Abstract
Background
Renal impairment is a common comorbidity and the strongest risk factor for poor prognosis in acute decompensated heart failure (ADHF). In clinical practice, renal function is labile during episodes of ADHF, and often worsens after discharge. The significance of worsening of renal function (WRF) after discharge has not been investigated as extensively as baseline renal function at admission or WRF during hospitalization.
Methods and Results
Among 611 consecutive patients with ADHF emergently admitted to our hospital, 233 patients with 3 measurements of serum creatinine (SCr) level measurements (on admission, at discharge, and 1 year after discharge) were included in the present study. Patients were divided into 2 groups according to the presence or absence of WRF at 1 year after discharge (1y‐WRF), defined as an absolute increase in SCr >0.3 mg/dL (>26.5 μmol/L) plus a ≥25% increase in SCr at 1 year after discharge compared to the SCr value at discharge. All‐cause and cardiovascular mortality were assessed as adverse outcomes. During a mean follow‐up of 35.4 months, 1y‐WRF occurred in 48 of 233 patients. There were 66 deaths from all causes. All‐cause and cardiovascular mortality were significantly higher in patients with 1y‐WRF (log‐rank P<0.0001 and P<0.0001, respectively) according to Kaplan–Meier analysis. In a multivariate Cox proportional hazards model, 1y‐WRF was a strong and independent predictor of all‐cause and cardiovascular mortality. Hemoglobin and B‐type natriuretic peptide at discharge, as well as left ventricular ejection fraction <50%, were independent predictors of 1y‐WRF.
Conclusions
In patients with ADHF, 1y‐WRF is a strong predictor of all‐cause and cardiovascular mortality.
Keywords: acute decompensated heart failure, prognosis, worsening of renal function after discharge
Introduction
In spite of great advances in the management of heart failure (HF), the prognosis of HF patients remains poor.1–2 The reasons for poor prognosis are not clear, but most HF patients have 1 or more disorders in addition to HF, such as chronic kidney disease, hypertension, chronic lung disease, and anemia, which possibly makes HF refractory to treatment. A large proportion of patients with acute decompensated HF (ADHF) have various degrees of heart and renal dysfunction concomitantly.3–4 Earlier cross‐sectional studies have demonstrated that baseline renal function, as reflected by the estimated glomerular filtration rate (eGFR), is a strong prognostic predictor in HF.5–7
However, during the management of ADHF, renal function often deteriorates.8 Reduced renal perfusion due to low cardiac output often leads to prerenal failure and the use of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers that can worsen renal function; also, hypovolemia secondary to loop diuretics usually elevate serum creatinine (SCr) level. Therefore, in addition to baseline renal function, worsening of renal function (WRF) has gained attention in recent years. Some previous studies have reported that WRF during the first hospitalization for ADHF is a strong and independent predictor of adverse outcomes.8–12 However, there were very few reports about WRF after discharge.13–14 Therefore, how WRF during long‐term follow‐up influences the prognosis of patients with ADHF remains unclear. In this context, the aim of the present study is to determine the clinical impact of WRF during the year after discharge (1y‐WRF) on prognosis in ADHF patients in the Nara Registry and Analyses for Heart Failure 2 (the NARA‐HF Study 2) cohort study.
Methods
Study Sample and Data Collection
The NARA‐HF Study 2 recruited 611 consecutive patients emergently admitted to our department or the coronary care unit at our hospital with documented ADHF (either acute new‐onset or acute‐on‐chronic HF) between January 2007 and December 2012. The diagnosis of HF was based on the Framingham Criteria.15 Patients with both reduced and preserved left ventricular ejection fraction (LVEF) were included, but patients with acute myocardial infarction, acute myocarditis, and acute HF with acute pulmonary embolism were excluded. The study protocol was approved by the ethics committee in Nara Medical University, and written informed consent was obtained from all patients according to the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects.
Of the 611 patients, 378 patients were excluded because 116 patients died within 1 year after discharge, 56 patients were treated with dialysis, 4 patients were prescribed vasopressin type 2 receptor antagonists, 186 patients did not have available SCr values at 1 year after discharge, and 16 patients were lost to follow‐up. Consequently, we included 233 patients in whom SCr levels were measured 3 times: on admission, at discharge, and at 1 year after discharge. For each patient, baseline data included age, sex, body mass index, HF etiology, medical history, vital signs, laboratory and echocardiographic data, and medications on admission and at discharge. For loop diuretics other than furosemide, we converted the dose to furosemide equivalent doses: 4 mg of torasemide and 30 mg of azosemide were considered equivalent to 20 mg of furosemide, respectively.16–17
Definitions
We measured SCr on admission, at discharge, and at 1 year after discharge. WRF was defined, according to previously published studies, as an absolute increase in SCr >0.3 mg/dL (>26.5 μmol/L) in combination with a ≥25% increase in SCr.10,12 We evaluated the occurrence of 1y‐WRF at the time point from discharge to 1 year of follow‐up. Patients were divided into the 1y‐WRF group (n=48) and the non‐WRF group (n=185) according to the presence or absence of 1y‐WRF.
Outcomes
The primary endpoints were all‐cause and cardiovascular mortality. Cardiovascular death was defined as death due to HF, acute myocardial infarction, sudden death, stroke, or vascular diseases such as aortic dissection. We reviewed medical records to determine vital status and the cause of death. When this information was unavailable, we telephoned patients or their families. Information regarding cardiovascular events such as nonfatal acute myocardial infarction, nonfatal stroke, and unexpected rehospitalization due to recurrence of ADHF was also obtained.
Statistical Analysis
Continuous variables were expressed as means ± SD, and between‐group differences were compared using Student t test. Categorical variables were summarized as percentages and analyzed using the χ2 test. Cumulative event‐free rates during follow‐up were derived using the method of Kaplan–Meier. Univariate and multivariable analyses of mortality were performed using Cox proportional hazards models. Multivariable Cox proportional hazards models performed using forced inclusion models incorporated the 8 prognostic factors that were identified during past studies in HF patients: age, sex, body mass index, hemoglobin, eGFR, B‐type natriuretic peptide (BNP), LVEF, and systolic blood pressure. We constructed 6 models adjusting for covariates: Model 1, unadjusted; Model 2, adjusted for age, sex, and body mass index; Model 3, adjusted for all factors in Model 2, plus hemoglobin, eGFR, and BNP; Model 4, adjusted for all factors in Model 3, plus LVEF and systolic blood pressure; Model 5, adjusted for the same factors as Model 4 except replacing eGFR at 1 year after discharge from eGFR at discharge; Model 6, adjusted for the same factors as Model 4 except replacing eGFR between hospital discharge and 1 year after discharge from eGFR at discharge. eGFR was calculated using the Japanese equations that take into account age, sex, and SCr.18 Multivariate logistic regression was used to identify independent predictors of 1y‐WRF.
Results were reported as hazard ratio (HR), 95% confidence interval (CI), and P values. HR for outcomes in the WRF group were compared with those in the non‐WRF group. A P value <0.05 was used as the criterion for variables to stay in the model. JMP version 10 for Windows (SAS Institute Inc, Cary, NC) was used for all statistical analyses.
Results
Baseline Characteristics
As shown in Table 1, the mean age was 72.2±11.6 (mean±SD) years, and 43.3% of the patients were women. Based on the aforementioned definition, 1y‐WRF occurred in 48 patients (20.6%). To investigate the impact of 1y‐WRF on ADHF prognosis, we divided patients into 2 groups according to the presence or absence of 1y‐WRF. Table 1 compares the baseline clinical characteristics of the 2 groups. Age, body mass index, and the sex distribution were similar in both groups. There were no significant differences in the etiology of HF or the proportion of comorbidities between the 2 groups. Moreover, New York Heart Association functional class, vital signs on admission, LVEF, and left ventricular end‐diastolic diameter were also similar. SCr on admission was equal between the 1y‐WRF group and the non‐WRF group (1.27 and 1.13 mg/dL, respectively, P=0.1163). There were also no significant differences in laboratory findings on admission. However, at discharge, the 1y‐WRF group had significantly lower hemoglobin and higher BNP compared to the non‐WRF group.
Table 1.
Baseline Characteristics of HF Patients With and Without 1y‐WRF
| Characteristic | Total (n=233) | Non‐WRF (n=185) | 1y‐WRF (n=48) | P Value |
|---|---|---|---|---|
| Demographic | ||||
| Age, y | 72.2±11.6 | 71.7±11.9 | 73.9±10.4 | 0.3178 |
| Female, % | 43.3 | 46.5 | 31.2 | 0.0577 |
| BMI, kg/m2 | 23.8±3.8 | 23.9±3.9 | 23.2±3.7 | 0.2804 |
| Cause of HF, % | ||||
| Ischemic | 44.6 | 42.2 | 54.2 | 0.1360 |
| Dilated cardiomyopathy | 19.3 | 20.5 | 14.6 | 0.3515 |
| Valvular | 16.3 | 16.2 | 16.7 | 0.9400 |
| Hypertensive | 3.9 | 4.3 | 2.1 | 0.4728 |
| Medical history, % | ||||
| Hypertension | 76.0 | 74.6 | 81.3 | 0.3363 |
| Diabetes mellitus | 45.1 | 46.0 | 41.7 | 0.5955 |
| Dyslipidemia | 45.5 | 43.8 | 52.1 | 0.3035 |
| Previous myocardial infarction | 32.2 | 29.7 | 41.7 | 0.1147 |
| Atrial fibrillation | 30.0 | 31.9 | 22.9 | 0.2268 |
| Procedures, % | ||||
| PCI | 27.9 | 26.0 | 35.4 | 0.1924 |
| CABG | 7.7 | 6.5 | 12.5 | 0.1644 |
| CRT/ICD | 3.0 | 2.2 | 6.3 | 0.1393 |
| NYHA class on admission, % | ||||
| III or IV | 88.8 | 87.0 | 95.8 | 0.0842 |
| Vital signs on admission | ||||
| Systolic blood pressure, mm Hg | 142.8±32.6 | 143.1±33.9 | 141.5±27.0 | 0.9655 |
| Diastolic blood pressure, mm Hg | 82.1±22.3 | 82.7±23.6 | 80.0±16.6 | 0.8090 |
| Heart rate, beats/min | 96.5±29.1 | 96.0±29.4 | 98.1±28.2 | 0.4998 |
| Echocardiographic parameters | ||||
| LVEF, % | 45.1±16.0 | 45.8±16.4 | 42.3±13.9 | 0.1954 |
| EF ≥50%, % | 38.2 | 41.1 | 27.1 | 0.0753 |
| LVEDD, mm | 55.4±10.3 | 55.4±10.4 | 55.5±9.8 | 0.9483 |
| Laboratory data on admission | ||||
| Hemoglobin, g/dL | 12.0±2.4 | 12.1±2.4 | 11.6±2.1 | 0.2235 |
| eGFR, mL/min per 1.73 m2 | 52.7±23.8 | 53.4±23.5 | 49.9±25.0 | 0.2653 |
| CKD stage 3A or 3B, % | 49.8 | 49.2 | 52.1 | 0.7208 |
| CKD stage 4 or 5, % | 16.3 | 15.1 | 20.8 | 0.3410 |
| Sodium, mEq/L | 139.3±3.3 | 139.4±3.2 | 138.8±3.5 | 0.3227 |
| Plasma BNP, pg/mL | 959±900 | 917±870 | 1122±998 | 0.0866 |
| Laboratory data at discharge | ||||
| Hemoglobin, g/dL | 11.8±2.1 | 11.9±2.2 | 11.2±1.7 | 0.0336 |
| eGFR, mL/min per 1.73 m2 | 49.8±24.2 | 49.7±24.2 | 50.1±24.3 | 0.7685 |
| Sodium, mEq/L | 138.6±3.6 | 138.6±3.5 | 138.5±3.7 | 0.9942 |
| Plasma BNP, pg/mL | 311±289 | 288±289 | 401±277 | 0.0023 |
Data are shown as percentages, means±SD. BMI indicates body mass index; BNP, B‐type natriuretic peptide; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; 1y‐WRF, worsening of renal function during the year after discharge.
Medications
Table 2 compares the medications on admission and at discharge of the patients in the 2 groups. The proportion of patients treated with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, β‐blockers, loop diuretics, mineralocorticoid receptor blockers, and calcium channel blockers were similar in the 2 groups, both on admission and at discharge. There were no significant differences in the furosemide equivalent dose at all time points (on admission, at discharge, and at 1 year after discharge) between the 1y‐WRF and non‐WRF groups. However, dose increases for loop diuretics between hospital discharge and 1 year afterwards were significantly larger in the 1y‐WRF group than in the non‐WRF group.
Table 2.
Medications on Admission and at Discharge, and Loop Diuretic Dose
| Medication | Total (n=233) | Non‐WRF (n=185) | 1y‐WRF (n=48) | P Value |
|---|---|---|---|---|
| Admission, % | ||||
| ACE inhibitor or ARB | 61.8 | 59.5 | 70.8 | 0.1484 |
| β‐blocker | 30.5 | 29.7 | 33.3 | 0.6289 |
| Loop diuretic | 50.2 | 49.2 | 54.2 | 0.5388 |
| MR blocker | 22.8 | 22.7 | 22.9 | 0.9749 |
| Ca channel blocker | 33.9 | 31.9 | 41.7 | 0.2024 |
| Statin | 29.2 | 27.6 | 35.4 | 0.2865 |
| Discharge, % | ||||
| ACE inhibitor or ARB | 91.9 | 91.9 | 91.7 | 0.9595 |
| β‐blocker | 57.9 | 57.8 | 58.3 | 0.9506 |
| Loop diuretic | 85.8 | 83.8 | 93.8 | 0.0776 |
| MR blocker | 38.2 | 35.1 | 50.0 | 0.0589 |
| Ca channel blocker | 27.0 | 26.0 | 31.3 | 0.4610 |
| Loop diuretic dose, mg | ||||
| On admission | 18.9±26.4 | 19.1±24.0 | 18.1±22.1 | 0.7579 |
| At discharge | 31.8±24.2 | 31.7±24.8 | 31.9±22.1 | 0.9372 |
| At 1 y after discharge | 34.9±25.8 | 33.7±26.4 | 39.8±23.2 | 0.1027 |
| Dose increased | 3.18±19.9 | 1.95±19.7 | 7.92±19.8 | 0.0464 |
Dose increased refers to an increase between discharge and 1 y afterwards. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; Ca, calcium; MR, mineralocorticoid receptor; 1y‐WRF, worsening of renal function during the year after discharge.
Prognosis and Outcome
During the mean follow‐up period of 35.4 months, 66 (28.3%) patients died; 38 (16.3%) were from cardiovascular causes. As shown in the Kaplan–Meier survival curves, the 1y‐WRF group had a much higher rate of all‐cause death (log‐rank P<0.0001) and cardiovascular death (log‐rank P<0.0001) (Figure 1). Table 3 shows the unadjusted and adjusted HRs for outcomes in the 2 groups: 1y‐WRF predicted all‐cause and cardiovascular mortality (HR, 3.136; 95% CI, 1.893–5.127; P<0.0001 and HR, 4.571; 95% CI, 2.388–8.783; P<0.0001, respectively). Even after adjusting for age, sex, and cardiovascular risk factors such as plasma BNP levels, LVEF, etc., associations between 1y‐WRF and all‐cause and cardiovascular mortality remained significant (Table 3). Moreover, in the models including the absolute value of eGFR at 1 year after discharge (Table 3, Model 5) and the ΔeGFR between hospital discharge and 1 year after discharge (Table 3, Model 6), 1y‐WRF remained a strong independent predictor of all‐cause and cardiovascular mortality.
Figure 1.
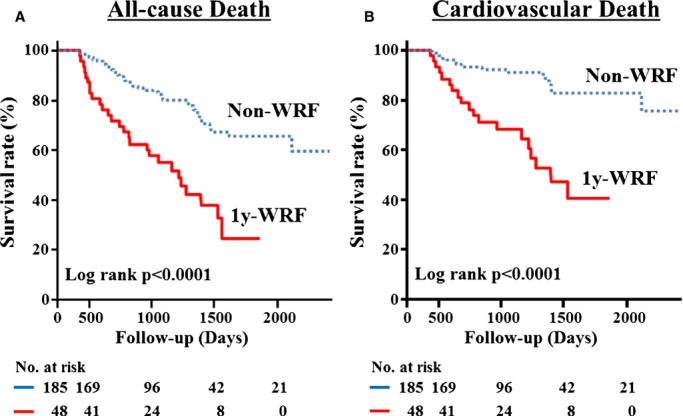
Kaplan–Meier event‐free survival curves for (A) all‐cause death and (B) cardiovascular death in patients with non‐WRF (dotted line; n=185) compared with patients with 1y‐WRF (solid line; n=48). WRF indicates worsening of renal function.
Table 3.
HR and 95% CI for All‐Cause and Cardiovascular Death According to 1y‐WRF Status
| All‐Cause Death | Cardiovascular Death | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model 1 | ||||
| 1y‐WRF | 3.136 (1.893 to 5.127) | <0.0001 | 4.571 (2.388 to 8.783) | <0.0001 |
| Model 2 | ||||
| 1y‐WRF | 2.990 (1.774 to 4.974) | <0.0001 | 4.641 (2.372 to 9.125) | <0.0001 |
| Age, y | 1.031 (1.007 to 1.058) | 0.0110 | 1.002 (0.974 to 1.033) | 0.9028 |
| Male sex | 0.877 (0.531 to 1.461) | 0.6103 | 0.903 (0.464 to 1.805) | 0.7663 |
| Model 3 | ||||
| 1y‐WRF | 2.622 (1.529 to 4.449) | 0.0006 | 4.561 (2.264 to 9.341) | <0.0001 |
| Age, y | 1.011 (0.984 to 1.041) | 0.4316 | 0.992 (0.960 to 1.028) | 0.6560 |
| Male sex | 1.209 (0.692 to 2.134) | 0.5063 | 1.215 (0.582 to 2.617) | 0.6064 |
| Hemoglobin, g/dL | 0.860 (0.731 to 1.008) | 0.0631 | 0.872 (0.711 to 1.062) | 0.1758 |
| eGFR, 10 mL/min per 1.73 m2 | 0.931 (0.813 to 1.054) | 0.2654 | 1.029 (0.876 to 1.193) | 0.7143 |
| Plasma BNP, 100 pg/mL | 1.132 (1.050 to 1.208) | 0.0020 | 1.123 (1.015 to 1.222) | 0.0259 |
| Model 4 | ||||
| 1y‐WRF | 2.423 (1.414 to 4.114) | 0.0015 | 4.500 (2.227 to 9.249) | <0.0001 |
| Age, y | 1.015 (0.987 to 1.046) | 0.3071 | 1.001 (0.967 to 1.038) | 0.9657 |
| Male sex | 1.155 (0.662 to 2.036) | 0.6123 | 1.227 (0.589 to 2.644) | 0.5881 |
| Hemoglobin, g/dL | 0.826 (0.695 to 0.976) | 0.0240 | 0.863 (0.699 to 1.055) | 0.1529 |
| eGFR, 10 mL/min per 1.73 m2 | 0.926 (0.806 to 1.053) | 0.2497 | 1.015 (0.863 to 1.178) | 0.8508 |
| Plasma BNP, 100 pg/mL | 1.126 (1.041 to 1.205) | 0.0042 | 1.107 (0.996 to 1.209) | 0.0581 |
| LVEF, % | 0.982 (0.961 to 1.003) | 0.0921 | 0.991 (0.963 to 1.017) | 0.4997 |
| SBP, mm Hg | 1.003 (0.984 to 1.021) | 0.7878 | 0.984 (0.960 to 1.008) | 0.1895 |
| Model 5 | ||||
| 1y‐WRF | 2.223 (1.217 to 4.070) | 0.0096 | 4.451 (1.989 to 10.354) | 0.0003 |
| Age, y | 1.017 (0.989 to 1.048) | 0.2459 | 1.000 (0.966 to 1.037) | 0.9991 |
| Male sex | 1.129 (0.648 to 1.986) | 0.6691 | 1.238 (0.596 to 2.659) | 0.5702 |
| Hemoglobin, g/dL | 0.825 (0.693 to 0.977) | 0.0251 | 0.865 (0.699 to 1.059) | 0.1634 |
| Plasma BNP, 100 pg/mL | 1.130 (1.045 to 1.209) | 0.0033 | 1.105 (0.995 to 1.207) | 0.0614 |
| LVEF, % | 0.983 (0.961 to 1.003) | 0.0996 | 0.991 (0.963 to 1.017) | 0.5134 |
| SBP, mm Hg | 1.003 (0.985 to 1.022) | 0.7455 | 0.984 (0.960 to 1.008) | 0.1818 |
| eGFR at 1 y, 10 mL/min per 1.73 m2 | 0.948 (0.803 to 1.104) | 0.5046 | 0.997 (0.806 to 1.205) | 0.9780 |
| Model 6 | ||||
| 1y‐WRF | 2.819 (1.470 to 5.421) | 0.0019 | 3.907 (1.713 to 9.151) | 0.0012 |
| Age, y | 1.018 (0.990 to 1.048) | 0.2053 | 1.001 (0.967 to 1.037) | 0.9757 |
| Male sex | 1.115 (0.641 to 1.960) | 0.7021 | 1.217 (0.585 to 2.618) | 0.6017 |
| Hemoglobin, g/dL | 0.815 (0.686 to 0.962) | 0.0151 | 0.867 (0.703 to 1.058) | 0.1612 |
| Plasma BNP, 100 pg/mL | 1.131 (1.046 to 1.210) | 0.0030 | 1.108 (0.997 to 1.208) | 0.0559 |
| LVEF, % | 0.981 (0.960 to 1.002) | 0.0705 | 0.991 (0.964 to 1.016) | 0.4935 |
| SBP, mm Hg | 1.003 (0.985 to 1.022) | 0.7425 | 0.985 (0.961 to 1.009) | 0.2163 |
| ΔeGFR, mL/min per 1.73 m2 | 1.009 (0.987 to 1.032) | 0.4507 | 0.992 (0.968 to 1.019) | 0.5574 |
Hemoglobin, plasma BNP and SBP values were at the time of discharge. eGFR values are at the time of discharge in Models 3 and 4 and at 1 year after discharge in Model 5. ΔeGFR is the change in eGFR between hospital discharge and 1 year after discharge in Model 6. BNP indicates B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; 1y‐WRF, worsening of renal function during the year after discharge.
Factors Affecting 1y‐WRF
Table 4 shows the multivariate analysis of factors associated with 1y‐WRF. Hemoglobin and BNP at discharge, as well as LVEF <50%, were independent risk factors for 1y‐WRF, but not age and eGFR at discharge.
Table 4.
Predictors of 1y‐WRF in the Multivariate Analysis
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| Age, y | 1.017 | 0.981 to 1.055 | 0.3605 |
| Hemoglobin, g/dL | 0.819 | 0.664 to 0.999 | 0.0491 |
| eGFR, mL/min per 1.73 m2 | 1.007 | 0.990 to 1.023 | 0.4303 |
| Plasma BNP, 100 pg/mL | 1.121 | 1.004 to 1.249 | 0.0421 |
| LVEF <50% | 2.219 | 1.025 to 5.087 | 0.0430 |
| Increase in loop diuretic dose, mg | 1.007 | 0.991 to 1.025 | 0.3947 |
Hemoglobin, plasma BNP, and eGFR values are at the time of discharge. Increase in loop diuretic dose refers to the increase in dose from the time of discharge to 1 year after discharge. BNP indicates B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; 1y‐WRF, worsening of renal function during the year after discharge.
Discussion
The present study demonstrates that 1y‐WRF is a strong and independent risk factor for all‐cause mortality and cardiovascular events in patients with ADHF. During the past decade, many studies reported a significant association between renal impairment and prognosis in HF. Many of these studies defined renal impairment as baseline SCr or WRF during hospitalization. In the present study, we evaluated longitudinal changes in renal function over the year after hospital discharge as a prognostic factor in ADHF. A large proportion of patients with ADHF have chronic kidney disease, which can exacerbate ADHF, and vice versa. This concept is currently accepted as the cardiorenal connection. More than half of the patients with ADHF in our study had eGFR <60 mL/min per 1.73 m2 at admission, and ≈20% of the patients who were alive for >1 year after discharge had WRF, defined as an absolute increase in SCr >0.3 mg/dL (>26.5 μmol/L) in combination with a ≥25% increase in SCr at 1 year after discharge. These figures are comparable to or slightly higher than those in previous studies, which were conducted in Europe and recruited patients with systolic heart failure. Many cross‐sectional studies have demonstrated that impaired renal function is an independent risk factor for poor outcomes in HF. In our study, however, the close association between 1y‐WRF and all‐cause mortality or cardiovascular events remained after adjustment for several factors including the absolute value of eGFR at 1 year after discharge. These observations provide clinically relevant information to physicians, namely, the importance of maintaining renal function when treating patients with HF. This concept is currently accepted as the cardiorenal connection, the mechanism of which may involve a complex interplay between HF and renal dysfunction through hemodynamic, pathological, and humoral dysregulation.
Although in‐hospital WRF was observed in ≈20% of the study patients, it was not significantly associated with all‐cause mortality or cardiovascular events (log‐rank P=0.7636 and log‐rank P=0.5908, respectively) (Figure 2). In prior studies, in‐hospital WRF was reported to be a risk factor for poor outcomes in HF.8–12 However, some recent reports showed it was not,14,19–20 which is consistent with our results. In our study, only 2 patients had both in‐hospital and 1y‐WRF; in other words, most of patients with in‐hospital WRF had preserved renal function at 1 year after discharge. Their transient WRF may be due to hemodynamic alterations rather than histological deterioration.
Figure 2.
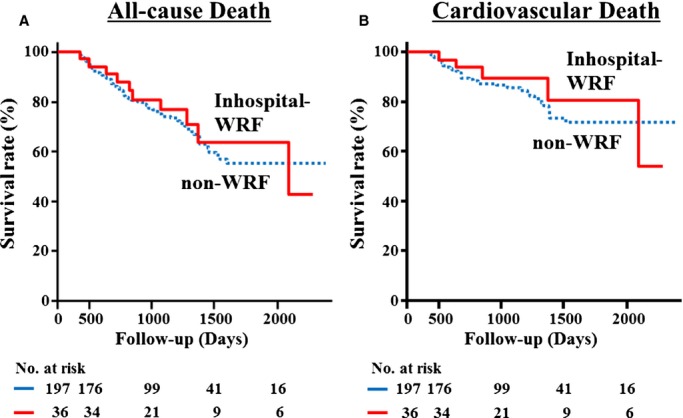
Kaplan–Meier event‐free survival curves for (A) all‐cause death and (B) cardiovascular death in patients with non‐WRF (dotted line; n=197) compared with patients with in‐hospital‐WRF (solid line; n=36). WRF indicates worsening of renal function.
The mechanisms for 1y‐WRF and in‐hospital WRF may differ, but this was not discernable from a clinical cohort study. The proportion of patients with hypertension and diabetes mellitus, as well as previous myocardial infarction, which are risk factors for WRF in outpatients, was similar in the 1y‐WRF and non‐WRF groups. There were no significant differences in LVEF or eGFR between the 2 groups. However, the plasma BNP level was significantly higher in the 1y‐WRF group than in the non‐WRF group, and multiple logistic regression showed that plasma BNP level, LVEF <50%, and anemia were significant risk factors for 1y‐WRF (Tables 1, 2, and 4). Thus, it is plausible that more advanced HF is more likely to be accompanied by WRF. Alternatively, the high levels of plasma BNP in the WRF group might be associated with continuing high venous pressure and negative effects on the kidney due to congestion.21 In our study, the furosemide equivalent dose of loop diuretics at discharge was similar in the 1y‐WRF and non‐WRF groups, but at 1 year, the 1y‐WRF group had a nonsignificantly higher dose compared to the non‐WRF group. As earlier reports reported that WRF has been attributed to hypoperfusion of the kidney due to intravascular volume depletion secondary to overdose of diuretics,12–13,12–23 patients with HF should be treated with the lowest effective dose of loop diuretics, to avoid WRF. In our institution, physicians would take BNP levels into account to prevent overuse of loop diuretics.
Since the definition of WRF is not uniform, there are many ways to assess changes in WRF. We chose a strict definition, an absolute SCr increase >0.3 mg/dL (>26.5 μmol/L) in combination with a ≥25% increase in SCr, which has been used by previous studies.10,12 Some investigators have used an absolute SCr increase >0.3 mg/dL from baseline. Therefore, we examined other definitions of 1y‐WRF, such as an absolute SCr increase >0.3 mg/dL between discharge and follow‐up at 1 year (53 patients with 1y‐WRF). Using this definition, the Kaplan–Meier survival analysis showed that the 1y‐WRF group had a much higher rate of all‐cause death (log‐rank P<0.0001) and cardiovascular death (log‐rank P<0.0001) (data not shown).
Study Limitations
There are several limitations to this study. The major limitation is that the sample size was moderate, the study was retrospective in nature, and that it was based at a single center. We did not collect data on variables that can potentially influence ADHF prognosis such as respiratory function and QRS complex widening on admission. We could not compare the influence of thiazides between the 2 groups because there are no official dose‐conversion formulas for converting between loop diuretics and thiazides.
Conclusions
WRF at 1 year after hospital discharge for ADHF is a strong predictor of all‐cause and cardiovascular death.
Disclosures
Yoshihiko Saito has the following conflicts of interest to disclose: Honoraria: MSD Co, Ltd; Mitsubishi Tanabe Pharma Corporation; Takeda Pharmaceutical Co; Daiichi Sankyo Company Ltd; Otsuka Pharmaceutical Co, Ltd; Pfizer Japan Inc. Research funding: Japan Heart Foundation, The Naito Foundation. Subsidies or donations: MSD Co, Ltd; Mitsubishi Tanabe Pharma Corporation; Daiichi Sankyo Company Ltd; Takeda Pharmaceutical Co, Ltd; Novartis Pharma K.K.; Shionogi & Co, Ltd; Astellas Pharma Inc; AstraZeneca K.K., Ltd; Otsuka Pharmaceutical Co, Ltd; St. Jude Medical Japan Co, Ltd; Kyowa Hakko Kirin Co Ltd. Endowed departments by commercial entities: MSD Co, Ltd. The other authors have no financial conflicts of interest to disclose.
Acknowledgments
The authors thank Yoko Wada for her support with data collection and data entry.
References
- 1.Bueno H, Ross JS, Wang Y, Chen J, Vidan MT, Normand SL, Curtis JP, Drye EE, Lichtman JH, Keenan PS, Kosiborod M, Krumholz HM. Trends in length of stay and short‐term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010; 303:2141-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pang PS, Komajda M, Gheorghiade M. The current and future management of acute heart failure syndromes. Eur Heart J. 2010; 31:784-793. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. The cardiorenal syndrome in heart failure. Prog Cardiovasc Dis. 2011; 54:144-153. [DOI] [PubMed] [Google Scholar]
- 4.Tang WH, Mullens W. Cardiorenal syndrome in decompensated heart failure. Heart. 2010; 96:255-260. [DOI] [PubMed] [Google Scholar]
- 5.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000; 35:681-689. [DOI] [PubMed] [Google Scholar]
- 6.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJCandesartan in Heart Failure: assessment of Reduction in M, Morbidity I. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006; 113:671-678. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski PGuidelines ESCCfP. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012; 14:803-869. [DOI] [PubMed] [Google Scholar]
- 8.Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014; 35:455-469. [DOI] [PubMed] [Google Scholar]
- 9.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004; 43:61-67. [DOI] [PubMed] [Google Scholar]
- 10.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM, Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008; 10:188-195. [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, Bradford WD, Horwitz RI. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol. 2000; 85:1110-1113. [DOI] [PubMed] [Google Scholar]
- 12.Belziti CA, Bagnati R, Ledesma P, Vulcano N, Fernandez S. Worsening renal function in patients admitted with acute decompensated heart failure: incidence, risk factors and prognostic implications. Rev Esp Cardiol. 2010; 63:294-302. [DOI] [PubMed] [Google Scholar]
- 13.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ. Both in‐ and out‐hospital worsening of renal function predict outcome in patients with heart failure: results from the coordinating study evaluating outcome of advising and counseling in heart failure (COACH). Eur J Heart Fail. 2009; 11:847-854. [DOI] [PubMed] [Google Scholar]
- 14.Blair JE, Pang PS, Schrier RW, Metra M, Traver B, Cook T, Campia U, Ambrosy A, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Konstam MA, Gheorghiade M. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J. 2011; 32:2563-2572. [DOI] [PubMed] [Google Scholar]
- 15.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993; 22:6A-13A. [DOI] [PubMed] [Google Scholar]
- 16.Kruck F, Bablok W, Besenfelder E, Betzien G, Kaufmann B. Clinical and pharmacological investigations of the new saluretic azosemid. Eur J Clin Pharmacol. 1978; 14:153-161. [DOI] [PubMed] [Google Scholar]
- 17.Diez J, Coca A, de Teresa E, Anguita M, Castro‐Beiras A, Conthe P, Cobo E, Fernandez E. TORAFIC study protocol: torasemide prolonged release versus furosemide in patients with chronic heart failure. Expert Rev Cardiovasc Ther. 2009; 7:897-904. [DOI] [PubMed] [Google Scholar]
- 18.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009; 53:982-992. [DOI] [PubMed] [Google Scholar]
- 19.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010; 122:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei Cas L. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012; 5:54-62. [DOI] [PubMed] [Google Scholar]
- 21.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009; 53:589-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossignol P, Cleland JG, Bhandari S, Tala S, Gustafsson F, Fay R, Lamiral Z, Dobre D, Pitt B, Zannad F. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone Post‐Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation. 2012; 125:271-279. [DOI] [PubMed] [Google Scholar]
- 23.Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, Cotter G, Teerlink JR, Greenberg BH, Filippatos G, Teichman SL, Metra M. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre‐RELAX‐AHF. Eur J Heart Fail. 2011; 13:961-967. [DOI] [PubMed] [Google Scholar]


