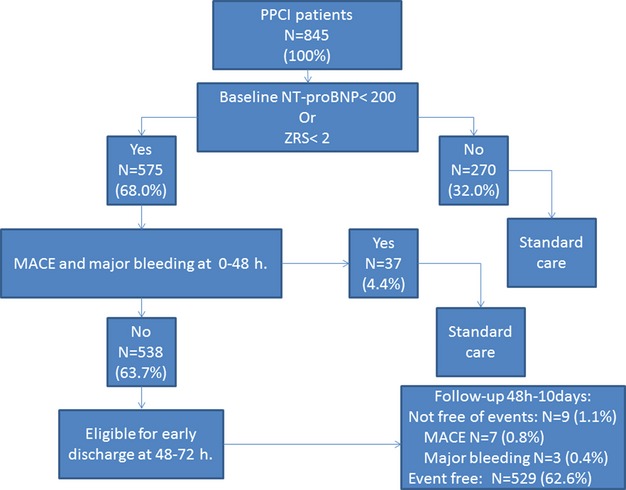
Feasibility of early discharge in 845 PPCI patients based on ZRS <2 or NT‐proBNP <200 pg/mL. MACE indicates major adverse cardiac events; NT‐proBNP, N‐terminal pro–brain natriuretic peptide; PPCI, primary percutaneous coronary intervention; ZRS, Zwolle Risk Score.
Discussion
Our study reveals that baseline NT‐proBNP values predict 30‐day mortality in patients with STEMI, treated with PPCI independently and even more strongly than ZRS alone. However, optimum predictive value was reached by a serial test combination, where NT‐proBNP (<200 pg/mL) or ZRS (<2) were able to identify a large group of patients (68% of the study population), with zero mortality at 30‐day follow‐up. Approximately 64% of the patients in our population would probably have been eligible for early discharge, with very low risk of MACEs, bleeding, or malignant ventricular arrhythmia in the first 10 days after admission.
Early Discharge After PPCI
Since the introduction of PPCI, hospitalization duration for patients with STEMI has been reduced considerably. Present guidelines now recommend that it is reasonable to discharge low‐risk PPCI patients early (after approximately 72 hours), providing that rehabilitation and follow‐up are properly arranged.18
Although several studies have shown that early discharge, particularly within 48 to 72 hours, is safe, feasible, and cost‐effective in uncomplicated PPCI patients,1–6 large, randomized trials, applying a structured risk strategy prospectively, are still scarce.
Traditionally, STEMI has been associated with severe cardiac complications, such as HF, malignant ventricular arrhythmias, mechanical complications, and, of course, reinfarction resulting from target segment‐related reocclusion or acute stent thrombosis. In addition, several noncardiac complications, such as severe (groin) bleeding, thromboembolic complications, or contrast agent‐mediated nephropathy, may occur. The possible occurrence of any of the complications mentioned above necessitates close monitoring at a coronary care unit for the first 24 to 48 hours of admission. In this setting, efforts to assess the moment and safety of discharge are often postponed until the moment that the patient can be moved to the cardiology ward.
Risk Stratification
In the current PPCI era, several risk strategies to identify STEMI patients eligible for early discharge have been proposed.1,5,7 The validated and accepted ZRS incorporates procedural factors and baseline clinical patients characteristics, without the use of time‐consuming imaging modalities. This relatively simple tool was efficient in identifying 74% of the PPCI patients at a very low mortality risk (0.2%) within 2 to 10 days. Approximately 61% of these patients would be eligible for discharge at 48 to 72 hours.7 In our study, baseline NT‐proBNP was also strongly predictive for 30‐day mortality, with a higher hazard ratio than the ZRS in multivariate analysis. Moreover, baseline NT‐proBNP had a highly predictive accuracy, with an AUC higher (although not reaching significance) than for the ZRS. Previous studies already suggested that, in STEMI patients, NT‐proBNP, drawn within 24 hours of the onset of chest pain, is more accurate in predicting mortality at 9 months than the Thrombolysis in Myocardial Infarction (TIMI) risk score.12 Even the final PCI result, in terms of TIMI flow and occurrence of the no‐reflow phenomenon, could be predicted by baseline levels of BNP or NT‐proBNP.19–20
In contrast with our previous report,7 we did not accept any mortality when defining cut‐off values, given that mortality is unacceptable when searching for shortening of hospitalization duration. Furthermore, we found a slightly higher mortality rate between 2 and 10 days (0.6% vs. 0.2%) for ZRS ≤3. We therefore set the cut‐off level at a ZRS of <2. None of the fatal cases in the study population had low baseline NT‐proBNP (all >200 pg/mL), and the mean NT‐proBNP for fatal cases was as high as 4606 pg/mL.
At present, it has been suggested that, in just 30 to 40% of low‐risk PPCI patients, discharge within 4 days is really effectuated.21–22 This may be improved by an additional simple baseline NT‐proBNP determination. Given our study results and the fact that biomarkers are more widely implemented at present, one could question whether ZRS could be replaced by simple baseline NT‐proBNP assessment. Alternatively, risk stratification could be optimized by the combination of ZRS and NT‐proBNP, and a larger group of patients could be targeted for early and safe discharge, as we have shown in our results.
Safety Issue
Since the introduction of the ZRS, PPCI techniques and accompanying periprocedural pharmacological treatment have been further improved. This has led to a decrease of ischemic time, higher rates of TIMI III flow before and after PPCI, improvement of myocardial blush grades,15,23–24 and a decrease of major bleeding.25 As a consequence, more patients will be potentially eligible for leaving the hospital safely, at an early stage.
Although the risk of mortality is an important factor in the identification of PPCI patients, when assessing for eligibility for early discharge, nonfatal MACEs, major bleeding, and malignant ventricular arrhythmias are conditions to be accounted for. This diversity of contraindications can neither be predicted by ZRS nor by baseline NT‐proBNP, but as we have shown, the frequencies of MACEs and major bleeding are very low in patients with baseline NT‐proBNP <200 pg/mL or ZRS <2. Moreover, these simple cut‐off values could facilitate early discharge of a large group of patients, at the cost of only few readmissions.
Limitations
First of all, the sample size for a study of this sort is relatively small as well as the number of deaths. These small numbers may limit the strength of the conclusions.
Moreover, several limitations in our methods have to be noted: Although, in every patient, baseline NT‐proBNP values were assessed by protocol, the ZRS was calculated afterward. All patients and outcome measures, however, were monitored longitudinally. NT‐proBNP levels were drawn at variable periods after onset of symptoms. This variability is accounted for by the ZRS, which is partially based on ischemic time. Based on the exclusion criteria, patients with higher Killip class, elderly patients, and young women were not included in the study.
We imputed missing values in order to have either a complete ZRS (74 patients) or NT‐proBNP (36 patients). The reason for this was that when we would exclude the patients with a missing value on ZRS and/or NT‐proBNP, we would miss 4 of 21 cases of death. When we excluded the 110 patients with imputed values, the results were comparable to the results of the imputed data set.
Although we succeeded in demonstrating the predictive accuracy and additional value of the assessment of baseline NT‐proBNP values, we did not effectuate hospital discharge after 48 hours. Also, we did not study the effect of real hospital length of stay on 30‐day mortality, although most patients (80%) were at low mortality risk according to the ZRS.
Prospective validation is therefore warranted in future studies, using the presently defined cut‐off values. Furthermore, the predictive value of consecutive NT‐proBNP levels, during and after PPCI, should be evaluated in order to optimize early discharge policy in successfully treated STEMI patients.
Conclusion
Baseline NT‐proBNP is highly predictive for short‐term mortality in PPCI patients and identifies a large group (64%) of very‐low‐risk patients (i.e., zero mortality at 30 days). These patients are potentially eligible for early discharge (48 hours after successful PPCI), with very low rate of nonfatal MACE and major bleeding up to 10 days after admission. Optimal predictive accuracy is reached by the combination of both ZRS <2 and baseline NT‐proBNP ≤200 pg/mL.
Disclosures
Prof Hamm has served as consultant for and received lecture fees from Iroko and Roche. Prof Giannitsis has served as consultant for and received lecture fees from Roche Diagnostics. The other authors declare they have no conflict of interest.
References
- 1.Grines CL, Marsalese DL, Brodie B, Griffin J, Donohue B, Costantini CR, Balestrini C, Stone G, Wharton T, Esente P, Spain M, Moses J, Nobuyoshi M, Ayres M, Jones D, Sachs D, Mason D, Grines LL, O'Neill W. Safety and cost‐effectiveness of early discharge after primary angioplasty in low risk patients with acute myocardial infarction. PAMI‐II investigators. Primary Angioplasty in Myocardial Infarction. J Am Coll Cardiol. 1998; 5:967-972. [DOI] [PubMed] [Google Scholar]
- 2.Branca G, Capodanno D, Capranzano P, Barbagallo R, Seminara D, Licciardello G, Tamburino C. Early discharge in acute myocardial infarction after clinical and angiographic risk assessment. J Cardiovasc Med (Hagerstown). 2008; 9:858-861. [DOI] [PubMed] [Google Scholar]
- 3.Jirmar R, Widimsky P, Capek J, Hlinomaz O, Groch L. Next day discharge after successful primary angioplasty for acute ST elevation myocardial infarction. An open randomized study “Prague‐5.”. Int Heart J. 2008; 49:653-659. [DOI] [PubMed] [Google Scholar]
- 4.Kotowycz MA, Cosman TL, Tartaglia C, Afzal R, Syal RP, Natarajan MK. Safety and feasibility of early hospital discharge in ST‐segment elevation myocardial infarction: a prospective and randomized trial in low risk primary percutaneous coronary intervention patients (The Safe‐Depart Trial). Am Heart J. 2010; 159:117.e1-117.e6. [DOI] [PubMed] [Google Scholar]
- 5.Laarman GJ, Dirksen MT. Early discharge after primary percutaneous coronary intervention. Heart. 2010; 96:584-587. [DOI] [PubMed] [Google Scholar]
- 6.Jones DA, Rathod KS, Howard JP, Gallagher S, Antoniou S, De Palma R, Guttmann O, Cliffe S, Colley J, Butler J, Ferguson E, Mohiddin S, Kapur A, Knight CJ, Jain AK, Rothman MT, Mathur A, Timmis AD, Smith EJ, Wragg A. Safety and feasibility of hospital discharge 2 days following primary percutaneous intervention for ST‐segment elevation myocardial infarction. Heart. 2012; 98:1722-1727. [DOI] [PubMed] [Google Scholar]
- 7.De Luca G, Suryapranata H, van ‘t Hof AWJ, de Boer MJ, Hoorntje JCA, Dambrink JHE, Gosselink ATM, Ottervanger JP, Zijlstra F. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004; 109:2737-2743. [DOI] [PubMed] [Google Scholar]
- 8.Heeschen C, Hamm CW, Mitrovic V, Lantelme NH, White HDPlatelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) investigators. N‐terminal pro‐B‐type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004; 110:3206-3212. [DOI] [PubMed] [Google Scholar]
- 9.Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, Sundsfjord JA, Dickstein K. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long‐term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N‐terminal proatrial natriuretic peptide. Circulation. 1996; 93:1963-1969. [DOI] [PubMed] [Google Scholar]
- 10.Tapanainen JM, Lindgren KS, Makikallio TH, Vuolteenaho O, Leppaluoto J, Huikuri HV. Natriuretic peptides as predictors of non‐sudden and sudden cardiac death after acute myocardial infarction in the beta‐blocking era. J Am Coll Cardiol. 2004; 43:757-763. [DOI] [PubMed] [Google Scholar]
- 11.Mega JL, Morrow DA, De Lemos JA, Sabatine MS, Murphy SA, Rifai N, Gibson CM, Antman EM, Braunwald E. B‐type natriuretic peptide at presentation and prognosis in patients with ST‐segment elevation myocardial infarction: an ENTIRE‐TIMI‐23 substudy. J Am Coll Cardiol. 2004; 44:335-339. [DOI] [PubMed] [Google Scholar]
- 12.Khan SQ, Quinn P, Davies JE, Ng LL. N‐terminal pro‐B‐type natriuretic peptide is better than TIMI risk score at predicting death after acute myocardial infarction. Heart. 2008; 94:40-43. [DOI] [PubMed] [Google Scholar]
- 13.Ang D, Wei L, Kao M, Lang C, Struthers A. A comparison between B‐type natriuretic peptide, global registry of acute coronary events (GRACE) score and their combination in ACS risk stratification. Heart. 2009; 95:1836-1842. [DOI] [PubMed] [Google Scholar]
- 14.Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliot J, Frampton C, Turner J, Crozier IG, Yandle TG. B‐type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation. 2003; 107:2786-2792. [DOI] [PubMed] [Google Scholar]
- 15.van ‘t Hof AWJ, ten Berg JM, Heestermans T, Dill T, Funck RC, van Werkum W, Dambrink JHE, Suryapranata H, van Houwelingen G, Ottervanger JP, Stella P, Giannitsis E, Hamm C. Prehospital initiation of tirofiban in patients with ST‐elevation myocardial infarction undergoing primary angioplasty (On‐Time 2): a multicentre, double‐blind, randomised controlled trial. Lancet. 2008; 372:537-546. [DOI] [PubMed] [Google Scholar]
- 16.van ‘t Hof AWJ, Hamm C, Rasoul S, Guptha S, Paolini JF, ten Berg JMOn‐TIME 2 investigators. Ongoing tirofiban in myocardial infarction evaluation (On‐TIME) 2 trial: rationale and study design. EuroInterv. 2007; 3:371-380. [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB. Multiple Imputation for Nonresponse in Surveys. 1987New York: John Wiley & Sons [Google Scholar]
- 18.The Task Force on the management of ST‐segment elevation myocardial infarction of the European Society of Cardiology (ESC). ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevationwww.escardio.org/guidelinesEur Heart J. 2012; 33:2569-2619. [DOI] [PubMed] [Google Scholar]
- 19.Grabowski M, Filipiak KJ, Karpinski G, Wretowski D, Rdzanek Z, Horszczaruk GJ, Kochman J, Rudowski R, Opolski G. Serum B‐type natriuretic peptide levels on admission predict not only short‐term death but also angiographic success of procedure in patients with acute ST‐elevation myocardial infarction treated with primary PCI. Am Heart J. 2004; 148:655-662. [DOI] [PubMed] [Google Scholar]
- 20.Hong SN, Ahn Y, Hwang SH, Yoon NS, Lee SR, Moon JY, Kim KH, Hong YJ, Park HW, Kim JH, Jeong MH, Cho JG, Park JC, Kang JC. Usefulness of preprocedural N‐terminal pre‐brain natriuretic peptide in predicting angiographic no‐reflow phenomenon during stent implantation in patients with ST‐segment elevation acute myocardial infarction. Am J Cardiol. 2007; 100:631-634. [DOI] [PubMed] [Google Scholar]
- 21.Kaul P, Newby LK, Fu Y, Mark DB, Califf RM, Topol EJ, Aylward P, Granger CB, Van de Werf F, Armstrong PW. International differences in evolution of early discharge. Lancet. 2004; 363:511-517. [DOI] [PubMed] [Google Scholar]
- 22.Barchielli A, Marchionni N, Carraba N, Margheri M, Santoro GM, Olivotto I, Buiatti EAMI‐Florence Working Group. Early discharge after acute myocardial infarction in the current clinical practice. Community data from the AMI‐Florence Registry, Italy. Int J Cardiol. 2007; 114:57-63. [DOI] [PubMed] [Google Scholar]
- 23.Heggunje PS, Harjai KJ, Stone GW, Mehta RH, Marsalese DL, Boura JA, O'Neill WW, Grines CL. Procedural success versus clinical risk status in determining discharge of patients after primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004; 44:1400-1407. [DOI] [PubMed] [Google Scholar]
- 24.Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun GA, Tan ES, Suurmeijer AJ, Zijlstra F. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008; 358:557-567. [DOI] [PubMed] [Google Scholar]
- 25.Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Wong SC, Nikolsky E, Gambone L, Vandertie L, Parise H, Dangas GD, Stone GW. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS‐AMI): 1‐year results of a randomised controlled trial. Lancet. 2009; 374:1149-1159. [DOI] [PubMed] [Google Scholar]


