Abstract
Background
Both supervised exercise (SE) and stenting (ST) improve functional status, symptoms, and quality of life compared with optimal medical care (OMC) in patients with claudication. The relative cost‐effectiveness of these strategies is not well defined.
Methods and Results
The Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study randomized patients with claudication due to aortoiliac stenosis to a 6‐month SE program, to ST, or to OMC. Participants who completed 6‐month follow‐up (n=98) were included in a health economic analysis through 18 months. Costs were assessed using resource‐based methods and hospital billing data. Quality‐adjusted life‐years were estimated using the EQ‐5D. Markov modeling based on the in‐trial results was used to explore the impact of assumptions about the longer term durability of observed differences in quality of life. Through 18 months, mean healthcare costs were $5178, $9804, and $14 590 per patient for OMC, SE, and ST, respectively. Measured quality‐adjusted life‐years through 18 months were 1.04, 1.16, and 1.20. In our base case analysis, which assumed that observed differences in quality of life would dissipate after 5 years, the incremental cost‐effectiveness ratios were $24 070 per quality‐adjusted life‐year gained for SE versus OMC, $41 376 for ST versus OMC, and $122 600 for ST versus SE. If the treatment effect of ST was assumed to be more durable than that of SE, the incremental cost‐effectiveness ratio for ST versus SE became more favorable.
Conclusions
Both SE and ST are economically attractive by US standards relative to OMC for the treatment of claudication in patients with aortoiliac disease. ST is more expensive than SE, with uncertain incremental benefit.
Clinical Trial Registration
URL: www.clinicaltrials.gov, Unique identifier: NCT00132743.
Keywords: claudication, cost–benefit analysis, exercise, peripheral vascular disease, stents
Introduction
Lower extremity peripheral arterial disease (PAD) affects 8.5 million Americans aged >40 years and >10% of the population aged >70 years, with incidence that is rising.1–3 PAD reduces functional status, impairs quality of life (QOL), and results in substantial morbidity and mortality, both directly and through its strong association with systemic atherosclerosis.4 In addition to these clinical concerns, PAD treatment also generates substantial costs for the healthcare system.5–6
Several treatments, including medical therapy,7 revascularization procedures,8–9 and structured exercise,10–11 have been shown to reduce claudication symptoms and improve exercise performance and QOL in PAD patients. Data on the comparative effectiveness of these treatments are limited.12–13
The Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) trial recently demonstrated that, for patients with claudication due to aortoiliac PAD, both a directly supervised exercise (SE) program and iliac stenting (ST) improve walking performance and QOL to a greater extent than medical therapy at 614 and 18 months (Tim Murphy, MD, unpublished data, 2014). To better inform policy and clinical decisions regarding treatment options in this patient population, we conducted a preplanned health economic study alongside the CLEVER trial.
Methods
The design and initial clinical results of the CLEVER trial have been reported previously.14–15 In brief, patients with moderate to severe claudication due to aortoiliac disease were randomized at a 2:2:1 ratio to receive iliac ST plus optimal medical care (OMC), SE plus OMC, or OMC alone. Iliac ST was done using standard techniques and stents approved by the US Food and Drug Administration. The SE program consisted of 3 directly supervised 1‐hour sessions per week for 26 weeks, supplemented by a subsequent 12‐month telephone‐based program designed to maintain adherence to exercise (calls twice per month for 6 months and then once per month for 6 months). All patients were treated with optimal medical therapy including cilostazol. Clinical follow‐up was continued for a total of 18 months. The study was approved by the institutional review committee at each enrolling site, and all patients provided informed consent.
Overview and Key Assumptions
Our economic evaluation was performed from a US societal perspective and included an in‐depth evaluation of QOL, resource utilization, and hospital billing data through 18 months. Key methodological assumptions were based on recommendations from the US Panel on Cost‐Effectiveness in Health and Medicine.16 Clinical benefits are expressed in quality‐adjusted life‐years (QALYs), and all costs are expressed in 2011 US dollars. Although there were no differences in mortality or other major clinical events at 18 months, persisting QOL differences were observed between groups (see Table A1). Accordingly, our base case analysis included the creation of a Markov model that was used to project costs and quality‐adjusted survival over a 5‐year time horizon, inclusive of the 18‐month in‐trial period.
Analysis Population
CLEVER enrolled 111 patients in the 3 treatment groups, 98 of whom had complete clinical and economic follow‐up through 6 months.14 All in‐trial portions of our analysis were based on those 98 participants because the 13 participants who withdrew or were lost to follow‐up within the first 6 months had very limited clinical and health economic follow‐up after enrollment.
Assessment of QALYs
Patients completed EQ‐5D questionnaires17 at baseline and at 6 and 18 months. Raw item responses on the questionnaires were converted to health state utilities based on an algorithm derived from a previous sampling of the US population.18 For each patient, QALYs from 0 to 6 and 6 to 18 months were calculated by multiplying observed survival duration by utility. For each interval, the transition between health states was assumed to occur at the midpoint of the interval, with the exception of the 0 to 6 month interval in the ST group. We assumed, based partly on previous literature,9 that QOL changes after ST would be more immediate than those observed with SE. Consequently, we set utilities for ST patients to their 6‐month value beginning 1 month after the intervention.
We observed an unexpected imbalance in the baseline EQ‐5D utility scores (see Table 1), with mean values highest in the ST group and lowest in the OMC group. To account for this, we assigned the mean baseline utility of the entire study population to each of the 3 study groups in our base case model. Subsequent changes in OMC group utility scores over time were based on the observed changes from baseline within that group, derived from paired t tests. The differences in 6‐ and 18‐month utility scores for the other 2 groups, relative to OMC, were then based on linear regression models of utility at those time points, adjusting for baseline scores.
Table 1.
Resource Utilization During 18‐Month Follow‐up
| Proportions | OMC | SE | ST | P Value (SE vs OMC) | P Value (ST vs OMC) | P Value (ST vs SE) |
|---|---|---|---|---|---|---|
| ER visits (vascular) | 5.0% (1/20) | 5.4% (2/37) | 7.3% (3/41) | 1.000 | 1.000 | 1.000 |
| ER visits (cardiac) | 5.0% (1/20) | 16.2% (6/37) | 4.9% (2/41) | 0.402 | 1.000 | 0.141 |
| ER visits (total) | 5.0% (1/20) | 21.6% (8/37) | 12.2% (5/41) | 0.139 | 0.653 | 0.364 |
| Outpatient clinic | 90.0% (18/20) | 89.2% (33/37) | 87.8% (36/41) | 1.000 | 1.000 | 1.000 |
| Home health services | 5.0% (1/20) | 5.4% (2/37) | 2.4% (1/41) | 1.000 | 1.000 | 0.601 |
| Physical therapy | 15.0% (3/20) | 5.4% (2/37) | 9.8% (4/41) | 0.332 | 0.674 | 0.678 |
| Nonacute residential care | 5.0% (1/20) | 2.7% (1/37) | 4.9% (2/41) | 1.000 | 1.000 | 1.000 |
| Coronary angiography | 0.0% (0/20) | 2.7% (1/37) | 2.4% (1/41) | 1.000 | 1.000 | 1.000 |
| Peripheral angiography | 0.0% (0/20) | 5.4% (2/37) | 2.4% (1/41) | 0.536 | 1.000 | 0.601 |
| Duplex ultrasound | 5.0% (1/20) | 8.1% (3/37) | 12.2% (5/41) | 1.000 | 0.653 | 0.715 |
| CT angiography | 0.0% (0/20) | 5.4% (2/37) | 9.8% (4/41) | 0.536 | 0.293 | 0.678 |
| MR angiography | 0.0% (0/20) | 0.0% (0/37) | 2.4% (1/41) | — | 1.000 | 1.000 |
| Exercise test | 0.0% (0/20) | 0.0% (0/37) | 7.3% (3/41) | — | 0.544 | 0.242 |
| Vascular intervention | 5.0% (1/20) | 5.4% (2/37) | 9.8% (4/41) | 1.000 | 1.000 | 0.678 |
CT indicates computed tomography; ER, emergency room; MR, magnetic resonance; OMC indicates optimal medical care; SE, supervised exercise; ST, stenting.
We assumed in our base case model that the utility differences between groups would gradually diminish such that no difference would be present at 5 years (owing to disease progression, restenosis, and/or loss of an exercise training effect); this was achieved by gradually decreasing the 18‐month utility values for the ST and SE groups to match the 18‐month value for the OMC group at year 5. Because the true durability of QOL benefit after ST and SE is unknown for this population, we varied the time over which utilities would equalize from 2 to 10 years in sensitivity analysis. We also explored scenarios in which the durability of the treatment effect provided by ST is longer than that provided by SE. Given that survival, QOL, and costs were assumed to be equal for all groups after 5 years, our base case analysis is conceptually equivalent to a model with a lifetime horizon.
Estimation of Costs
Costs were estimated during the in‐trial period using a combination of resource‐based accounting and hospital billing data.19 For all iliac stent procedures, procedural costs were calculated by multiplying measured units of resource consumption (guidewires, catheters, percutaneous transluminal angioplasty balloons, stents, vascular closure devices, intravascular ultrasound, specialty catheters, and procedure duration) by unit prices derived from 2 study hospitals. The remaining costs of the iliac ST encounters were derived from hospital bills for the 72% of patients who had complete billing data available. In those cases, nonprocedural charges were converted to costs using cost center–specific cost:charge ratios obtained from each enrolling hospital's Medicare cost report. When hospital bills were unavailable, nonprocedural costs were estimated with a linear regression model derived from the patients with complete billing data.
The costs of the SE program were estimated for both the individual participants and the facilities. At the patient level, the actual number of sessions attended and the estimated travel time to and from each session were measured. The participants' time cost for participation in the program was calculated as the total time spent in SE participation multiplied by the nominal US wage rate.20 The facility costs for the SE program were estimated and varied in sensitivity analysis. For our base case, we assumed facility costs of $40 per hour, based on a detailed analysis of cardiac rehabilitation costs at 3 programs in 2 cities. This cost is similar to current Medicare reimbursement for cardiac rehabilitation with ECG monitoring (CPT code 93798). For sensitivity analysis, we used a lower bound of $19 per hour, which is the current valuation for PAD rehabilitation (CPT code 93668), and an upper bound of $60 per hour (50% more than the base case). We also included an estimate of the costs for the professional time expended on telephone maintenance for each participant in months 6 to 12.
The costs of cardiovascular hospitalizations during the 18‐month follow‐up period were obtained from hospital summary bills, which were available for 86% of observed admissions. Charges were converted to costs using department‐specific cost:charge ratios. Costs for the small number of cardiovascular hospitalizations without available billing data were estimated based on average national Medicare reimbursement for the most likely diagnosis‐related groups (Medicare Provider Analysis and Review).
During the 18‐month trial period, resource utilization data were collected on a quarterly basis for PAD‐related outpatient care, residential care, and medications. Costs for these items were estimated by multiplying resource measures by representative unit costs, generally based on Medicare reimbursement. Prescription drug costs for cilostazol and clopidogrel were included based on average wholesale prices. Other drug classes did not differ in utilization among the CLEVER study arms and were not included.
Total costs for each group were aggregated into intervals of 0 to 6 and 6 to 18 months. In our base case (5‐year) model, the empirically derived costs were used through 18 months, with the assumption that costs would be evenly distributed across months 6 to 18 within each group. Because there were minimal differences between groups in total costs during months 6 to 18, we assumed that monthly costs thereafter would be the same for each group.
Statistical Analysis
Categorical data are reported as frequencies, and continuous data are reported as mean±SD. Discrete variables were compared by Fisher exact test. Cost data are reported as both mean and median values and were compared by 2 sample t tests, given our focus on comparing mean costs between groups (rather than the underlying distributions). All probability values were 2‐sided. Between‐group differences in costs and QALYs were estimated for the 18‐month in‐trial period using bootstrap resampling.
Cost‐Effectiveness Model
Using the health state utility and cost data from the 18‐month in‐trial period, we developed a Markov model to project results over a longer time horizon, using the assumptions regarding longer term QOL and costs described above. The model used a 1‐month cycle length and was programmed using TreeAge Pro software (TreeAge Software). All future costs and benefits were discounted at 3% per year in accordance with US methodological standards.21 The model included a background mortality rate for patients with PAD reported from the Reduction of Atherothrombosis for Continued Health (REACH) registry.22 Because only 1 death was observed during the trial, there was no basis for differing the background mortality rate between groups.
We performed sensitivity analyses on the hourly facility cost for an SE program, the time course over which QOL differences between groups were assumed to dissipate, and the durability of ST relative to SE in terms of health state utilities. To assess uncertainty in our results, we also performed a probabilistic sensitivity analysis of our base case model by replacing all model inputs with probability distributions and sampling from those distributions over 1000 model iterations.23 The results of the probabilistic sensitivity analysis were then plotted as a cost‐effectiveness acceptability curve.24 We also considered the impact of ignoring the time costs for patients to participate in the SE program; this would be the case in an analysis from the perspective of a third‐party payer or health system.
Results
ST and SE Costs
As previously reported, CLEVER enrolled 111 patients, 98 of whom completed 6‐month follow‐up and form the basis for the current analysis. The mean ankle‐brachial index of the target limb for all patients was 0.67 and did not differ across treatment groups. Of the 41 participants in our analysis assigned to ST, 38 completed iliac ST procedures. The mean preintervention stenosis severity for all treated lesions was 83.5%. Mean encounter costs for these 41 patients (including 2 patients who underwent only angiography and 1 who had no procedure) were $9211±4778, including physician fees. A slight majority of the procedures entailed overnight observation; the mean length of stay was 0.8±0.8 days (median 1).
Patients in the SE group completed a mean of 56±24 (of 78) exercise visits, and 36 patients participated in a mean of 11±5 (of 18) telephone counseling sessions during the maintenance phase. Using our base case assumptions, the mean total cost for the SE program was $4447±2017 per patient (median $5069), of which $2067±1016 (median $1997) was attributable to the time cost to the patients.
Follow‐up Resource Utilization and Costs
Inpatient and outpatient healthcare resource utilization through the 18‐month follow‐up period did not differ among the 3 groups, as shown in Table 1. Overall, <10% of patients in each group had a cardiovascular hospitalization or underwent nonprotocol revascularization procedures or diagnostic testing for PAD.
Healthcare costs for the 0‐ to 6‐month and 6‐ to 18‐month periods are shown in Tables 2 and 3. During this time frame, there were no major differences between groups in overall nonprotocol inpatient, outpatient, or medication costs. Consequently, observed differences in cumulative 18‐month costs were attributable primarily to the cost of the SE intervention and the ST procedures, with costs lowest in the OMC group and highest in the ST group (mean $5179±5658, $9805±7072, and $14 590±8898 for OMC, SE, and ST, respectively; P<0.02 for all 2‐way comparisons of means).
Table 2.
Summary of Healthcare Costs, 0 to 6 Months
| OMC (n=20) | SE (n=37) | ST (n=41) | P Value (SE vs OMC) | P Value (ST vs OMC) | P Value (ST vs SE) | |
|---|---|---|---|---|---|---|
| Index procedure/admission, $ | — | — | 9211±4778 (8966) | — | — | — |
| Exercise intervention, $ | — | 4088±1834 (4699) | — | — | — | — |
| Outpatient & residential care, $ | 680±785 (436) | 1121±1131 (654) | 1442±1631 (1091) | 0.127 | 0.017 | 0.312 |
| Cardiovascular admissions, $ | 701±2387 (0) | 0±0 (0) | 22±105 (0) | 0.204 | 0.218 | 0.217 |
| Medications, $ | 266±152 (234) | 233±132 (234) | 231±159 (234) | 0.399 | 0.411 | 0.937 |
| Total, $ | 1647±2585 (793) | 5442±2162 (5803) | 10 904±5595 (10 012) | <0.001 | <0.001 | <0.001 |
Values represent mean±SD; median values in parentheses. OMC indicates optimal medical care; SE, supervised exercise; ST, stenting.
Table 3.
Summary of Healthcare Costs, 6 to 18 Months
| OMC (n=20) | SE (n=37) | ST (n=41) | P Value (SE vs OMC)* | P Value (ST vs OMC)* | P Value (ST vs SE)* | |
|---|---|---|---|---|---|---|
| Exercise intervention, $ | — | 360±285 (359) | — | — | — | — |
| Outpatient & residential care, $ | 2380±2853 (1091) | 2129±2619 (1309) | 1737±2398 (873) | 0.739 | 0.360 | 0.493 |
| Cardiovascular admissions, $ | 739±2600 (0) | 1522±5057 (0) | 1537±6193 (0) | 0.443 | 0.482 | 0.990 |
| Medications, $ | 413±318 (449) | 352±318 (356) | 412±366 (463) | 0.498 | 0.992 | 0.450 |
| Total, $ | 3531±4849 (1490) | 4363±6169 (2213) | 3686±6746 (1341) | 0.604 | 0.927 | 0.646 |
Values represent mean±SD; median values in parentheses. OMC indicates optimal medical care; SE, supervised exercise; ST, stenting.
P values from 2‐sample t‐tests.
Quality‐Adjusted Life Expectancy
EQ‐5D scores at baseline and at 6 and 18 months, along with adjusted between‐group differences, are shown in Table 4. At baseline, the mean EQ‐5D score for the full population was 0.72; mean values were highest in the ST group and lowest in the OMC group. Within the ST and SE groups, EQ‐5D scores increased by 0.06 to 0.08 from baseline to 6 months (P<0.05 for paired comparisons within both groups) and did not change significantly between 6 and 18 months. Utilities in both the SE and ST groups were roughly 0.07 to 0.10 higher than in the OMC group at 6 and 18 months after adjustment for baseline values.
Table 4.
EQ‐5D Utility Scores, 0 to 18 Months
| Raw Data | Adjusted Difference in Means* | |||||
|---|---|---|---|---|---|---|
| OMC (n=20) | SE (n=37) | ST (n=41) | SE‐OMC | ST‐OMC | ST‐SE | |
| Baseline | 0.69±0.20 (0.78) | 0.72±0.17 (0.78) | 0.75±0.13 (0.78) | — | — | — |
| 6 months | 0.68±0.20 (0.74) | 0.80±0.12 (0.78) | 0.81±0.17 (0.79) | 0.097 (0.02 to 0.17) P=0.02 |
0.084 (−0.01 to 0.17) P=0.08 |
0.001 (−0.06 to 0.06) P=0.97 |
| 18 months | 0.72±0.17 (0.77) | 0.79±0.12 (0.79) | 0.81±0.17 (0.81) | 0.066 (−0.01 to 0.14) P=0.10 |
0.079 (−0.01 to 0.17) P=0.08 |
0.02 (−0.04 to 0.08) P=0.59 |
Raw mean±SD (median) values are shown on the left and differences in means, adjusted for baseline values, are shown on the right. OMC indicates optimal medical care; SE, supervised exercise; ST, stenting.
Adjusted for baseline values.
QALYs through 18 months, based on the observed data, were 1.04±0.24, 1.16±0.15, and 1.20±0.20 for the OMC, SE, and ST groups, respectively (P<0.05 for both SE versus OMC and ST versus OMC). In our base case model, which adjusted for the baseline difference in utility scores and incorporated background mortality and 3% discounting, 18‐month QALYs were 1.05, 1.14, and 1.16, respectively (Table 5).
Table 5.
Incremental Costs and QALYs, Observed and Projected
| OMC | Structured Exercise | Stent | ||||
|---|---|---|---|---|---|---|
| Cost | QALYs | Cost | QALYs | Cost | QALYs | |
| Observed data | $5179 | 1.04 | $9804 | 1.16 | $14 590 | 1.20 |
| 18 months* | $4953 | 1.05 | $9466 | 1.14 | $14 304 | 1.16 |
| 2 years | $6684 | 1.39 | $11 197 | 1.50 | $16 035 | 1.52 |
| 3 years | $10 004 | 2.04 | $14 516 | 2.18 | $19 355 | 2.21 |
| 4 years | $13 140 | 2.66 | $17 653 | 2.82 | $22 491 | 2.86 |
| 5 years | $16 103 | 3.24 | $20 616 | 3.43 | $25 454 | 3.47 |
| 10 years | $28 641 | 5.71 | $33 154 | 6.01 | $37 992 | 6.08 |
OMC indicates optimal medical care; QALYs, quality adjusted life years.
Based on Markov model, adjusting for baseline differences in utility and incorporating background mortality and 3% discount rate.
Cost‐Effectiveness
Under our base case assumptions, with a 5‐year time horizon, the SE strategy increased quality‐adjusted life expectancy compared with OMC by 0.19 QALY, whereas ST resulted in an additional gain of 0.04 QALY compared with SE. Over the same time frame, OMC was the least costly approach. SE increased costs by ≈$4500 relative to OMC, and ST increased costs by an additional ≈$5000 relative to SE (Table 5). The incremental cost‐effectiveness ratios (ICERs) for SE versus OMC and ST versus OMC at 5 years (the point at which QOL was assumed to equalize) were $24 070 per QALY gained and $41 376 per QALY gained, respectively. The ICER for ST versus SE at 5 years was $122 600 per QALY gained.
Sensitivity and Uncertainty Analyses
The result of our primary sensitivity analysis on the duration of QOL benefit is shown in Figure 1. As expected, both SE and ST became more cost‐effective relative to OMC the longer the differences in QOL were assumed to persist. The ICER for SE versus OMC was <$50 000 per QALY, even if the QOL difference between groups lasted no more than 18 months. The ICER for ST versus OMC became <$50 000 per QALY gained if the QOL benefit persisted at least 3.75 years.
Figure 1.
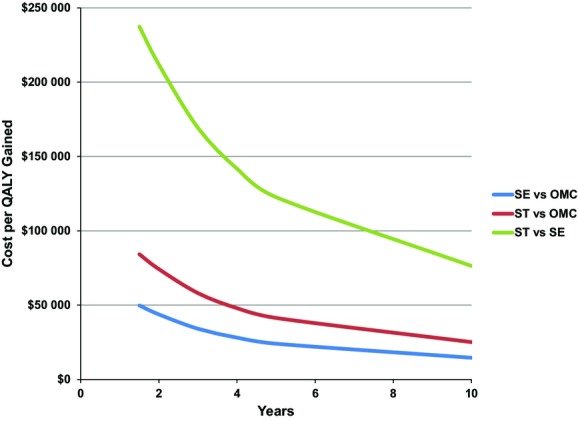
Sensitivity analysis on model time horizon. Incremental cost‐effectiveness ratios for SE vs OMC (blue line), ST vs OMC (red line), and ST vs SE (green line) are shown. In these scenarios, the health state utilities for ST and SE are assumed to become equivalent to OMC after the number of years shown on the x‐axis (base case=5 years). OMC indicates optimal medical care; QALY, quality‐adjusted life‐year; SE, supervised exercise; ST, stenting.
If QOL beyond 18 months was assumed to decrease more slowly for ST than SE, then the ICER for ST versus SE became more favorable than in the base case. It is possible, in fact, to project scenarios in which ST would be preferred under the principle of extended dominance (ie, the effectiveness of ST was greater than that for SE, whereas the ICER for ST versus OMC was lower than the ICER for SE versus OMC). If utilities for the ST and OMC groups, for example, were assumed to equalize at 5 years (as in the base case) but SE and OMC equalized after only 3 years, the ICER for ST versus SE decreased to ≈$51 000 per QALY gained. Alternatively, if there was no decrement in utilities at 5 years after ST and the treatment effect of SE over OMC persisted for less than 49 months, ST would be preferred (Figure 2).
Figure 2.
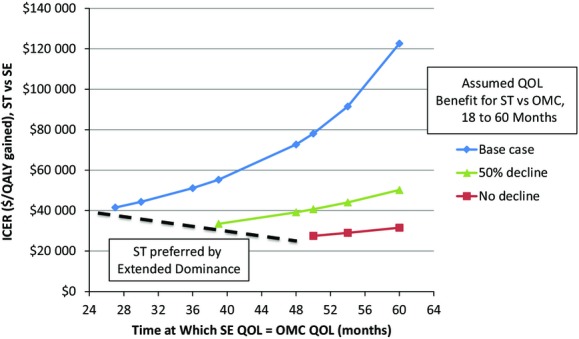
Sensitivity analysis on the relative durability of QOL benefits. ICERs for ST vs SE are plotted on the y‐axis with varying assumptions about the durability of QOL benefit for each relative to OMC. The 3 lines represent 3 scenarios for ST: no decline in QOL over 5 years, a 50% decline, or full decline to the level of OMC. The number of months at which QOL with SE becomes equal to OMC is plotted on the x‐axis. For each ST scenario, a threshold can be defined at which ST is economically preferable to SE based on extended dominance (dashed line). ICERs indicates incremental cost‐effectiveness ratios; OMC, optimal medical care; QALY, quality adjusted life years; QOL, quality of life; SE, supervised exercise; ST, stenting.
When the facility costs of SE were assumed to be $19 per hour (roughly half of our base case estimate), the ICER for SE versus OMC improved to $17 834 per QALY gained at 5 years, whereas the ICER for ST versus SE increased to $152 225 per QALY gained. In contrast, when the facility costs for SE were assumed to be $60 per hour, the ICER for SE versus OMC increased to $30 024 per QALY gained and the ICER for ST versus SE decreased to $94 315 per QALY gained.
The probability that each treatment option is cost‐effective across a range of willingness to pay thresholds, based on probabilistic sensitivity analysis of our base case model, is shown in Figure 3. At very low ICER thresholds (<$20 000 per QALY gained), OMC was the preferred option from a health economic perspective. In the range of ≈$30 000 to $80 000 per QALY gained, there was at least a 60% likelihood that SE would be the preferred option. At thresholds above ≈$120 000 per QALY gained, a slightly greater proportion of model iterations favored ST than SE.
Figure 3.
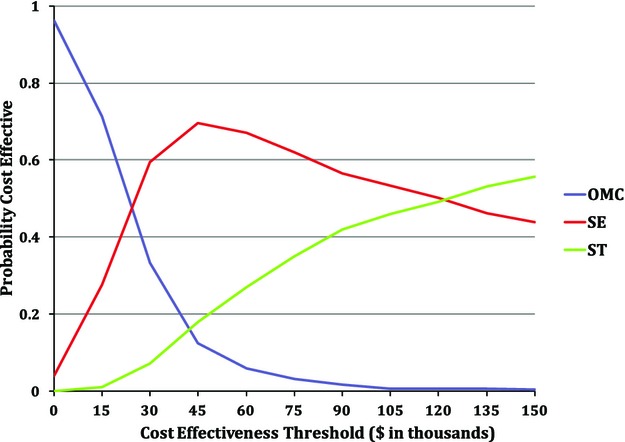
Probabilistic sensitivity analysis. Starting with the base case assumptions, all model parameters were replaced with probabibility distributions that were sampled independently over many model iterations. The probability that each option would be preferred at a given willingness‐to‐pay threshold is plotted across a range of such thresholds. OMC indicates optimal medical care; SE, supervised exercise; ST, stenting.
If patient time costs for participating in the SE intervention were excluded from the base case analysis (as in a payer or health system perspective), the 5‐year ICER for SE versus OMC became slightly more favorable at $13 080 per QALY gained, and the ICER for ST versus SE became less favorable at $177 051 per QALY gained.
Discussion
In this preplanned health economic analysis of the CLEVER trial, we found that both SE and ST increased overall health care costs and improved quality‐adjusted life expectancy relative to OMC, with ICERs for both treatments of <$50 000 per QALY gained relative to OMC over a 5‐year time horizon. The incremental QALY gain for ST relative to SE in the CLEVER trial was small and uncertain over 18 months, whereas ST increased costs relative to SE by about $5000 per patient. Consequently, the cost‐effectiveness of ST relative to SE was uncertain and would most likely represent high value25 only if the treatment effect of ST were more durable than that of SE.
There are few randomized studies assessing the comparative effectiveness and cost‐effectiveness of therapies for claudication. Previous health economic models based on syntheses of mostly nonrandomized data have suggested that endovascular intervention is likely more expensive than exercise therapy, with inconsistent findings regarding effectiveness and cost‐effectiveness.26–27 A previous single‐center trial13 randomized 151 patients with claudication due to iliac or femoropopliteal stenosis to endovascular revascularization or a 24‐week SE program. In a health economic analysis of that trial,28 endovascular intervention was found to be more expensive (by ≈€2300 per patient) than SE and was associated with only slightly higher EQ‐5D scores and, hence, QALYs (point estimate of 0.01 QALY gained). The authors concluded that endovascular intervention was not a good value relative to SE.
CLEVER is the only randomized trial to have directly compared SE and ST with OMC and thus provides the only empirically derived estimates of cost‐effectiveness among these strategies. Consistent with previous reports from the CLEVER trial, our analysis clearly indicates that both SE and ST improve QOL at 6 months14 and that these improvements persist for at least 18 months (Table A1). With relatively conservative assumptions about the projected durability of QOL benefit beyond 18 months, both SE and ST appear to provide these benefits at reasonable incremental costs from a US societal perspective.
Uncertainty remains about whether ST increases QALYs by a meaningful amount relative to SE. In CLEVER, although maximum walking distance was improved to a greater degree with SE than with ST at 6 months, improvement on a number of disease‐specific QOL measures was greater with ST than with SE. A potential interpretation of these findings is that ST relieves claudication either more quickly or completely than SE.29 However, there were no statistically significant differences between generic QOL measures (SF‐36 or EQ‐5D) at 6 or 18 months, as seen in 1 prior randomized study.28
The small difference in QALYs between ST and SE (0.02 at 18 months; 0.04 at 5 years) in our analysis emerges in part because of the assumption that QOL improves more quickly after revascularization than with exercise. Although early QOL data were not assessed in CLEVER, we believe this assumption is justifiable, given that a previous randomized trial reported a significantly higher clinical success rate (defined as a ≥1‐step improvement in Rutherford scale after treadmill walking) for ST versus SE within 1 week after initiation of treatment13 and that a recently reported study found >1‐month improvements in QOL for the combination of ST plus SE compared with SE alone.30
Although ST may improve QOL to a greater extent than SE, our analysis suggests that the cost‐effectiveness of this approach is uncertain. Although the ICER for ST versus SE was >$100 000 per QALY in our base case analysis, the ICER clearly would be more favorable if ST provided a more durable treatment effect than SE. Indeed, our model suggests that with a difference of >1 year in the duration of QOL benefit, the ICER for ST versus SE would fall well below $100 000 per QALY gained. Additional factors suggest that ST might provide reasonable or even high value to relative to SE: improved disease‐specific QOL with ST; the potential preference on the part of patients for more rapidly acting treatment effects; the results of our probabilistic sensitivity analysis, which indicates significant uncertainty regarding the preferred strategy at higher willingness‐to‐pay thresholds; and the possibility that the trial, by requiring patients to accept randomization of their treatments, systematically enrolled patients with weaker treatment preferences than might be found in the general population of patients with claudication.
Our findings have important implications for coverage and reimbursement policies. Peripheral arterial ST in patients with claudication is widely available. At present, the Medicare program covers cardiac rehabilitation programs nationally following acute myocardial infarction or coronary artery bypass grafting and recently extended coverage to patients with chronic heart failure, but Medicare has no national coverage policy establishing access to SE programs for claudication. In the current analysis, we found that SE would be cost‐effective relative to OMC even if the facility costs were 50% greater than the current reimbursement for cardiac rehabilitation with ECG monitoring—and this is before taking into account that a substantial portion of the intervention's “cost” is borne by the patients (in terms of their time). Given the increased expense and marginal benefits of ST relative to SE, there would appear to be no rational justification for covering ST but not SE for the treatment of claudication.
Our analysis should be interpreted with several important limitations in mind. CLEVER enrolled a small sample of patients and lacked the statistical power to detect small differences between groups, a fact that particularly hinders the comparisons between ST and SE. Many more patients were screened for CLEVER than enrolled. Consequently, the enrolled patients may not well represent the full universe of patients with aortoiliac disease and claudication, and the excellent results with SE observed in the trial may not be easy to replicate in other settings. Other patients who declined to participate in the study could have had different pre‐existing treatment preferences or symptomatology, and this could have affected our QOL results. QOL was measured at only 3 time points that were spaced rather widely apart; therefore, the time course of changes in QOL, particularly early after intervention, was not well defined. Although CLEVER had a longer follow‐up duration than most previous studies in this area, the assessment of cost‐effectiveness for the interventions under study requires projection over a longer time horizon than the trial, and that introduces further uncertainty into the results. As shown, differences in the durability of ST relative to SE could substantially alter the base case cost‐effectiveness estimates.
Finally, although by design the CLEVER trial evaluated SE and ST as mutually exclusive treatments for claudication due to PAD, ST and SE can certainly be combined in clinical practice. Recently reported and perhaps future studies may help clarify the potentially synergistic effects of combining SE and ST.30
Conclusions
In this 18‐month randomized trial, we found that both SE and ST are economically attractive by conventional US standards relative to OMC for the treatment of claudication due to aortoiliac stenosis. Because ST is more costly and provides marginal additional benefit over SE, SE may provide better value, at least in the short term. Longer term results are uncertain. In light of these findings, we believe that treatment guidelines and healthcare policy should be adjusted to ensure that patients have ready access to SE as an option for the treatment of aortoiliac claudication.
Appendix
A1.
Quality‐of‐Life End Points at Baseline and 18 Months
| Measure | OMC (n=20) (Mean±SD) | SE (n=37) (Mean±SD) | ST (n=41) (Mean±SD) | Difference [95% CI], (P Value)* | ||
|---|---|---|---|---|---|---|
| SE vs OMC | ST vs OMC | ST vs SE | ||||
| SF‐12 physical | ||||||
| Baseline | 31.6±10.5 (20) | 32.5±9.0 (37) | 34.3±9.3 (41) | 4.6 [0.2 to 9.0], (P=0.02) |
3.5 [−1.2 to 8.1], (P=0.07) |
1.1 [−3.0 to 5.2], (P=0.52) |
| 18 months | 30.2±7.2 (19) | 36.7±9.9 (37) | 36.9±9.4 (40) | |||
| Baseline to 18‐month change | −0.4±7.7 (19) | 4.2±8.2 (37) | 3.0±10.1 (40) | |||
| WIQ pain severity | ||||||
| Baseline | 28.4±20.8 (20) | 31.8±26.1 (37) | 33.7±27.5 (41) | 18.9 [−1.7 to 39.5], (P=0.03) |
20.6 [−0.4 to 41.6], (P=0.03) |
−1.6 [−20.3 to 17.1], (P=0.81) |
| 18 months | 42.2±24.8 (16) | 65.4±26.8 (34) | 71.7±28.6 (38) | |||
| Baseline to 18‐month change | 15.6±32.8 (16) | 34.6±38.4 (34) | 36.2±42.6 (38) | |||
| WIQ walking distance | ||||||
| Baseline | 22.9±26.8 (20) | 13.9±12.0 (37) | 17.9±15.5 (41) | 34.0 [17.1 to 50.8], (P<0.001) |
38.6 [21.3 to 55.9], (P<0.001) |
−4.7 [−20.2 to 10.9], (P=0.53) |
| 18 months | 18.7±20.3 (16) | 43.5±33.1 (33) | 52.5±37.0 (38) | |||
| Baseline to 18‐month change | −3.4±26.7 (16) | 30.6±31.1 (33) | 35.3±35.6 (38) | |||
| PAQ physical limitation | ||||||
| Baseline | 32.9±27.0 (20) | 31.5±18.0 (35) | 30.5±19.5 (41) | 10.3 [−3.4 to 24.0], (P=0.12) |
20.5 [5.7 to 35.4], (P=0.01) |
−10.2 [−23.6 to 3.1], (P=0.12) |
| 18 months | 28.5±16.9 (18) | 44.2±24.4 (36) | 53.9±31.4 (40) | |||
| Baseline to 18‐month change | 0.7±22.6 (17) | 11.0±25.4 (34) | 21.3±32.2 (38) | |||
| PAQ symptoms | ||||||
| Baseline | 43.3±19.7 (20) | 40.5±19.4 (37) | 48.2±21.1 (41) | 9.7 [−0.7 to 20.2], (P=0.10) |
17.0 [6.0, to 28.0], (P=0.02) |
−7.3 [−18.9 to 4.3], (P=0.18) |
| 18 months | 49.7±21.3 (19) | 57.0±25.2 (36) | 73.3±26.9 (41) | |||
| Baseline to 18‐month change | 7.9±15.5 (19) | 17.6±24.0 (36) | 24.9±27.9 (41) | |||
| PAQ quality of life | ||||||
| Baseline | 44.4±25.5 (20) | 44.0±19.7 (36) | 46.1±19.4 (41) | 6.6 [−7.3 to 20.5], (P=0.34) |
19.7 [5.3 to 34.2], (P=0.01) |
−13.1 [−25.3 to −1.0], (P=0.02) |
| 18 months | 48.4±27.5 (18) | 56.5±24.8 (36) | 71.0±25.5 (41) | |||
| Baseline to 18‐month change | 5.8±24.5 (18) | 12.4±24.5 (35) | 25.5±29.5 (41) | |||
| PAQ summary | ||||||
| Baseline | 45.6±23.3 (20) | 43.6±16.4 (37) | 45.3±18.3 (41) | 13.5 [3.1 to 23.9], (P=0.02) |
23.8 [12.0 to 35.6], (P=0.001) |
−10.3 [−21.3 to 0.7], (P=0.05) |
| 18 months | 43.5±21.6 (19) | 56.0±22.0 (37) | 68.2±24.8 (41) | |||
| Baseline to 18‐month change | −1.1±17.7 (19) | 12.4±20.8 (37) | 22.7±28.4 (41) | |||
Results are shown using all available data. P values are based on analysis of covariance adjusting for study center, baseline cilostazol use, and baseline value of the end point. For each of these scales, higher scores indicate fewer symptoms or better quality of life. OMC indicates optimal medical care; PAQ, peripheral artery questionnaire; SE, supervised exercise; SF‐12, short‐form 12; ST, stent; WIQ, walking impairment questionnaire.
P values for change from baseline to 18 months are based on ANCOVA with adjustment for study center, baseline cilostazol use, and baseline score.
Sources of Funding
The Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study was sponsored primarily by the National Heart Lung and Blood Institute (grants HL77221 and HL081656) and received financial support from Cordis/Johnson & Johnson, eV3, and Boston Scientific. Otsuka America Inc donated cilostazol for all study participants throughout the study. Omron Healthcare Inc donated pedometers. Krames Staywell donated print materials for study participants on exercise and diet.
Disclosures
Dr Murphy reports research grant support from Abbott Vascular, Cordis/Johnson&Johnson, and Otsuka Pharmaceuticals. Dr Cutlip reports research contract or grant support (paid to institution) from Medtronic, Boston Scientific and Abbott Vascular. Dr Cohen reports research grant support from Medtronic, Boston Scientific, Abbott Vascular, and Cardiovascular Systems, Inc and is a consultant to Medtronic, Abbott Vascular, and Cardiovascular Systems, Inc. Dr Reynolds is a consultant to Medtronic, Inc. Dr Hirsch reports research grants from Abbott Vascular, Aastrom Biosciences, Viromed, and AstraZeneca and is a consultant to Novartis and Merck. The remaining authors report no disclosures.
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013; 382:1329-1340. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013; 127:e6-e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criqui MH, Fronek A, Barrett‐Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985; 71:510-515. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WRC, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor JLM, White CJ, White J, White RA, Antman EM, Smith JSC, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease). J Am Coll Cardiol. 2006; 47:e1-e192. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008; 13:209-215. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010; 3:642-651. [DOI] [PubMed] [Google Scholar]
- 7.Regensteiner JG, Ware JE, McCarthy WJ, Zhang P, Forbes WP, Heckman J, Hiatt WR. Effect of cilostazol on treadmill walking, community‐based walking ability, and health‐related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta‐analysis of six randomized controlled trials. J Am Geriatr Soc. 2002; 50:1939-1946. [DOI] [PubMed] [Google Scholar]
- 8.Murphy TP, Soares GM, Kim HM, Ahn SH, Haas RA. Quality of life and exercise performance after aortoiliac stent placement for claudication. J Vasc Interv Radiol. 2005; 16:947-953. [DOI] [PubMed] [Google Scholar]
- 9.Safley DM, House JA, Laster SB, Daniel WC, Spertus JA, Marso SP. Quantifying improvement in symptoms, functioning, and quality of life after peripheral endovascular revascularization. Circulation. 2007; 115:569-575. [DOI] [PubMed] [Google Scholar]
- 10.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996; 23:104-115. [DOI] [PubMed] [Google Scholar]
- 11.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002; 347:1941-1951. [DOI] [PubMed] [Google Scholar]
- 12.Mazari FA, Gulati S, Rahman MN, Lee HL, Mehta TA, McCollum PT, Chetter IC. Early outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg. 2010; 24:69-79. [DOI] [PubMed] [Google Scholar]
- 13.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital‐based exercise training–randomized controlled trial. Radiology. 2009; 250:586-595. [DOI] [PubMed] [Google Scholar]
- 14.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman J, Treat‐Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas U, Hirsch AT. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six‐month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012; 125:130-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bronas UG, Hirsch AT, Murphy T, Badenhop D, Collins TC, Ehrman JK, Ershow AG, Lewis B, Treat‐Jacobson DJ, Walsh ME, Oldenburg N, Regensteiner JG. Design of the multicenter standardized supervised exercise training intervention for the claudication: exercise vs endoluminal revascularization (CLEVER) study. Vasc Med. 2009; 14:313-321. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost‐Effectiveness in Health and Medicine. 1996New York, NY: Oxford University Press [Google Scholar]
- 17.The EuroQol Group. Euro‐Qol: a new facility for measurement of health‐related quality of life. Health Policy. 1990; 16:199-208. [DOI] [PubMed] [Google Scholar]
- 18.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ‐5D health states: development and testing of the D1 valuation model. Med Care. 2005; 43:203-220. [DOI] [PubMed] [Google Scholar]
- 19.Cohen DJ, Krumholz HM, Sukin CA, Ho KK, Siegrist RB, Cleman M, Heuser RR, Brinker JA, Moses JW, Savage MP, Detre K, Leon MB, Baim DS. Stent Restenosis Study Investigators. In‐hospital and one‐year economic outcomes after coronary stenting or balloon angioplasty. Results from a randomized clinical trial. Circulation. 1995; 92:2480-2487. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Bureau of Labor Statistics. National compensation survey: occupational earnings in the United States, 2010. 2011;Bulletin 2753.
- 21.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost‐effectiveness in health and medicine. JAMA. 1996; 276:1253-1258. [PubMed] [Google Scholar]
- 22.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Rother J, Smith SC, Jr, Salette G, Contant CF, Massaro JM, Steg PG. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010; 304:1350-1357. [DOI] [PubMed] [Google Scholar]
- 23.Critchfield GC, Willard KE. Probabilistic analysis of decision trees using Monte Carlo simulation. Med Decis Making. 1986; 6:85-92. [DOI] [PubMed] [Google Scholar]
- 24.Lothgren M, Zethraeus N. Definition, interpretation and calculation of cost‐effectiveness acceptability curves. Health Econ. 2000; 9:623-630. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63:2304-2322. [DOI] [PubMed] [Google Scholar]
- 26.de Vries SO, Visser K, de Vries JA, Wong JB, Donaldson MC, Hunink MG. Intermittent claudication: cost‐effectiveness of revascularization versus exercise therapy. Radiology. 2002; 222:25-36. [DOI] [PubMed] [Google Scholar]
- 27.Treesak C, Kasemsup V, Treat‐Jacobson D, Nyman JA, Hirsch AT. Cost‐effectiveness of exercise training to improve claudication symptoms in patients with peripheral arterial disease. Vasc Med. 2004; 9:279-285. [DOI] [PubMed] [Google Scholar]
- 28.Spronk S, Bosch JL, den Hoed PT, Veen HF, Pattynama PM, Hunink MG. Cost‐effectiveness of endovascular revascularization compared to supervised hospital‐based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg. 2008; 48:1472-1480. [DOI] [PubMed] [Google Scholar]
- 29.Murphy TP, Reynolds MR, Cohen DJ, Regensteiner JG, Massaro JM, Cutlip DE, Mohler ER, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Hirsch AT. Correlation of patient‐reported symptom outcomes and treadmill test outcomes after treatment for aortoiliac claudication. J Vasc Interv Radiol. 2013; 24:1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fakhry F; ERASE Trial Investigators. Results from the endovascular revascularization and supervised exercise for claudication study (abstract). Presented November 2013 at American Heart Association Scientific Sessions. Dallas, TX. [Google Scholar]


