Abstract
Background
Arterial blood pressure is dependent on interactions between the heart and arteries. Resistive and pulsatile components of arterial load can be assessed by systemic vascular resistance (SVR, a microvascular property) and the ratio of stroke volume to pulse pressure (a surrogate of total arterial compliance, TAC), respectively. The relationship between arterial function and cardiovascular events in populations without cardiovascular disease is unknown.
Methods and Results
We studied 4806 adults enrolled in the Multi‐Ethnic Study of Atherosclerosis who were free of clinical cardiovascular disease at baseline. SVR and stroke volume/pulse pressure (SV/PP) were derived by sphygmomanometry and magnetic resonance imaging. The relationship between these measures of arterial function and incident cardiovascular events was assessed using Cox regression. With a mean follow‐up of 7.5 years, cardiovascular events occurred in 358 participants (7.4%). There was no relationship between SVR and subsequent cardiovascular events. However, increased stroke volume/pulse pressure was associated with reduced event rate in unadjusted (hazard ratio=0.67, 95% CI=0.58 to 0.77, P<0.001) and analyses that adjusted for multiple confounders (HR=0.75; 95% CI=0.62 to 0.90; P<0.001).
Conclusions
Greater total arterial compliance, manifest by higher stroke volume/pulse pressure is associated with a reduced incidence of subsequent CVE. In contrast, SVR was not independently associated with CVE in subjects free of overt cardiovascular disease at baseline. These findings support the concept that alternations in the large conduit vessels, rather than changes in microvascular resistance, are primarily related to incident cardiovascular disease.
Keywords: arterial compliance, pulsatile hemodynamics, microvascular, resistance
Introduction
Arterial blood pressure is determined by the interaction between the left ventricle and the load imposed by systemic arteries (arterial load). There are 2 broad components to arterial load: resistance, provided largely by the microcirculation, and pulsatile load, dependent on conduit vessels. The resistive component of arterial load (systemic vascular resistance, SVR) can be computed simply as the ratio of mean arterial pressure/cardiac output. Pulsatile load is complex and time varying, and can be assessed with detailed modeling of aortic pressure‐flow relations.1 A more readily available index of pulsatile arterial load, mainly related to total arterial compliance, is the ratio of stroke volume/pulse pressure (SV/PP).
The relationship between these resistive and pulsatile components of arterial load and cardiovascular events (CVE) in healthy populations has not been thoroughly assessed. Although elevated SVR and arterial pressure are clearly relevant for cardiovascular outcomes, a derangement in pulsatile arterial hemodynamics may predict cardiovascular risk earlier, more accurately, or be associated with a different spectrum of morbidities.2–5 Most previous studies have assessed pulse pressure compared with mean arterial pressure as a predictor of cardiovascular outcomes.6–8 However, the determinants of pulse pressure and mean arterial pressure extend beyond large arterial properties and microvascular resistance, and depend also on the flow generated by the heart (stroke volume and cardiac output). SVR and TAC are, in contrast, measures of arterial function. An understanding of arterial properties that determine mean or pulsatile arterial pressure may provide incremental risk stratification,9 refinement of cardiovascular disease phenotypes,10–11 or even identify suitable targets for earlier preventive strategies.
The goal of the present study is to assess the relationship between SVR and SV/PP to cardiovascular events (CVE) in the Multi‐Ethnic Study of Atherosclerosis (MESA).
Methods
Study Population
The MESA study design has been previously described.12 Briefly, MESA is a prospective observational cohort study designed to identify the prevalence, risk factors, and progression of subclinical atherosclerosis in a diverse population. Individuals from different ethnic groups (white, Chinese, black, Hispanic) aged 45 to 84 years, without clinically apparent cardiovascular disease, were recruited between July 2000 and August 2002 from 6 geographical centers across the United States. All participants were between the ages 44 and 84 and provided informed consent. MESA was approved by the institutional review boards at each recruiting center.
Data and Laboratory Collection
Demographic and clinical variables (medical history, ethnicity, medication use) were obtained by standardized questionnaires. Smoking was determined by patient history, and categorized as current, former, or never. Cardiovascular events were defined as any of the following: myocardial infarction, fatal coronary heart disease, angina if followed by revascularization, resuscitated cardiac arrest, stroke, and other atherosclerotic death. Follow‐up events were derived from telephone contact every 9 to 12 months. Moreover, 2 physicians independently reviewed copies of medical records and death certificates for hospitalizations and outpatient diagnoses using pre‐specified criteria.13 Fasting blood samples were collected for determination of total, high‐density lipoprotein (HDL) cholesterol and triglyceride levels; low‐density lipoprotein cholesterol concentration was calculated by the Friedewald equation. Hypertension was defined per JNC‐8 criteria14 (≥140/90 or current anti‐hypertensive medication use), and diabetes mellitus was defined as elevated fasting glucose (>126 mg/dL) or the use of oral or subcutaneous hypoglycemic.
Measures of Arterial Load
Left ventricular stroke volume was assessed by magnetic resonance imaging (MRI) at 6 centers using 1.5‐Tesla magnets during a separate visit, with low inter‐observer variability as previously described.15 Stroke volume was defined as the difference between left ventricular end‐diastolic and end‐systolic volume measured in a stack of gradient‐echo short‐axis cine acquisitions. Total arterial compliance (mL/mm Hg) was estimated using the left ventricular stroke volume divided by brachial arterial pulse pressure (average of systolic–diastolic blood pressure before and after MRI). Systemic vascular resistance was derived as the ratio of mean brachial arterial pressure (2×diastolic blood pressure/systolic blood pressure, averaged from pre‐ and post‐MRI values) divided by MRI‐derived cardiac output.
Statistical Analyses
Categorical variables were expressed as percentages and continuous variables as medians with the inter‐quartile range (25th to 75th percentiles) or means (standard deviation) as appropriate. Because both SVR and SV/PP are dependent on and bear an approximate linear allometric relationship with body surface area,16 they were normalized and are expressed SVR index (SVRi) and SV/PP index (SV/PPi) where appropriate. For comparison of high, normal, and low values, SVRi and SV/PPi were divided into tertiles. For multivariate analysis the non‐indexed SVR and SV/PP values were used, since height and weight were included as covariates. The relationship between SVRi or SV/PPi (assessed at baseline) and the occurrence of CVE was analyzed with Cox regression. Covariates included established cardiovascular risk factors and other potential confounders including: age, gender, race, height, weight, hypertension, total and HDL cholesterol, triglycerides, diabetes, smoking status, estimated glomerular filtration rate, hypertension therapy, statin use, and heart rate. The glomerular filtration rate was determined by the Modification of Diet in Renal Disease formula.17 Sequential models with Cox proportional hazards were employed as follows: Model 1 is corrected for age, gender, race, height, and weight; Model 2 additionally incorporates hypertension, total LDL and HDL cholesterol, triglycerides, diabetes, smoking status, eGFR, and hypertension therapy. Tertiles of SVR and SV/PP were compared using analysis of variance. In order to investigate whether or not arterial load differed with respect to demographic or clinical risk factors, we divided the spectrum of SVR and SV/PP into tertiles. We chose to deem the middle tertiles referent to avoid potential confusion by introducing presumed lowest (top tertile for SVR and bottom tertile for SV/PP) or highest (bottom tertile for SV/PP and the top SVR tertile) risk categories. All tests were 2‐sided with alpha=0.05. Analyses were performed with SPSS (v 19.0; SPSS Inc, Chicago, IL). Model discrimination was assessed with the Harrel's c‐index (which is analogous to the area under the receiver‐operator‐characteristic curve).18–19 Improvements in subject reclassification by the hemodynamic parameters of interest was further assessed using the category‐free net reclassification improvement (NRI), which depends on the increased probability that a new model will categorize case subjects as higher‐risk and decreased probability that it will categorize control subjects as lower‐risk, compared to a base model.18–20 We also computed the integrated discrimination improvement (IDI), which expresses the improvement in discrimination slopes (mean difference in predicted probabilities between case and control participants) between the base model and new model.18–21 NRI and IDI were computed at 5 years of follow‐up using the R package survIDINRI.22
Results
There were 4806 participants in MESA with cardiac MRI data. Among these, the median age was 61 and 48% were female while 39% were Caucasian, 25% African American, 22% Hispanic and 13% Chinese. Three hundred and fifty‐eight participants (7.4%) had a CVE at a mean follow‐up of 7.5 years (Table 1).
Table 1.
Baseline Characteristics
| Entire Study Population (n=4806) | Cardiovascular Event (n=358) | |
|---|---|---|
| Age | 61 (46 to 76) | 66 (52 to 80) |
| Sex | ||
| Male | 2521 (52) | 127 (35) |
| Female | 2285 (48) | 230 (64) |
| Ethnicity | ||
| White | 1884 (39) | 166 (46) |
| African American | 1219 (25) | 86 (24) |
| Chinese American | 638 (13) | 29 (8) |
| Hispanic American | 1065 (22) | 76 (21) |
| Body measurements | ||
| Height, cm | 166 (152 to 181) | 167 (153 to 181) |
| Weight, kg | 77 (56 to 98) | 80 (60 to 100) |
| BMI, kg/m2 | 27 (22 to 34) | 28 (22 to 35) |
| Hemodynamic variables | ||
| Brachial SBP, mm Hg | 134 (107 to 162) | 144 (115 to 172) |
| Brachial DBP, mm Hg | 77 (63 to 91) | 79 (64 to 95) |
| Heart rate, bpm | 62 (49 to 75) | 64 (51 to 77) |
| Laboratory analysis | ||
| Total cholesterol | 194 (150 to 238) | 194 (25 to 250) |
| LDL‐cholesterol | 117 (77 to 157) | 119 (75 to 163) |
| HDL‐cholesterol | 51 (32 to 70) | 47 (31 to 63) |
| Triglycerides | 131 (47 to 215) | 143 (50 to 236) |
| Estimated GFR, mL/min×1.73−2 | 81 (59 to 103) | 79 (54 to 104) |
| Risk factors | ||
| Diabetes mellitus | 545 (11) | 79 (22) |
| Past smoker | 1717 (36) | 139 (39) |
| Current smoking | 610 (12) | 66 (18) |
| Hypertension | 2026 (42) | 237 (66) |
| Hypertension medication use | 1691 (35) | 187 (52) |
| Statin use | 691 (14) | 71 (20) |
| All cardiovascular events | 358 (7.4) | |
Values are median (interquartile range) or n (%). DBP indicates diastolic blood pressure; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure.
The mean SVR across the study population was 18.0±5.4 mm Hg×min/L (SVRi 10.0±3.6 mm Hg×min/L), and mean SV/PP was 1.8±0.6 mL/mm Hg (SV/PPi 0.96±0.30 mL/beat×m2×mm Hg). In order to investigate whether or not arterial load differs with respect to demographic or clinical risk factors, we divided the spectrum of SVR and SV/PP into tertiles and deemed the middle tertile referent. Those with increased arterial load (high SVR tertile, lowest SV/PP tertile; Table 2) tended to be older with higher mean arterial pressure and lower eGFR. Females tended to have higher SVR and lower SV/PP, even when indexed to body surface area. There was no relationship between SVR tertiles and conventional cardiovascular risk factors. In contrast, participants with reduced SV/PP were more likely diabetic with a history of hypertension (Table 2).
Table 2.
Baseline Characteristics by Tertiles of SVR or SV/PP
| Systemic Vascular Resistance Tertile (Median) | Stroke Volume/Pulse Pressure Tertile (Median) | |||||
|---|---|---|---|---|---|---|
| Lowest (6.94) | Reference (9.30) | Highest (12.92) | Lowest (0.61) | Reference (0.83) | Highest (1.17) | |
| Age, y | 58.2±9.3* | 61.0±9.9 | 65.0±9.9 | 67.0±9.1* | 61.2±9.5 | 55.6±8.4* |
| Female | 586 (37) | 851 (53) | 1084 (68) | 948 (64)* | 803 (54) | 575 (39)* |
| Race | ||||||
| White | 720 (45) | 594 (37) | 570 (36) | 577 (39) | 566 (38) | 600 (40) |
| African American | 432 (27) | 410 (26) | 454 (24) | 441 (30) | 382 (26) | 283 (19) |
| Chinese American | 102 (6) | 221 (14) | 315 (20) | 135 (9) | 218 (15) | 258 (18) |
| Hispanic American | 348 (22) | 277 (24) | 340 (21) | 339 (23) | 327 (22) | 352 (24) |
| Hemodynamic variables | ||||||
| Mean arterial pressure, mm Hg | 93.9±12.3* | 95.9±12.4 | 99.4 + 13.5* | 101.0±13.8* | 95.6±12.2 | 92.4±11.2* |
| Heart rate, bpm | 64.1±9.4* | 62.8±9.3 | 61.6±9.4* | 63.0±9.6 | 63.1±9.2 | 62.3±9.3 |
| Laboratory analysis | ||||||
| LDL‐cholesterol, mg/dL | 116.5±30.4 | 117.9±32.1 | 117.3±31.4 | 117.2±31.6 | 117.3±30.2 | 116.9±31.8 |
| HDL‐cholesterol, mg/dL | 48.4±13.9 | 51.0±14.9 | 54.3±15.5 | 51.6±14.5 | 51.2±14.9 | 50.5±14.9 |
| Triglycerides, mg/dL | 132.5±89.1 | 132.7±89.7 | 128.5±74.0 | 140.1±90.1* | 128.9±73.4 | 125.1±77.7 |
| eGFR, mL/min per 1.73 m2 | 83.7±17.6* | 81.3±17.0 | 78.3±16.8* | 77.3±17.9* | 82.0±17.2 | 84.1±15.8* |
| Risk factors | ||||||
| Diabetes mellitus | 200 (13) | 187 (12) | 157 (10) | 239 (16)* | 174 (12) | 97 (7) |
| Never smoker | 754 (47) | 835 (52) | 876 (55) | 767 (52) | 758 (51) | 795 (53) |
| Past smoker | 626 (39) | 566 (35) | 525 (33) | 555 (37) | 530 (36) | 508 (34) |
| Current smoking | 219 (14) | 197 (12) | 194 (12) | 167 (11) | 200 (13) | 189 (13) |
| Hypertension | 519 (32) | 556 (35) | 616 (39) | 944 (63)* | 593 (40) | 335 (22)* |
Values are mean±SD or percentages. There are n=1602 participants per group. Systemic vascular resistance and stroke volume/pulse pressure are indexed to body surface area, and units are mm Hg×min/L and mL/beat×m2×mm Hg, respectively. The median value for each tertile is listed in parenthesis. eGFR indicates estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SV/PP, stroke volume/pulse pressure; SVR, systemic vascular resistance.
P<0.01 compared to reference tertile.
SV/PP, but not SVR, was independently associated with incident CVEs in unadjusted models. After adjustment for age, gender, race, height and weight, hypertension, total LDL and HDL cholesterol, triglycerides, diabetes, smoking status, eGFR, and hypertension therapy, a 1‐SD increase in SV/PP remained was associated with a reduced risk of CVEs (HR 0.73; 95% CI 0.61 to 0.88; P<0.001; Table 3). There was no significant interaction between SV/PP and hypertension, hypertension stage (I to III), diabetes, or current smoking (all P>0.05). The addition of SV/PP to a model containing age, gender, race, weight, height, LDL cholesterol, HDL cholesterol, triglycerides, smoking, diabetes mellitus, hypertension, antihypertensive medication use, estimated glomerular filtration rate, and SVR, resulted in a category‐free NRI of 0.20 (95%CI=0.05 to 0.38; P=0.02) and an integrated discrimination improvement of 0.004 (95%CI=0.001 to 0.01; P=0.007). The Harrel's C of the full model was 0.77.
Table 3.
Cox Proportional Hazards of the Relationship of Baseline Arterial Load and Subsequent Cardiovascular Events
| Unadjusted | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR | P Value | HR | P Value | HR | P Value | |
| Systemic vascular resistance | ||||||
| All CVE | 1.03 (0.93 to 1.14) | 0.30 | 1.01 (0.91 to 1.13) | 0.76 | 1.01 (0.91 to 1.13) | 0.78 |
| Stroke volume/pulse pressure | ||||||
| All CVE | 0.62 (0.54 to 0.72) | <0.001 | 0.63 (0.53 to 0.76) | <0.001 | 0.73 (0.61 to 0.88) | 0.001 |
Standardized hazard ratios represent difference per standard deviation of the dependent variable. All CVE includes myocardial infarction, fatal coronary heart disease, angina if followed by revascularization, resuscitated cardiac arrest, stroke, and other atherosclerotic death. Model 1 is corrected for age, gender, race, height, and weight; Model 2 additionally incorporates hypertension, LDL and HDL cholesterol, triglycerides, diabetes, smoking status, eGFR, and hypertension therapy. CVE indicates cardiovascular events; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HR, hazard ratio; LDL, low‐density lipoprotein.
When only hard CVEs (cardiovascular death or myocardial infarction) were considered, there remained a significant association with SV/PP in unadjusted (HR 0.58; 95% CI 0.46 to 0.73; P<0.001) and fully adjusted models (HR 0.60; 95% CI 0.45 to 0.81; P=0.001). There was no significant relationship between SVR and hard CVE (P>0.05 for all models). Figure shows Kaplan–Meier curves for CVE‐free survival among participants stratified by tertiles of SV/PP and SVR. The greatest incidence of CVE was observed among those with the lowest SV/PP.
Figure 1.
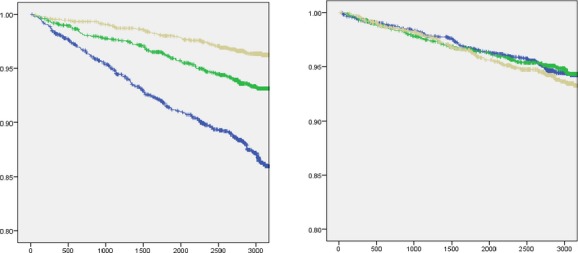
Event free‐survival stratified by tertiles of SV/PP (left) and SVR (right). Individuals with tertiles of low (blue), intermediate (green), and high SV/PP or SVR. For these analyses SV/PP and SVR were indexed to body surface area. Events were censored at 3000 days. SV/PP indicates stroke volume/pulse pressure; SVR, systemic vascular resistance.
Discussion
Herein we demonstrate a significant relationship between SV/PP, and subsequent CVE in a healthy population without known cardiovascular disease. This relationship was independent of conventional cardiovascular risk factors including hypertension. In contrast to pulsatile arterial load, resistive arterial load (SVR) was not related to subsequent CVE.
Hypertension is an established risk factor for cardiovascular disease.23–25 Although derangements in the resistive (SVR) and pulsatile (SV/PP) components of blood pressure are observed in tandem with aging and hypertension,26 SVR and SV/PP have anatomical and physiological distinctions. The pre‐capillary small vessels, or “resistance” vessels moderate the greatest hydrostatic pressure decrease in the circulation and are thus the primary determinants of SVR.27 In contrast, larger conduit vessels do not provide considerable resistance, but rather serve to accommodate and buffer stroke volume, which under normal circumstances protects the microcirculation from wide swings in pulse pressure.28 In addition to these distinctions, SVR and SV/PP may respond differently to pharmacological and lifestyle interventions.29–31 Therefore, blood pressure is a composite measure, and it is important to assess the pathophysiologic determinants of blood pressure that primarily relate to cardiovascular risk.
Others have identified relationships between SV/PP and CVE among those with cardiovascular disease. For example, reduced SV/PP is an independent predictor of CVE and all‐cause mortality among patients with hypertension.32–34 Among individuals with type 2 diabetes reduced SV/PP was associated with overall mortality independent of blood pressure.35 To our knowledge, our study is the first to assess the predictive value of SV/PP versus SVR in a population without established cardiovascular disease. We demonstrate that greater total arterial compliance, but not SVR, is independently associated with CVE in this large multi‐ethnic population. Our findings support the concept that alternations in the large conduit vessels, rather than changes in microvascular resistance, are primarily related to incident cardiovascular disease. Our findings are consistent with previous studies demonstrating that increased carotid‐femoral pulse wave velocity (a measure of aortic wall stiffness) is predictive of CVE and mortality in healthy and at‐risk populations.28,36 However, these previous studies did not simultaneously assess SVR. Furthermore, we note that SV/PP and carotid‐femoral pulse wave velocity are not interchangeable. SV/PP is dependent on the compliance of the entire arterial tree, which is dependent on arterial wall stiffness, arterial size and is composed of contributions of both large and muscular arteries.1
Reduced arterial compliance occurs with aging, and is more common among those with diabetes, renal disease, atherosclerosis28,31,37 and those at risk for future hypertension.38 In the present study, reduced SV/PP was associated with advanced age, and a greater prevalence of risk factors for atherosclerosis (diabetes and hypertension). Although we did not include subclinical atherosclerosis in the present analysis, reduced SV/PP was reported among those with occult lower extremity peripheral vascular disease in the same cohort.39 Similarly, others have reported a significant inverse relationship between the amount of thoracic aortic calcium and aortic distensibility.40 Although arterial stiffness and atherosclerosis frequently are identified together, alterations in arterial load and CVE have been described independent of atherosclerosis and often precede the clinical emergence of many risk factors for atherosclerosis.41–42
Our study should be interpreted in the context of its strengths and limitations. The strengths of the present study include its large, multiethnic sample, highly accurate stroke volume measures (based on cardiac MRI) and the availability of temporally proximate assessments of stroke volume and blood pressure measurements. The concepts presented here should be studied in other cohorts to assess external validity. The observational nature of the study, however, does not permit assessments of causality. The absence of known cardiovascular disease was a criterion for participation in MESA, the observations herein cannot necessarily be extended to individuals or populations with known cardiovascular disease. Furthermore, we did not perform detailed central arterial pressure‐flow analyses, which would have allowed a more detailed assessment of pulsatile arterial load and incident risk. This should be a goal for future research.
In conclusion, increased arterial load manifest by reduced SV/PP, but not increased SVR, is associated with CVE in adults from the general population without clinically evident cardiovascular disease. This implicates early derangements in the conduit vessels, not microvascular resistance, as partial determinates of future cardiovascular risk.
Sources of Funding
This research was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐RR‐025005 from NCRR. Zamani received research and salary support from 5‐T32‐HL007843‐17 and UL1RR024134.
Disclosures
None.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This manuscript was approved for submission by the Presentations and Publications Committee.
References
- 1.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: Part 2: arterial pressure‐flow and pressure‐volume relations in humans. Hypertension. 2010; 56:563-570. [DOI] [PubMed] [Google Scholar]
- 2.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (multiethnic study of atherosclerosis). J Am Coll Cardiol. 2012; 60:2170-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992; 86:513-521. [DOI] [PubMed] [Google Scholar]
- 4.Chirinos JA, Segers P, Raina A, Saif H, Swillens A, Gupta AK, Townsend R, Emmi AG, Jr, Kirkpatrick JN, Keane MG, Ferrari VA, Wiegers SE, St John Sutton MG. Arterial pulsatile hemodynamic load induced by isometric exercise strongly predicts left ventricular mass in hypertension. Am J Physiol Heart Circ Physiol. 2010; 298:H320-H330. [DOI] [PubMed] [Google Scholar]
- 5.Davis JT, Rao F, Naqshbandi D, Fung MM, Zhang K, Schork AJ, Nievergelt CM, Ziegler MG, O'Connor DT. Autonomic and hemodynamic origins of pre‐hypertension: central role of heredity. J Am Coll Cardiol. 2012; 59:2206-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, Glynn RJ. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000; 36:801-807. [DOI] [PubMed] [Google Scholar]
- 7.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999; 33:951-958. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the framingham heart study. Circulation. 2010; 122:1379-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haluska BA, Jeffries L, Carlier S, Marwick TH. Measurement of arterial distensibility and compliance to assess prognosis. Atherosclerosis. 2010; 209:474-480. [DOI] [PubMed] [Google Scholar]
- 10.Eleid MF, Rihal CS, Gulati R, Bell MR. Systematic use of transradial PCI in patients with ST‐segment elevation myocardial infarction: a call to “arms”. JACC Cardiovasc Interv. 2013; 6:1145-1148. [DOI] [PubMed] [Google Scholar]
- 11.Eleid MF, Nishimura RA, Sorajja P, Borlaug BA. Systemic hypertension in low‐gradient severe aortic stenosis with preserved ejection fraction. Circulation. 2013; 128:1349-1353. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871-881. [DOI] [PubMed] [Google Scholar]
- 13.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the mesa (multi‐ethnic study of atherosclerosis) study. J Am Coll Cardiol. 2008; 52:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA. 2014; 311:507-520. [DOI] [PubMed] [Google Scholar]
- 15.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi‐ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006; 186:S357-S365. [DOI] [PubMed] [Google Scholar]
- 16.Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, Segers PAsklepios investigators. Arterial load and ventricular‐arterial coupling: physiologic relations with body size and effect of obesity. Hypertension. 2009; 54:558-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente FChronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006; 145:247-254. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008; 27:157-172. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009; 150:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011; 30:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010; 48:1703-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013; 32:2430-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewington S, Clarke R, Qizilbash N, Peto R, Collins RProspective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002; 360:1903-1913. [DOI] [PubMed] [Google Scholar]
- 24.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996; 275:1557-1562. [PubMed] [Google Scholar]
- 25.Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens. 1994; 7:7S-12S. [DOI] [PubMed] [Google Scholar]
- 26.Slotwiner DJ, Devereux RB, Schwartz JE, Pickering TG, de Simone G, Roman MJ. Relation of age to left ventricular function and systemic hemodynamics in uncomplicated mild hypertension. Hypertension. 2001; 37:1404-1409. [DOI] [PubMed] [Google Scholar]
- 27.Levy BI, Ambrosio G, Pries AR, Struijker‐Boudier HA. Microcirculation in hypertension: a new target for treatment? Circulation. 2001; 104:735-740. [DOI] [PubMed] [Google Scholar]
- 28.Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011; 57:1511-1522. [DOI] [PubMed] [Google Scholar]
- 29.Kelly RP, Gibbs HH, O'Rourke MF, Daley JE, Mang K, Morgan JJ, Avolio AP. Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J. 1990; 11:138-144. [DOI] [PubMed] [Google Scholar]
- 30.Levy BI, Duriez M, Phillipe M, Poitevin P, Michel JB. Effect of chronic dihydropyridine (isradipine) on the large arterial walls of spontaneously hypertensive rats. Circulation. 1994; 90:3024-3033. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000; 102:1270-1275. [DOI] [PubMed] [Google Scholar]
- 32.Fagard RH, Pardaens K, Staessen JA, Thijs L. The pulse pressure‐to‐stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol. 2001; 38:227-231. [DOI] [PubMed] [Google Scholar]
- 33.de Simone G, Roman MJ, Koren MJ, Mensah GA, Ganau A, Devereux RB. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999; 33:800-805. [DOI] [PubMed] [Google Scholar]
- 34.Lind L, Andren B, Sundstrom J. The stroke volume/pulse pressure ratio predicts coronary heart disease mortality in a population of elderly men. J Hypertens. 2004; 22:899-905. [DOI] [PubMed] [Google Scholar]
- 35.Mohty D, Pibarot P, Echahidi N, Poirier P, Dagenais GR, Dumesnil JG. Reduced systemic arterial compliance measured by routine Doppler echocardiography: a new and independent predictor of mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2012; 225:353-358. [DOI] [PubMed] [Google Scholar]
- 36.Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton‐Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum HT, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014; 63:636-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadi N, Nabavi V, Hajsadeghi F, Flores F, Azmoon S, Ismaeel H, Shavelle D, Mao SS, Ebrahimi R, Budoff MJ. Impaired aortic distensibility measured by computed tomography is associated with the severity of coronary artery disease. Int J Cardiovasc Imaging. 2011; 27:459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, Heiss G. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999; 34:201-206. [DOI] [PubMed] [Google Scholar]
- 39.Lilly SM, Jacobs DR, Jr, Kronmal R, Bluemke DA, Criqui M, Lima J, Allison M, Duprez D, Segers P, Chirinos JA. Arterial compliance across the spectrum of ankle‐brachial index: the multiethnic study of atherosclerosis. Atherosclerosis. 2014; 233:691-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al‐Mallah MH, Nasir K, Katz R, Takasu J, Lima JA, Bluemke DA, Hundley G, Blumenthal RS, Budoff MJ. Thoracic aortic distensibility and thoracic aortic calcium (from the multi‐ethnic study of atherosclerosis [MESA]). Am J Cardiol. 2010; 106:575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the baltimore longitudinal study of aging. J Am Coll Cardiol. 2008; 51:1377-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laurent S. Arterial stiffness in arterial hypertension. Curr Hypertens Rep. 2006; 8:179-180. [DOI] [PubMed] [Google Scholar]


