Abstract
Background
Clinical studies show that metformin attenuates all‐cause mortality and myocardial infarction compared with other medications for type 2 diabetes, even at similar glycemic levels. However, there is paucity of data in the euglycemic state on the vasculoprotective effects of metformin. The objectives of this study are to evaluate the effects of metformin on ameliorating atherosclerosis.
Methods and Results
Using ApoE−/− C57BL/6J mice, we found that metformin attenuates atherosclerosis and vascular senescence in mice fed a high‐fat diet and prevents the upregulation of angiotensin II type 1 receptor by a high‐fat diet in the aortas of mice. Thus, considering the known deleterious effects of angiotensin II mediated by angiotensin II type 1 receptor, the vascular benefits of metformin may be mediated, at least in part, by angiotensin II type 1 receptor downregulation. Moreover, we found that metformin can cause weight loss without hypoglycemia. We also found that metformin increases the antioxidant superoxide dismutase‐1.
Conclusion
Pleiotropic effects of metformin ameliorate atherosclerosis and vascular senescence.
Keywords: aging, angiotensin, atherosclerosis, metformin
Introduction
Cardiovascular disease (CVD) is a leading cause of mortality and morbidity among those with chronic diseases of aging as manifested by atherosclerosis.1 However, age‐related changes in blood vessels, such as senescence of vascular cells and the inflammatory milieu mediated by stimuli such as angiotensin II (Ang II) or a high‐fat diet (HFD), can enable the development of atherosclerosis.2–7 Hence, therapeutic strategies targeting vascular senescence and, ultimately, atherosclerosis are of special interest.
Biguanides, notably metformin, have been used extensively as the first‐line medication for treating type 2 diabetes mellitus (DM2) during the past 50 years.8–9 However, the mechanisms of action of metformin, especially those relevant to many of the observed benefits beyond its antihyperglycemic effects, are incompletely understood.9–10 The UK Prospective Diabetes Study (UKPDS) Group in 1998 reported the survival benefit and cardiovascular protection of metformin compared with other conventional treatments for DM2, including diet, sulfonylurea, and insulin.11 While the cardiovascular benefits of metformin found in the UKPDS were not seen in a study with shorter follow‐up periods, A Diabetes Outcome Progression Trial (ADOPT),12 the vascular benefits of metformin have been further confirmed in a meta‐analysis,13 as well as in a report of the Reduction of Atherothrombosis for Continued Health (REACH) Registry.14 In the REACH study, metformin was found to be advantageous even in patients with renal insufficiency or heart failure that were historically thought to be at higher risk of lactic acidosis, a side effect of biguanides.14–15 Moreover, several reports endorse metformin in contrast to phenformin, the first‐generation biguanide, as a safe medication with no evidence of lactic acidosis in nondiabetic patients, even at an advanced age.16–18 Additionally, metformin has been suggested as a treatment for obesity,19–20 a condition that is associated with increased mortality.21–22 Despite promising preclinical data as long ago as 30 years on the vasculoprotective effects of metformin even in the absence of diabetes,10,23–24 there is a paucity of information describing the cardiovascular benefits of metformin for nondiabetic patients who are at high risk of cardiovascular events. Thus, there is a need for further preclinical data to elaborate possible underlying mechanisms for such cardiovascular benefits of metformin. Here, we show, in an atherosclerotic‐prone, nondiabetic mouse model, novel evidence of the protective role of metformin in attenuating atherosclerosis and/or vascular senescence induced by an HFD.
It is widely accepted that the antihyperglycemic effect of metformin occurs mainly through a mild and transient inhibition of the mitochondrial respiratory‐chain complex‐1,25 which increases AMP:ATP ratios, leading to activation of AMP kinase. Activated AMP kinase switches cells from an anabolic to a catabolic state, resulting in inhibition of glucose, lipid, and protein synthesis while promoting the oxidation of fatty acids and glucose uptake associated with weight loss and lower triglyceride levels.9 This pathway has been confirmed in several cell types involved directly in metabolism and energy expenditure such as hepatocytes,26 skeletal muscle cells,27 and pancreatic cells.28 However, there is little known on the effects of metformin on vascular cells that are directly involved in the processes of atherosclerosis.4 While there is evidence from clinical studies that metformin improves endothelial vascular reactivity29–30 and ameliorates hypertension,31–32 there is insufficient knowledge on underlying mechanisms. Also, metformin has been shown to decrease the extent of cardiac damage and improve survival in myocardial ischemia studies on nondiabetic murine models of heart failure, again with little knowledge on the underlying mechanism(s).33–34
Ang II signaling plays a critical role in regulating many of the stimuli and signals that govern vascular senescence and, ultimately, atherogenesis.35 Ang II has been reported to accelerate senescence of vascular smooth muscle cells (VSMCs).36 Indeed, several studies have shown that Ang II, through binding to Ang II type 1 receptor (AT1R), is involved in the progression of cardiovascular diseases including atherosclerosis, hypertension, cardiac hypertrophy, and heart failure.37–38 Furthermore, disruption or inhibition of the AT1R by Ang II receptor blockers promotes longevity in rodents.39–41 Disruption of AT1R becomes even more clinically beneficial if we take into account that HFD upregulates AT1R.42 Here, we show that metformin decreases the expression of AT1R in the aortas of mice and attenuates vascular senescence and atherosclerosis induced by an HFD, suggesting that AT1R downregulation, at least in part, mediates the protective effect of metformin in the vascular system. These studies will further elucidate whether metformin can be used as a preventive therapy for patients at risk for or who have cardiovascular complications even in the absence of diabetes and suggest that metformin could have a broader effect in other age‐related diseases.
Methods
Animal Model and Experiments
All animal studies were approved by the Emory University Institutional Animal Care and Use Committee in accordance with the guidelines set forth by the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” In this study homozygous ApoE‐deficient (C57BL/6 background) male mice were purchased from the Jackson Laboratory. Mice aged 2, 3, and 6 months were used. Metformin hydrochloride (Sigma‐Aldrich) was delivered intraperitoneally (IP) or via gastric gavage (orally) at 100 mg/kg per day, the dosage calculated based on body surface area equivalent to therapeutic human dosing (2 g/day) as used previously,43 for 2 or 4 weeks to mice fed standard chow diet (rodent diet No. 5001; LabDiet) either with Ang II or with Modified Paigen's Atherogenic Purified Rodent Diet (Research Diets, Inc). The Modified Paigen's atherogenic diet was made of purified components and was designed to match the original Paigen's Atherogenic Rodent Diet.44 The components per kilogram as listed by the manufacturer are as follows: 75 g casein, 130 g soy protein, 2 g dl‐methionine, 275 g corn starch, 150 g maltodextrin 10, 30 g sucrose, 90 g cellulose, 50 g soy bean oil, 75 g cocoa butter, 35 g coconut oil, 35 g salt mix S10001, 5.5 g calcium carbonate, 8 g sodium chloride, 10 g potassium citrate, 10 g vitamin mix V10001, 2 g choline bitartrate, 12.5 g cholesterol USP, and 5 g sodium cholic acid. Control group animals were fed Low Fat Control for Modified Paigen's Atherogenic Rodent Diet or chow diet and received 100 μL of normal saline daily IP or orally. In the Ang II study, mice received Ang II infusions via a subcutaneously implanted osmotic minipump (model 1002; Alzet) for 10 days. The mice were anesthetized with ketamine‐xylazine (maximal dose: ketamine 100 mg/kg and xylazine 10 mg/kg; Sigma Chemical Co). An osmotic pump containing Ang II dissolved in a solution of 0.15 mol/L NaCl and 0.01 N acetic acid at a concentration calculated to deliver 0.7 mg/kg per day of drug was inserted into a subcutaneous pocket. The dose of Ang II was selected to provide a plasma concentration of Ang II similar to that reported in patients with renovascular hypertension.45 As placebo, the controls in the Ang II study set received osmotic minipumps containing normal saline alone. The animals were euthanized by CO2 inhalation at the set time points. The heart and aorta were pressure‐perfused with saline solution.
Blood samples were collected via right ventricular puncture to measure blood glucose levels using a standard glucometer and lipid concentrations. Mouse aortas were surgically removed, washed in cold PBS, and homogenized on ice in lysis buffer to prepare tissue lysates.
Systolic Blood Pressure Measurement
Systolic blood pressure (SBP) was measured by using a computerized, noninvasive, tail‐cuff system (BP 2000; Visitech Systems). One set of 10 measurements was obtained for each animal, and the mean SBP was calculated. Animals were habituated to the device 1 week before measurement of the pressures to ensure accurate measurements.
Atherosclerotic Plaque Quantification
Excised aortas were fixed in 0.2% glutaraldehyde (Sigma‐Aldrich) overnight and then opened and pinned to black wax. Images of aortas were taken, and the amounts of atherosclerotic plaque areas were quantified using image‐processing software (Image J) in a semiautomated technique.
Senescence‐Associated β‐Galactosidase Assay
Senescence‐associated β‐galactosidase assay was performed with a kit obtained from Marker Gene Technologies, Inc that uses the high‐sensitivity substrate fluorescein di‐β‐d‐galactopyranoside to quantify senescence‐associated β‐galactosidase activity. In brief, aortas fixed in 0.2% glutaraldehyde overnight were incubated for 16 hours at 37°C in 1 mL of freshly prepared fluorescein di‐β‐d‐galactopyranoside solutions (pH 6.0). The fluorescence at excitation/emission, 485 nm/535 nm, from each sample was read after transferring 100 μL of the supernatant from each well to a 96‐well plate for fluorescence measurements in triplicate. The average reading for each aorta sample was adjusted for the weight of the sample. A similar procedure was performed using 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐galactopyranoside after fixation of the aortas in 0.2% glutaraldehyde overnight to qualitatively evaluate the senescence in the harvested aortas.
Lipid Profile Measurements
Plasma lipids were analyzed at the Cardiovascular Specialty Laboratories (Atlanta, GA). Total cholesterol in plasma samples was measured by using an enzymatic method with a chemistry autoanalyzer (AU 480; Beckman). Triglyceride levels were measured using Beckman reagents and direct measurements of high‐density lipoprotein cholesterol were performed by using Sekisui diagnostics reagents. Low‐density lipoprotein was calculated based on the Friedewald formula.46
Western Blotting
Frozen mice aorta samples were lysed in lysis buffer (50 mmol/L HEPES, pH 7.4, 50 mmol/L NaCl, 1% Triton X‐100, 5 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 10 mmol/L sodium pyrophosphate, 50 mmol/L sodium fluoride, and 1 mmol/L sodium orthovanadate) plus protease inhibitors cocktail (Sigma) and 50 μg of homogenates separated on 4% to 20% SDS‐PAGE Criterion precast gels (Bio‐Rad). Protein expression was determined by enhanced chemiluminescence with specific antibodies. Quantification of band intensities by densitometry was carried out by using Labworks software.
RT‐PCR and Quantitative Real‐Time PCR
Total RNA from aortic tissue was extracted by using TRI Reagent LS from Sigma according to the manufacturer's instructions. The concentration and purity of RNA were determined with a spectrophotometer at 260 and 280 nm. RT was performed by using the Enhanced Avian RT First Stand Synthesis kit from Sigma. Two micrograms of total RNA were used as a template for subsequent RT. The cDNA samples were amplified in the LightCycler (Roche Applied Science) real‐time thermocycler with the use of SYBR Green JumpStart Taq ReadyMix (Sigma) with the following PCR primers: sense 5′‐GTGTTCCTGCTCACGTGTCT‐3′ and antisense 5′‐TAATGAAAAGCGCAAACAGT‐3′ for mouse AT1R (NM_177322); sense 5′‐ACAACTTTGGCATTGTGGAA‐3′ and antisense 5′‐ GATGCAGGGATGATGTTCTG‐3′ for mouse GAPDH (NM_008084). The results of relative expression were normalized to GAPDH mRNA levels in each sample.
Statistics
Data are presented as mean±SEM. Statistical significance between experimental groups was calculated by using the nonparametric Wilcoxon rank sum test between 2 groups with use of the statistical software SPSS (IBM). Significance was accepted at P<0.05.
Results
Metformin Causes Weight Loss and Reduces Blood Glucose of ApoE−/− C57BL/6J Mice
To evaluate whether metformin can attenuate vascular senescence, we used Ang II, a strong stimulator of vascular aging and disease,35 in an ApoE−/− C57BL/6J mice model in which Ang II induces accelerated senescence of vascular cells.35 Animals were treated daily with IP injections of metformin (100 mg/kg) or saline for 7 days and then infused with or without Ang II for an additional 10 days through the use of osmotic minipumps. We found that vascular senescence, measured as increased levels of senescence‐associated β‐galactosidase activity, and hypertension were induced by Ang II infusion. However, these effects of Ang II were not completely attenuated by metformin (Figure 1A and 1B). The effect of metformin was associated with a significant weight loss in both control and Ang II–treated groups (Figure 1C). Although metformin caused a reduction in plasma glucose levels in the control group, no significant differences in plasma glucose levels were observed in the Ang II–treated groups (Figure 1D). Further, no metformin‐induced hypoglycemia was observed. Plasma lipid levels were relatively unchanged for total cholesterol and low‐ and high‐density lipoprotein. Triglyceride levels, however, showed a non–statistically significant trend to be lower in metformin‐treated groups compared with their control groups (Figure 1E through 1H).
Figure 1.

Metformin causes weight loss and reduces blood glucose of ApoE−/− C57BL/6J mice. Vascular senescence was evaluated using fluorescein di‐β‐d‐galactopyranoside to perform senescence‐associated β‐galactosidase assay (A). Systolic blood pressure (SBP) was measured by using tail‐cuff plethysmography method (B). Weight of animals was measured at the beginning and end of the study and weight changes were calculated and presented compared with the initial weights (C). Plasma glucose (D) and lipid profile (E through H) of animals were measured at the time of death (*P<0.05). HDL indicates high‐density lipoprotein; Ang II, angiotensin II; LDL, low‐density lipoprotein; TG, triglyceride.
Metformin Attenuates HFD‐Induced Atherosclerosis in ApoE−/− C57BL/6J Mice
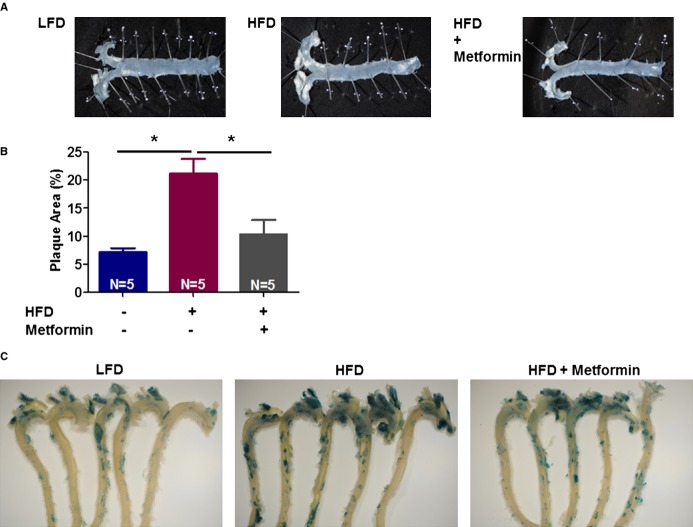
Vascular senescence contributes to cardiovascular disease, including atherosclerosis.35 To test whether metformin mitigates vascular senescence and atherosclerosis, we fed ApoE−/− mice an HFD. Metformin was delivered via an IP or oral approach. Metformin (IP) significantly attenuates HFD‐induced atherosclerosis and vascular senescence in 2 (Figure 2A through 2C) or 4 weeks (Figure 3A through 3C) study sets. We found that the majority of the senescent cells in this study model were present in the aortic arch, an area with high atherosclerotic plaque burden (Figure 3C). Also, oral administration of metformin via gavage attenuated atherosclerosis and vascular senescence (Figure 4). We observed trends, with no statistical significance, toward weight loss and less food intake by metformin in HFD‐fed animals (Figure 2D through 2E). Furthermore, there was no significant difference in plasma glucose or lipids (Figure 2F through 2J). Also, SBPs were similar in all groups (Figure 2K).
Figure 2.
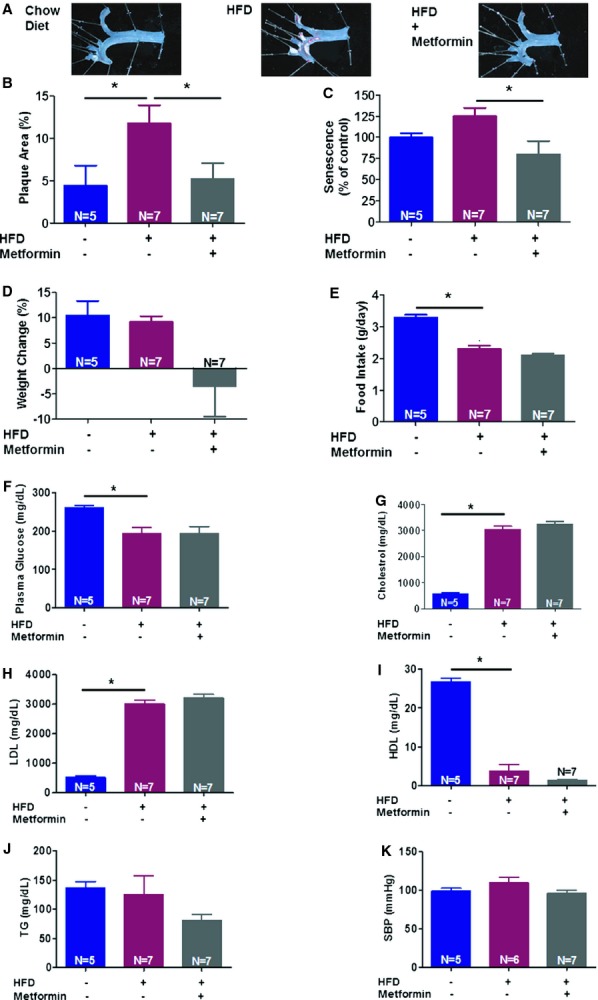
Metformin (delivered intraperitoneally for 2 weeks) attenuates high‐fat diet (HFD)‐induced atherosclerosis in ApoE−/− C57BL/6J mice. Atherosclerotic plaques images (A) and their quantification in aortic arch and bifurcations using Image J (B) are presented. Vascular senescence was evaluated using fluorescein di‐β‐d‐galactopyranoside to measure senescence‐associated β‐galactosidase activity in aortas (C). Also, metformin use was associated with modest weight loss (D) and slight decrease in food intake (E) in HFD‐fed mice in this study. Glucose (F), lipid profile (G through J), and systolic blood pressure (SBP) (K) of the studied animals were measured as previously explained (*P<0.05). HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein; TG, triglyceride.
Figure 3.
Metformin (delivered intraperitoneally for 4 weeks) attenuates high‐fat diet (HFD)‐induced atherosclerosis and vascular aging in ApoE−/− C57BL/6J mice. Atherosclerotic plaque images (A) and their quantification using Image J (B). Vascular aging was evaluated using 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐galactopyranoside (C) to assess senescence‐associated β‐galactosidase activity in aortas (*P<0.05). LFD indicates low‐fat diet.
Figure 4.
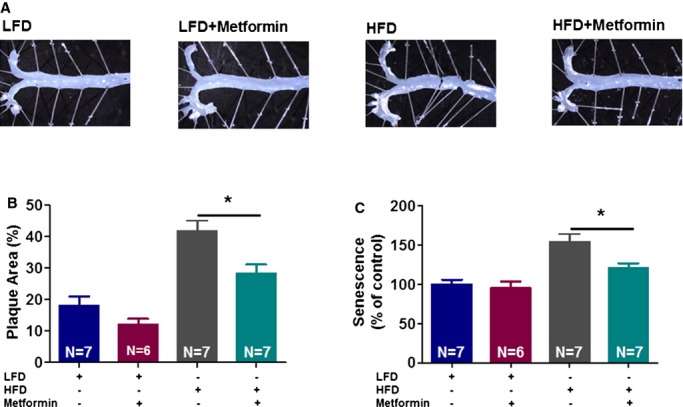
Metformin (delivered orally for 4 weeks) attenuates high‐fat diet (HFD)‐induced atherosclerosis in ApoE−/− C57BL/6J mice. Atherosclerotic plaque images (A) and their quantification in aortic arch and bifurcations using Image J (B) are presented. Vascular senescence was evaluated using fluorescein di‐β‐d‐galactopyranoside to measure senescence‐associated β‐galactosidase activity in aortas (C) (*P<0.05). LFD indicates low‐fat diet.
Metformin Does Not Reverse the HFD‐Induced Atherosclerosis in ApoE−/− C57BL/6J Mice
To test whether metformin prevents atherosclerosis by delaying the formation of plaque or by accelerating the regression of established plaque, we treated ApoE−/− mice in the following conditions: (1) low‐fat diet for 3 months as baseline control, (2) HFD in the first 2 months and low‐fat diet for the last month plus daily saline as placebo, and (3) diet similar to group 2 plus metformin daily (IP). We found that 4 weeks of metformin treatment did not reverse atherosclerosis or vascular senescence in the mice fed an HFD (Figure 5).
Figure 5.
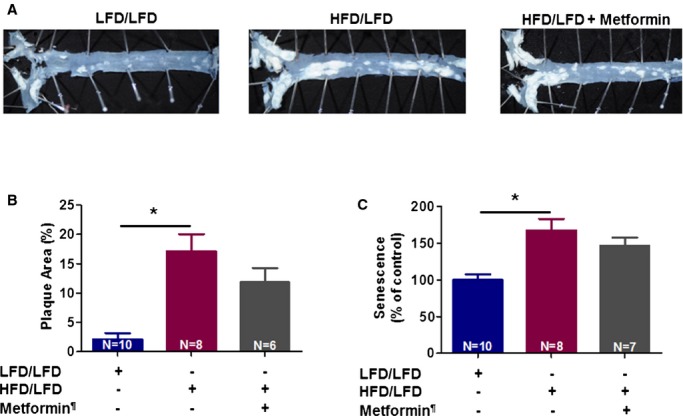
Metformin does not reverse the high‐fat diet (HFD)‐induced atherosclerosis in ApoE−/− C57BL/6J mice. Atherosclerotic plaque images (A) and quantification of plaque burden in descending aortas using Image J (B) are presented. Vascular senescence was evaluated using fluorescein di‐β‐d‐galactopyranoside to measure senescence‐associated beta‐galactosidase activity in aortas (C) (*P<0.05). LFD/LFD indicates low‐fat diet for all 12 weeks; HFD/LFD, high‐fat diet for 8 weeks then switched to low‐fat diet for 4 weeks. ¶Metformin was given only during the last 4 weeks in the metformin‐treated group.
Metformin Attenuates Upregulation of HFD‐Induced AT1R in Aortas of ApoE−/− C57BL/6J Mice
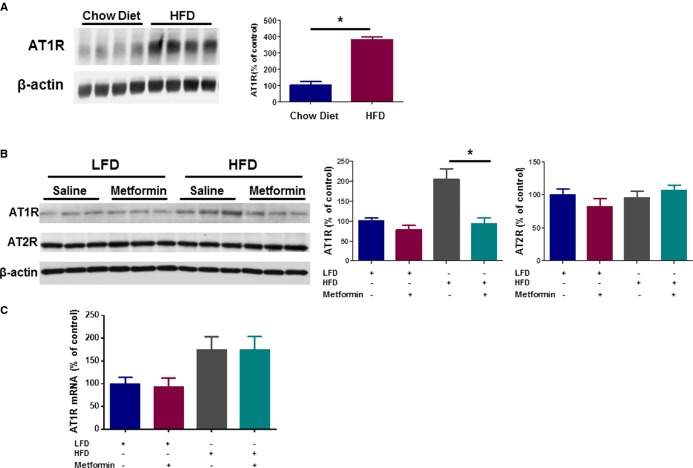
There is evidence that Ang II through its effect on AT1R enhances aging and that blocking AT1R by Ang II receptor blockers can induce longevity.6,35,39,41,47–48 Our observations that metformin may prevent Ang II–induced vascular senescence, an event associated with AT1R activation,37 and that HFD increases AT1R expression at the mRNA level,42 led us to investigate whether metformin could downregulate AT1R in the aortas of ApoE−/− C57BL/6J mice. We found that aortas of mice fed an HFD show an increase in AT1R protein levels compared with low‐fat diet–fed controls (Figure 6A) that was decreased by metformin (Figure 6B). There is evidence that Ang II type 2 receptors (AT2Rs) mediate the cardioprotective effects of Ang II receptor blockers.49 Therefore, we evaluated whether metformin could also upregulate AT2R expression. No change in AT2R protein levels was observed in aortas of low‐fat diet– or HFD‐fed mice in the presence or absence of metformin (Figure 6B). We also found that HFD increases the AT1R mRNA expression level; however, metformin does not affect the expression of AT1R at the mRNA level (Figure 6C).
Figure 6.
Metformin attenuates upregulation of high‐fat diet (HFD)‐induced angiotensin II receptor type 1 (AT1R) in aortas of ApoE−/− C57BL/6J mice. Western blot analysis for AT1R was performed on cytosolic fractions of cell lysates obtained from aortas of 6‐month‐old ApoE−/− C57BL/6J mice fed chow diet versus HFD for 2 weeks (N=4 per group) (A). Also, similarly in younger, 2‐month‐old mice fed low‐fat diet (LFD) versus HFD for 2 weeks in the absence and presence of metformin treatment, the expression level of both AT1R and AT2R was measured by using Western blot analysis on total cell lysates obtained from aortas of the studied animals (N=6 per group) (B). Level of protein expression was adjusted for the β‐actin level in each sample. A similar in vivo experiment was repeated and in mice aortic tissues, level of mRNA for AT1R, adjusted for GAPDH, was measured using qPCR (N=6 per group) (C). (*P<0.05).
Metformin Increases Superoxide Dismutase‐1, but Not Superoxide Dismutase‐2 or Catalase
AT1R activation is associated with decreased antioxidant enzyme levels and increased inflammation.6 To further investigate the underlying mechanism(s) of the vasculoprotective roles of metformin, we measured the level of antioxidant enzymes in the aortas of mice treated with or without metformin. We found that 2 weeks of treatment with metformin increase the expression of superoxide dismutase‐1, but not superoxide dismutase‐2 or catalase, in aortas of animals fed chow diet (Figure 7A through 7D).
Figure 7.
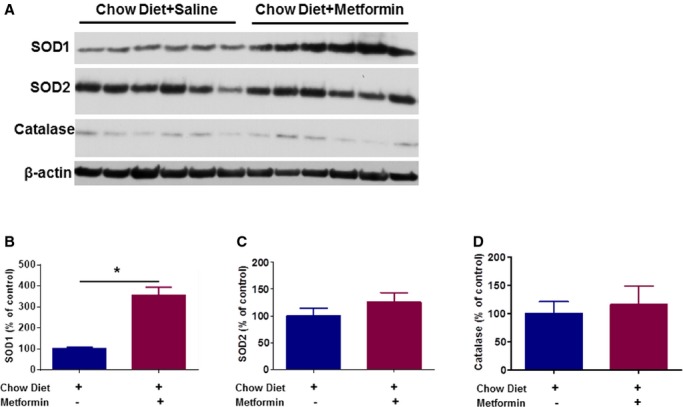
Metformin increases expression levels of SOD1, but not SOD2 or catalase, in aortas of ApoE−/− C57BL/6J mice fed chow diet. Western blot analyses for SOD1, SOD2, and catalase were performed on total cell lysates obtained from aortas of 3‐month‐old ApoE−/− C57BL/6J mice fed chow diet for 2 weeks in the absence and presence of metformin treatment (N=6 per group) (*P<0.05). SOD indicates superoxide dismutase.
Discussion
In this study using nondiabetic ApoE−/− C57BL/6J mice models, we show novel evidence of the vasculoprotective effects of metformin in attenuating atherosclerosis and some of its underlying mechanisms. We found that metformin indeed can effectively modulate the process of atherosclerosis induced by an HFD. Also, metformin attenuated vascular senescence induced by an HFD in ApoE−/− mice and perhaps the hypertensive and vascular senescence effects of Ang II. The effect of metformin in reducing vascular senescence is particularly important considering the growing evidence suggesting that cellular senescence promotes atherosclerosis.50 The senescence phenomenon is characterized by reduced cell proliferation, irreversible growth arrest, elevated DNA damage, epigenetic modifications, and telomere shortening and dysfunction.50 Senescent cells express specific markers such as senescence‐associated β‐galactosidase, a lysosomal enzyme seen in senescence of multiple human cell types that have been used extensively to study senescent cells and tissues,51 as used in our study.
We found a weight loss effect for metformin that is consistent with several previous clinical reports on the weight loss effect of metformin on patients with or without DM2.11,18–19,31,52 Furthermore, we provide mechanistic insights by showing that metformin attenuates AT1R at the protein level in the aortas of ApoE−/− mice. We found that metformin does not affect AT1R mRNA levels, thereby suggesting that decrease in AT1R protein likely results from primary degradation of the receptor protein. Future studies will elucidate whether AT1R downregulation is sufficient to recapitulate the protective effects of metformin in preventing senescence and atherosclerosis.
Metformin is a widely used medication to treat DM2. There are several clinical studies that strongly suggest its superiority in improving survival compared with other standard medications in patients with DM2 even at similar glycemic levels.14,18,53 Also, data from clinical studies confirm a modest effect of metformin on attenuation of blood pressure.54 However, data are scarce on whether cardiovascular benefits of metformin can be seen in nondiabetic conditions such as in patients with risk factors for CVD in the absence of DM2. Two recent clinical studies on metformin use for nondiabetic patients, Carotid Atherosclerosis: Metformin for insulin ResistAnce (CAMERA) study55 and Glycometabolic Intervention as Adjunct to Primary Percutaneous Intervention in ST Elevation Myocardial Infarction (GIPS)‐III Trial,56 did not show beneficial effects for metformin in very high risk populations. In the CAMERA study, nondiabetic patients with an average age of 63 who were receiving statins were randomized to receive metformin or placebo for 18 months, and the progression of mean distal carotid intima‐media thickness was found to be similar in the 2 groups at the conclusion of the study. Of note, about half of patients in CAMERA had had a myocardial infarction and about a third had undergone coronary artery bypass grafting before the study. Considering that carotid intima‐media thickness is a controversial surrogate marker for evaluation of drug effects in CVD,55 the value of the findings of CAMERA is very limited. Also, in the GIPS‐III trial in nondiabetic patients who underwent primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction, the use of metformin compared with placebo did not result in improved left ventricular ejection fraction after 4 months; however, both groups had normal left ventricular ejection fraction at the conclusion of the study.56 Hence, it would be informative to study the effects of metformin at early stages of CVDs.
The weight loss effect of metformin is perhaps due to the activation of AMP kinase, which switches cells from anabolic to catabolic states.9,15,57–58 It is unknown whether the vasculoprotective effects of metformin are independent of its effect on weight loss; however, weight loss by itself has survival benefits.20,22 Although limited clinical data support a modest favorable effect on lipid profiles for metformin,59–60 we did not find any significant effect for metformin on lipid profiles.
In brief, we found that metformin exerts its vasculoprotective benefits most likely in a pleiotropic manner (Figure 8). This pleiotropic mechanism, hypothetically due to mediators such as improvement in mitochondrial function, may explain the beneficial effects of metformin in attenuation of atherosclerosis independent of cholesterol metabolism. However, more detailed studies on each of the proposed pathways, and their relative importance in contribution to the final end points, are beyond the scope of this study and present open questions for us and other investigators. From the translational point of view, the results here demonstrated the feasibility and benefits of metformin beyond its glycemic control properties in nondiabetic mice models. Based on current standard of care, metformin is used for the treatment of patients with DM2 and a few other conditions such as polycystic ovary syndrome; however, as discussed earlier, it seems possible that metformin can be used as a primary preventive strategy for a broader population of patients at risk for CVD. Indeed, the findings of this study justify additional randomized clinical trials toward finding new therapeutic horizons for metformin for CVD prevention. In conclusion, we found that metformin provides pleiotropic benefits leading to the amelioration of vascular disease.
Figure 8.

Schematic proposed model for the mechanisms underlying vasculoprotective roles of metformin. By disrupting AT1R, metformin can attenuate many of the pathways that can lead to atherosclerosis, including the ones that can be triggered by HFD, and result in worsening vascular senescence. Moreover, perhaps through its effect on activation of AMPK, metformin can lead to weight loss by switching cells from an anabolic state to a catabolic energy consuming state. Also, some of the vasculoprotective effects of metformin can be through its effect on upregulation of SOD1, an antioxidant enzyme. (Dotted lines indicate the possibility of mediators for the presented effects of metformin.) AMPK indicates AMP kinase; AT1R, angiotensin II type 1 receptor; HFD, high‐fat diet; SOD1, superoxide dismutase‐1.
Sources of Funding
Dr Forouzandeh is the recipient of an American Heart Association (AHA) Postdoctoral Fellowship.
Disclosures
None.
References
- 1.Burnett JR. Lipids, lipoproteins, atherosclerosis and cardiovascular disease. Clin Biochem Rev. 2004; 25:2. [PMC free article] [PubMed] [Google Scholar]
- 2.Nazarewicz RR, Salazar G, Patrushev N, San Martin A, Hilenski L, Xiong S, Alexander RW. Early endosomal antigen 1 (EEA1) is an obligate scaffold for angiotensin ii‐induced, PKC‐alpha‐dependent akt activation in endosomes. J Biol Chem. 2011; 286:2886-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Chen KJ. Atherosclerosis, vascular aging and therapeutic strategies. Chin J Integr Med. 2012; 18:83-87. [DOI] [PubMed] [Google Scholar]
- 4.Orlandi A, Bochaton‐Piallat ML, Gabbiani G, Spagnoli LG. Aging, smooth muscle cells and vascular pathobiology: implications for atherosclerosis. Atherosclerosis. 2006; 188:221-230. [DOI] [PubMed] [Google Scholar]
- 5.Minamino T, Miyauchi H, Yoshida T, Tateno K, Komuro I. The role of vascular cell senescence in atherosclerosis: antisenescence as a novel therapeutic strategy for vascular aging. Curr Vasc Pharmacol. 2004; 2:141-148. [DOI] [PubMed] [Google Scholar]
- 6.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007; 292:C82-C97. [DOI] [PubMed] [Google Scholar]
- 7.Xiong S, Salazar G, Patrushev N, Ma M, Forouzandeh F, Hilenski L, Alexander RW. Peroxisome proliferator‐activated receptor gamma coactivator‐1alpha is a central negative regulator of vascular senescence. Arterioscler Thromb Vasc Biol. 2013; 33:988-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, Nicholson WK, Hutfless S, Bass EB, Bolen S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med. 2011; 154:602-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond). 2012; 122:253-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isoda K, Young JL, Zirlik A, MacFarlane LA, Tsuboi N, Gerdes N, Schonbeck U, Libby P. Metformin inhibits proinflammatory responses and nuclear factor‐kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006; 26:611-617. [DOI] [PubMed] [Google Scholar]
- 11. Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) group. Lancet. 1998; 352:854-865. [PubMed] [Google Scholar]
- 12.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006; 355:2427-2443. [DOI] [PubMed] [Google Scholar]
- 13.Selvin E, Bolen S, Yeh HC, Wiley C, Wilson LM, Marinopoulos SS, Feldman L, Vassy J, Wilson R, Bass EB, Brancati FL. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Arch Intern Med. 2008; 168:2070-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roussel R, Travert F, Pasquet B, Wilson PWF, Smith SC, Goto S, Ravaud P, Marre M, Porath A, Bhatt DL, Steg PG, Continu RA. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. 2010; 170:1892-1899. [DOI] [PubMed] [Google Scholar]
- 15.Wong AK, Howie J, Petrie JR, Lang CC. Amp‐activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond). 2009; 116:607-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin PJ, Stambolic V. Obesity and insulin resistance in breast cancer—chemoprevention strategies with a focus on metformin. Breast. 2011; 20suppl 3:S31-S35. [DOI] [PubMed] [Google Scholar]
- 17.Hadad S, Iwamoto T, Jordan L, Purdie C, Bray S, Baker L, Jellema G, Deharo S, Hardie DG, Pusztai L, Moulder‐Thompson S, Dewar JA, Thompson AM. Evidence for biological effects of metformin in operable breast cancer: a pre‐operative, window‐of‐opportunity, randomized trial. Breast Cancer Res Treat. 2011; 128:783-794. [DOI] [PubMed] [Google Scholar]
- 18.Berstein LM. Metformin in obesity, cancer and aging: addressing controversies. Aging (Albany NY). 2012; 4:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levri KM, Slaymaker E, Last A, Yeh J, Ference J, D'Amico F, Wilson SA. Metformin as treatment for overweight and obese adults: a systematic review. Ann Fam Med. 2005; 3:457-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leblanc ES, O'Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care‐relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011; 155:434-447. [DOI] [PubMed] [Google Scholar]
- 21.Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979; 32:563-576. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4:579-591. [DOI] [PubMed] [Google Scholar]
- 23.Marquie G. Comparative effects of metformin and phenformin on the progression and regression of cholesterol induced atherosclerosis in rabbits. Paroi Arterielle. 1979; 5:209-218. [PubMed] [Google Scholar]
- 24.Marquie G. Metformin action on lipid metabolism in lesions of experimental aortic atherosclerosis of rabbits. Atherosclerosis. 1983; 47:7-17. [DOI] [PubMed] [Google Scholar]
- 25.Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22:820-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El‐Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000; 275:223-228. [DOI] [PubMed] [Google Scholar]
- 27.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004; 53:1052-1059. [DOI] [PubMed] [Google Scholar]
- 28.Hinke SA, Martens GA, Cai Y, Finsi J, Heimberg H, Pipeleers D, Van de Casteele M. Methyl succinate antagonises biguanide‐induced AMPK‐activation and death of pancreatic beta‐cells through restoration of mitochondrial electron transfer. Br J Pharmacol. 2007; 150:1031-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Aguiar LG, Bahia LR, Villela N, Laflor C, Sicuro F, Wiernsperger N, Bottino D, Bouskela E. Metformin improves endothelial vascular reactivity in first‐degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006; 29:1083-1089. [DOI] [PubMed] [Google Scholar]
- 30.Jensterle M, Sebestjen M, Janez A, Prezelj J, Kocjan T, Keber I, Pfeifer M. Improvement of endothelial function with metformin and rosiglitazone treatment in women with polycystic ovary syndrome. Eur J Endocrinol. 2008; 159:399-406. [DOI] [PubMed] [Google Scholar]
- 31.Luque‐Ramirez M, Mendieta‐Azcona C, Alvarez‐Blasco F, Escobar‐Morreale HF. Effects of metformin versus ethinyl‐estradiol plus cyproterone acetate on ambulatory blood pressure monitoring and carotid intima media thickness in women with the polycystic ovary syndrome. Fertil Steril. 2009; 91:2527-2536. [DOI] [PubMed] [Google Scholar]
- 32.Helvaci MR, Sevinc A, Camci C, Yalcin A. Treatment of white coat hypertension with metformin. Int Heart J. 2008; 49:671-679. [DOI] [PubMed] [Google Scholar]
- 33.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK‐eNOS‐mediated signaling. Diabetes. 2008; 57:696-705. [DOI] [PubMed] [Google Scholar]
- 34.Gundewar S, Calvert JW, Jha S, Toedt‐Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya‐Cisneros M, Tian R, Lefer DJ. Activation of AMP‐activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009; 104:403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21‐dependent pathway. Circulation. 2006; 114:953-960. [DOI] [PubMed] [Google Scholar]
- 36.Minamino T, Yoshida T, Tateno K, Miyauchi H, Zou Y, Toko H, Komuro I. Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation. 2003; 108:2264-2269. [DOI] [PubMed] [Google Scholar]
- 37.Ichiki T, Miyazaki R, Kamiharaguchi A, Hashimoto T, Matsuura H, Kitamoto S, Tokunou T, Sunagawa K. Resveratrol attenuates angiotensin II‐induced senescence of vascular smooth muscle cells. Regul Pept. 2012; 177:35-39. [DOI] [PubMed] [Google Scholar]
- 38.Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens. 2011; 20:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009; 119:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linz W, Heitsch H, Scholkens BA, Wiemer G. Long‐term angiotensin II type 1 receptor blockade with fonsartan doubles lifespan of hypertensive rats. Hypertension. 2000; 35:908-913. [DOI] [PubMed] [Google Scholar]
- 41.de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011; 89:31-40. [DOI] [PubMed] [Google Scholar]
- 42.Cole BK, Keller SR, Wu R, Carter JD, Nadler JL, Nunemaker CS. Valsartan protects pancreatic islets and adipose tissue from the inflammatory and metabolic consequences of a high‐fat diet in mice. Hypertension. 2010; 55:715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habib A, Karmali V, Polavarapu R, Akahori H, Nakano M, Yazdani S, Otsuka F, Pachura K, Davis T, Narula J, Kolodgie FD, Virmani R, Finn AV. Metformin impairs vascular endothelial recovery after stent placement in the setting of locally eluted mammalian target of rapamycin inhibitors via S6 kinase‐dependent inhibition of cell proliferation. J Am Coll Cardiol. 2013; 61:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985; 57:65-73. [DOI] [PubMed] [Google Scholar]
- 45.Roguska J, Simon NM, del Greco F. Pressor response to angiotensin II in hypertension. Correlation with plasma renin activity and response to norepinephrine and metaraminol. Am J Cardiol. 1968; 21:705-713. [DOI] [PubMed] [Google Scholar]
- 46.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499-502. [PubMed] [Google Scholar]
- 47.Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B. Angiotensin II‐mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere‐dependent and independent pathways. Circ Res. 2008; 102:201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillespie EL, White CM, Kardas M, Lindberg M, Coleman CI. The impact of ace inhibitors or angiotensin II type 1 receptor blockers on the development of new‐onset type 2 diabetes. Diabetes Care. 2005; 28:2261-2266. [DOI] [PubMed] [Google Scholar]
- 49.Oishi Y, Ozono R, Yoshizumi M, Akishita M, Horiuchi M, Oshima T. At2 receptor mediates the cardioprotective effects of at1 receptor antagonist in post‐myocardial infarction remodeling. Life Sci. 2006; 80:82-88. [DOI] [PubMed] [Google Scholar]
- 50.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012; 111:245-259. [DOI] [PubMed] [Google Scholar]
- 51.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira‐Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995; 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non‐diabetic individuals with obesity. Exp Clin Endocrinol Diabetes. 2013; 121:27-31. [DOI] [PubMed] [Google Scholar]
- 53.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577-1589. [DOI] [PubMed] [Google Scholar]
- 54.Giugliano D, De Rosa N, Di Maro G, Marfella R, Acampora R, Buoninconti R, D'Onofrio F. Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care. 1993; 16:1387-1390. [DOI] [PubMed] [Google Scholar]
- 55.Preiss D, Lloyd SM, Ford I, McMurray JJ, Holman RR, Welsh P, Fisher M, Packard CJ, Sattar N. Metformin for non‐diabetic patients with coronary heart disease (the camera study): a randomised controlled trial. Lancet Diabetes Endocrinol. 2013; 2:116-124. [DOI] [PubMed] [Google Scholar]
- 56.Lexis CP, van der Horst IC, Lipsic E, Wieringa WG, de Boer RA, van den Heuvel AF, van der Werf HW, Schurer RA, Pundziute G, Tan ES, Nieuwland W, Willemsen HM, Dorhout B, Molmans BH, van der Horst‐Schrivers AN, Wolffenbuttel BH, ter Horst GJ, van Rossum AC, Tijssen JG, Hillege HL, de Smet BJ, van der Harst P, van Veldhuisen DJ, Investigators G‐I. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the gips‐III randomized clinical trial. JAMA. 2014; 311:1526-1535. [DOI] [PubMed] [Google Scholar]
- 57.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk‐Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP‐activated protein kinase in mechanism of metformin action. J Clin Invest. 2001; 108:1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulligan JD, Gonzalez AA, Stewart AM, Carey HV, Saupe KW. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J Physiol. 2007; 580:677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pentikainen PJ, Voutilainen E, Aro A, Uusitupa M, Penttila I, Vapaatalo H. Cholesterol lowering effect of metformin in combined hyperlipidemia: placebo controlled double blind trial. Ann Med. 1990; 22:307-312. [DOI] [PubMed] [Google Scholar]
- 60.Robinson AC, Burke J, Robinson S, Johnston DG, Elkeles RS. The effects of metformin on glycemic control and serum lipids in insulin‐treated niddm patients with suboptimal metabolic control. Diabetes Care. 1998; 21:701-705. [DOI] [PubMed] [Google Scholar]