Abstract
Background
Hypertrophic cardiomyopathy (HCM) is a primary myopathic process in which regional left ventricular dysfunction may exist without overt global left ventricular dysfunction. In obstructive HCM patients who underwent surgical myectomy (SM), we sought to determine if there is a significant association between echocardiographic longitudinal strain, histopathology, and in vitro myocardial performance (resting tension and developed tension) of the surgical specimen.
Methods and Results
HCM patients (n=122, 54±14 years, 54% men) undergoing SM were prospectively recruited. Longitudinal systolic strain and diastolic strain rates were measured at that basal septum (partially removed at SM) by using velocity vector imaging on preoperative echocardiography. Semiquantitative histopathologic grading of myocyte disarray and fibrosis and in vitro measurements of resting tension and developed tension were made in septal tissue obtained at SM. Mean basal septal systolic strain and diastolic strain rate were −8.3±5% and 0.62±0.4/s, while mild or greater degree of myocyte disarray and interstitial fibrosis were present in 85% and 87%, respectively. Mean resting tension and developed tension were 2.8±1 and 1.4±0.8 g/mm2. On regression analysis, basal septal systolic strain, diastolic strain rate, disarray, and fibrosis were associated with developed tension (β=0.19, 0.20, −0.33, and −0.40, respectively, all P<0.01) and resting tension (β=0.21, 0.22, −0.25, and −0.28, respectively, all P<0.01).
Conclusion
In obstructive HCM patients who underwent SM, left ventricular mechanics (echocardiographic longitudinal systolic strain and diastolic strain rates), assessed at the basal septum (myocardium removed during myectomy) and histopathologic findings characteristic for HCM (disarray and fibrosis) were significantly associated with in vitro myocardial resting and developed contractile performance.
Keywords: contractile performance, HCM, histopathology, strain
Introduction
Hypertrophic cardiomyopathy (HCM) is characterized by variable clinical expression ranging from symptomatic left ventricular (LV) outflow tract (LVOT) obstruction to progressive heart failure and sudden death, with a vast majority of subjects having near normal life expectancy.1 It is increasingly understood that while the phenotypic expression of hypertrophy, obstruction, and diastolic dysfunction occur later in HCM, regional LV dysfunction predates overt morphologic changes.2–3 Histopathologically, it is characterized by myocyte hypertrophy, disarray, interstitial fibrosis, and small intracoronary arteriole dysplasia (SICAD).4–6 Multiple previous studies have elucidated the characteristic histopathologic abnormalities (albeit in hearts of patients with sudden cardiac death6–8) and demonstrated regional myocardial dysfunction in HCM patients.2–3,2–11 In a recent observational study, we have reported on association between regional LV function (measured at the basal septum by using speckle tracking echocardiography12) and histopathology (of the basal septal specimen) in living HCM patients who underwent surgical myectomy for relief of LVOT obstruction.13
The basal septal tissue, removed at the time of surgical myectomy, provides a unique opportunity to obtained detailed insight into myocardial contractile performance, similar to what has described previously in other models of heart failure, where investigators measured resting and developed tension (RT and DT, respectively).14–16 In vitro analysis of the myectomy specimen can potentially be useful as the associations can be determined in a living patient, as opposed to inferences from a postmortem study. For the current study, we hypothesized that there would be a significant association between in vivo basal septal strain (measured with preoperative echocardiography), histology, and in vitro myocardial contractile performance in HCM patients. The aim of this study was to determine the clinical and echocardiographic (including longitudinal LV strain) predictors of in vitro myocardial performance in adult HCM patients who underwent surgical myectomy to relieve LVOT obstruction.
Methods
Study Population
This was a prospective study of 122 patients with documented obstructive HCM (refractory to maximal medical therapy) who underwent surgical myectomy at our tertiary care center. These were consecutive patients who provided written informed consent to have their myectomy tissue analyzed, at the time of surgery. All patients had a preoperative echocardiogram performed as part of their clinical workup. The study was approved by the institutional review board. We excluded patients with fixed obstruction (subaortic membrane or aortic stenosis) or those with an alternative diagnosis (eg, Fabry's/amyloidosis). HCM was defined as a hypertrophied and nondilated left ventricle in the absence of another cardiac or systemic disease that could result in a similar magnitude of hypertrophy.17 Baseline clinical and imaging data, along with histopathologic data, were collected. Patients underwent myectomy as previously described.18–19 Subsequently, all histopathologic data were recorded, as described later. Additionally, all myectomy specimens were further analyzed for in vitro muscle performance, as detailed later. Clinical, echocardiographic, histopathologic, and muscle performance data on all patients were available for analysis.
Echocardiography
All patients underwent comprehensive echocardiograms using commercially available instruments (Philips Medical Systems, NA, Bothell, Wash and Siemens Medical Solution USA, Inc). End‐diastolic interventricular septal, posterior wall thickness, LV ejection fraction, and volumes were measured according to guidelines.20 Resting LVOT peak velocity was measured by using continuous‐wave Doppler echocardiography, and resting LVOT pressure gradient was estimated by using a simplified Bernoulli equation.21 In patients with resting LVOT gradients <30 mm Hg, provocative maneuvers, including Valsalva, amyl nitrite, and exercise echocardiography, were also used to measure a provocable LVOT gradient. Maximal LVOT gradient was recorded and defined as the highest recorded gradient (either resting or provoked) in a given patient. Resting peak systolic pressure was estimated as resting LVOT gradient plus systolic blood pressure (mm Hg). Resting mitral regurgitation (none to severe) and diastolic grades were assessed according to guidelines.22–23
Additionally, by using speckle tracking echocardiography, longitudinal systolic strain and strain rates (systolic and early diastolic) were measured in 2‐ and 4‐chamber views (40 frames/s, Velocity Vector Imaging; Siemens).9–10 Endocardial borders were manually traced in end systole, with the software automatically tracking myocardial deformation. If poor tracking occurred, endocardial borders were readjusted manually until satisfactory tracking was achieved. Strain curves for each segment (summed average strain) were recorded. These measurements were made at apical, mid, and basal levels of visualized LV walls. However, for the purpose of comparison with histopathologic and in vitro myocardial contractile performance data, only the values from the basal septum (at the site of myectomy) were used (Figure 1A through 1C). We wanted to understand the association of regional LV mechanics during both systole and diastole. As a result, we recorded the following parameters: longitudinal systolic strain and diastolic strain rates. Reproducibility of such analyses in HCM patients undergoing myectomy has been previously reported by our group.13 All echocardiographic analysis was performed blinded to in vitro analyses described next.
Figure 1.
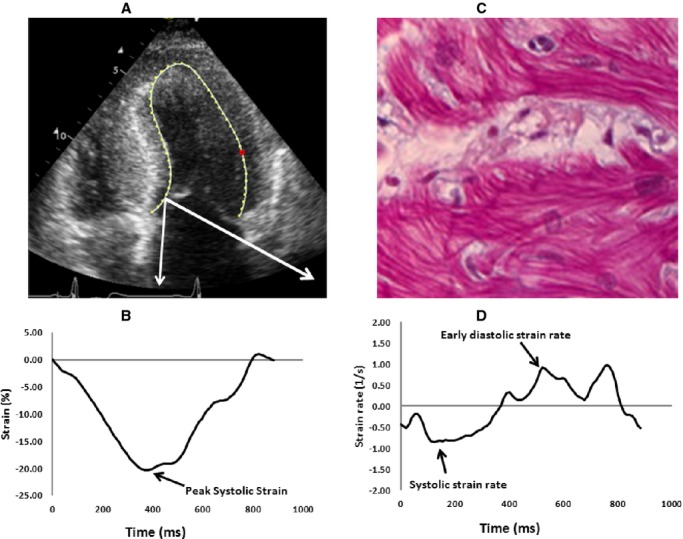
A, FOur‐chamber echocardiographic view of a patient with hypertrophic cardiomyopathy. The white arrows represent the segment removed at myectomy, where the strain measurements were made. B, Systolic strain measurement of the septal segment removed at surgical myectomy (dark arrow). C, Early diastolic strain rate measurement of the septal segment removed at surgical myectomy (dark arrow). D, Histopathologic appearance of myocyte hypertrophy, disarray, and interstitial fibrosis.
Contractile Performance of Isolated Muscles
Myectomy specimens, obtained at the time of surgery, were transported to the laboratory in cold cardioplegia, as previously described.24–25 Once in the laboratory, tissue was placed in room‐temperature, oxygenated Krebs‐Henseleit buffer (composition [in mmol/L]: NaCl 100.0, KCl 4.0, MgSO4 1.5, NaHCO3 20.0, NaH2PO4 1.5, NaC2H3O2 20.0, glucose 10.0, ascorbic acid 0.1, and CaCl2 2.5 and insulin 5.0 IU/L) for dissection. Long, thin trabecular muscles were dissected from the LV surface of the septum. Isometric muscle contraction studies were performed as we have previously described at 37°C, stimulation rate 1.0 Hz, duration 5 ms, and voltage 20% above threshold24–25 (Figure 2). When the muscle was initially mounted in the bath, it was mounted at a slack length, adjusted to a resting tension of ≈0.5 g. This allowed the muscle to rest comfortably and not be stretched as it was allowed to reequilibrate the ion gradients and warm up to 37°C in the bath. After ≈45 to 60 minutes, the length of the muscle was increased in 0.1‐mm increments, using a micrometer attached to the hook that held the top of the muscle. Each time the muscle was lengthened, it produced a slightly greater DT. We continued to increase the length until we began to observe that DT did not change significantly. The point where DT remained constant through 2 additions of length was defined as Lmax, where the muscle rested for the remainder of the experiment. It is a physiologically defined length that is used to normalize muscle performance between various preparations, since contraction is length dependent in cardiac muscle. We recorded the following contractile parameters: RT, DT, time to peak tension (TPT), time to half‐relaxation (THR), maximal rate of tension rise (+dT/dt), and maximal rate of tension fall (−dT/dt). At the end of the experiment, muscle cross‐sectional area was recorded, and tension values were normalized for muscle size.
Figure 2.
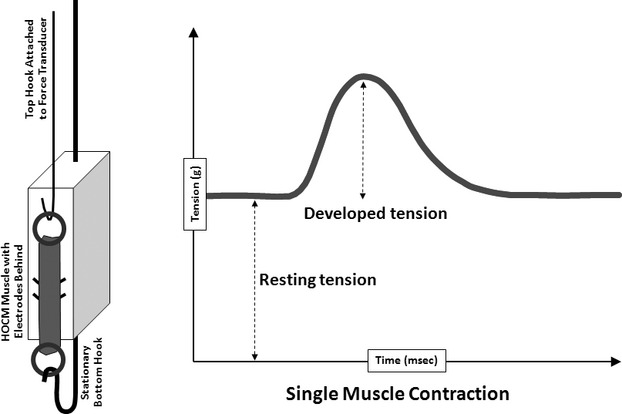
Experimental design and contractility data for cardiac muscle. Shown on the left is a schematic of a left ventricular trabecular muscle stretched between a stationary bottom hook and a top hook attached to a force transducer. The muscle is stimulated to contract by direct‐contact electrodes located behind the muscle. Shown on the right is a recording of a single cardiac muscle contraction. Resting tension (RT) is reflective of the muscle's innate stiffness. Developed tension (DT) is the amount of force generated by the muscle upon stimulation.
Histopathology of the Myectomy Specimen
All specimens were fixed in 10% formalin. Formalin fixed tissues were processed for paraffin embedding. Initially, sectioning was done along the longitudinal myocardial fascicles to assess for myocyte disarray. All sections were stained with hematoxylin–eosin and Movat pentachrome stains. Histologic sections were evaluated for the presence and extent of myocyte hypertrophy, disarray, interstitial fibrosis, and SICAD (Figure 1D). Myocyte hypertrophy and disarray were classified as none, mild (1% to 25%), moderate (26% to 50%), and severe (> 50%).6 SICAD was classified as none (absent to 1 vessel involvement), mild (1% to 25% of arterial involvement), moderate (26% to 50%), and severe (>50%).6,24 Similarly, myocardial fibrosis was assessed as normal, mild, moderate, and severe as described elsewhere.6 Reproducibility of such analyses in HCM patients undergoing myectomy has been previously reported.13 Histopathologic analysis was blinded from all other analyses.
Statistics
Baseline variables are summarized for the group. Continuous variables are expressed as a mean and SD. Categorical data are presented using percentage frequencies. Differences between groups were determined by ANOVA for continuous variables (Mann–Whitney test for nonparametric variables) and χ2 test for categorical variables. Correlation was assessed between continuous variables. In addition, univariable and stepwise multivariable regression analyses (using P<0.1 as entry cutoff) were performed to test association between dependent variables and various potential predictors. A value of P<0.05 was considered significant. Statistical analysis was performed with SPSS version 11.5.
Results
The clinical and echocardiographic data of the study population (n=122) are shown in Table 1. Because all patients underwent surgical myectomy for symptomatic improvement, maximal basal septal thickness was severely increased (2.2±0.6 cm) and all patients had severe maximal LVOT obstruction (98±32 mm Hg). Basal septal systolic strain (−8.3±5%) and basal septal diastolic strain rate (0.62±0.4/s) were also significantly abnormal, compared with normal values reported by using velocity vector imaging (Table 1). There was no correlation between maximal basal septal thickness and septal systolic strain (r=0.004, P=0.9) or septal diastolic strain rate (r=−0.01, P=0.9).
Table 1.
Clinical and Echocardiographic Data of the Study Population
| N=122 | |
|---|---|
| Clinical | |
| Age, y | 54±14 |
| Male sex | 66 (54%) |
| Hypertension | 42 (34%) |
| Coronary artery disease | 24 (20%) |
| Syncope | 21 (17%) |
| New York Heart Association Class | |
| II | 62 (51%) |
| III | 58 (48%) |
| IV | 2 (1.6%) |
| Family history of hypertrophic cardiomyopathy | 33 (27%) |
| Internal cardioverter‐defibrillator | 9 (7%) |
| Atrial fibrillation | 25 (21%) |
| β‐Blockers | 110 (90%) |
| Calcium channel blockers | 19 (16%) |
| Echocardiographic data | |
| LV ejection fraction, % | 62±5 |
| Maximal septal thickness, cm | 2.2±0.6 |
| Left atrial dimensions, cm | 4.5±0.8 |
| LV end‐diastolic dimensions, cm | 4±0.6 |
| LV end‐systolic dimensions, cm | 2.3±0.5 |
| Resting mitral regurgitation | |
| None or trivial | 15 (12%) |
| 1+ | 52 (43%) |
| II+ | 30 (25%) |
| III+ | 24 (20%) |
| IV+ | 1 (1%) |
| Diastolic function | |
| Impaired relaxation | 55 (45%) |
| Pseudonormal | 53 (43%) |
| Restrictive filling | 14 (12%) |
| Resting LVOT gradient | 67±43 mm Hg |
| Maximal LVOT gradient | 98±32 mm Hg |
| Resting LV peak systolic pressure | 191±65 mm Hg |
| Basal septal systolic strain, % | −8.3±5 (normal −17.1±5) |
| Basal septal diastolic strain rate, 1/s | 0.62±0.4 (normal 1.1±0.6) |
Results of histopathologic and muscle contractile parameters on the myectomy specimen are shown in Table 2. The majority of patients had at least mild myocyte disarray (85%), interstitial fibrosis (87%), and SICAD (63%) on histologic examination. Associations between various muscle contractile and histopathologic parameters (of the tissue removed at myectomy) and strain parameters (portion of the basal septum corresponding to tissue removed during myectomy) are shown in Table 3. Association of septal systolic strain and septal diastolic strain rate (Figure 3) was more significant with DT (β=−0.56 and 0.48, respectively) than with RT (β=−0.29 and 0.27, respectively). Similarly, association of myocyte disarray and interstitial fibrosis rate was more significant with DT (β=−0.74 and −0.77, respectively) than with RT (β=−0.26 and −0.37, respectively). There were significant differences in basal septal longitudinal systolic strain and early diastolic strain rate among the study population, separated on the basis of worsening degree of myocyte disarray (Figure 4A and 4B) and interstitial fibrosis (Figure 5A and 5B), as seen on histologic examination (all P<0.01). Similarly, there were significant differences in both RT and DT among the study population, separated on the basis of worsening degree of myocyte disarray (Figure 4C and 4D) and interstitial fibrosis (Figure 5C and 5D), on histologic examination (all P<0.01). Associations of various muscle contractile parameters with each other are shown in the Table 4.
Table 2.
Histopathologic and In Vitro Baseline Contractile Parameters in the Myectomy Specimens of the Study Population (N=122)
| Variable | |
|---|---|
| Histopathology | |
| Myocyte disarray | |
| None to minimal | 18 (15%) |
| Mild | 46 (38%) |
| Moderate | 42 (34%) |
| Severe | 16 (13%) |
| Interstitial fibrosis | |
| None to minimal | 16 (13%) |
| Mild | 61 (50%) |
| Moderate | 31 (25%) |
| Severe | 14 (12%) |
| SICAD | |
| None | 43 (37%) |
| Mild | 57 (47%) |
| Moderate | 20 (16%) |
| Severe | 2 (2%) |
| Baseline contractile parameters at 37°C and 1.0 Hz | |
| RT, g/mm2 | 2.8±1.1 (normal 2.7±1.1) |
| DT, g/mm2 | 1.4±0.8 (normal 1.6±0.8) |
| TPT, ms | 210±30 (normal 178±35) |
| THR, ms | 150±20 (normal 134±19) |
| +dT/dt, g/mm2 per second | 9.1±4.1 (normal 12.5±5) |
| −dT/dt, g/mm2 per second | 8.2±3.9 (normal 10.9±4) |
Normal values for contractile parameters (±SD) were derived from nonfailing normal hearts (obtained from healthy organ donors deemed unsuitable for transplantation but who had no history of cardiac disease). Values given as n (%) or mean±SD. Please see reference 14. SICAD, small intramural coronary arteriole dysplasia; RT, resting tension; DT, developed tension; THR, time to half relaxation; TPT, time to peak tension; +dT/dt indicates maximum rate of tension rise; –dT/dt, maximum rate of tension fall.
Table 3.
Association Between Left Ventricular Basal Septal Strain, Various Markers of In Vitro Myocardial Contractile Performance, and Histology of the Septal Specimen Removed at Myectomy (N=122)
| β | |||||
|---|---|---|---|---|---|
| Septal Systolic Strain | Septal Diastolic SR | Disarray | Interstitial Fibrosis | SICAD | |
| RT | −0.29* | 0.27* | −0.26* | −0.37* | −0.21* |
| DT | −0.56* | 0.48* | −0.74* | −0.77* | −0.44* |
| TPT | −0.25* | 0.13 | −0.52* | −0.42* | −0.34* |
| THR | −0.07 | 0.15 | −0.07 | 0.05 | 0.09 |
| +dT/dt | −0.55* | 0.48* | −0.67* | −0.75* | −0.41* |
| −dT/dt | −0.57* | 0.44* | −0.70* | −0.76* | −0.40* |
SR indicates strain rate; SICAD, small intramural coronary arteriole dysplasia; DT, developed tension; RT, resting tension; TPT, time to peak tension; THR, time to half relaxation; +dT/dt indicates maximum rate of tension rise; –dT/dt, maximum rate of tension fall.
P<0.05.
Figure 3.
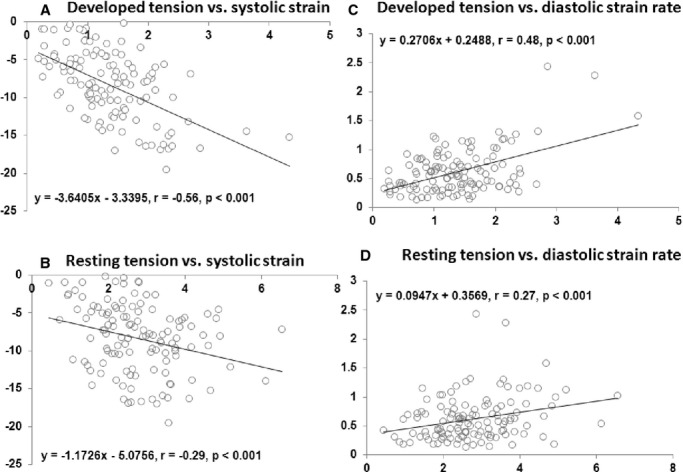
Associations between various longitudinal strain measurements at basal septum and muscle contractile performance. A, Longitudinal systolic strain vs developed tension (DT). B, Longitudinal systolic strain vs resting tension (RT). C, Longitudinal diastolic strain rate vs DT. D, Longitudinal diastolic strain rate vs RT.
Figure 4.
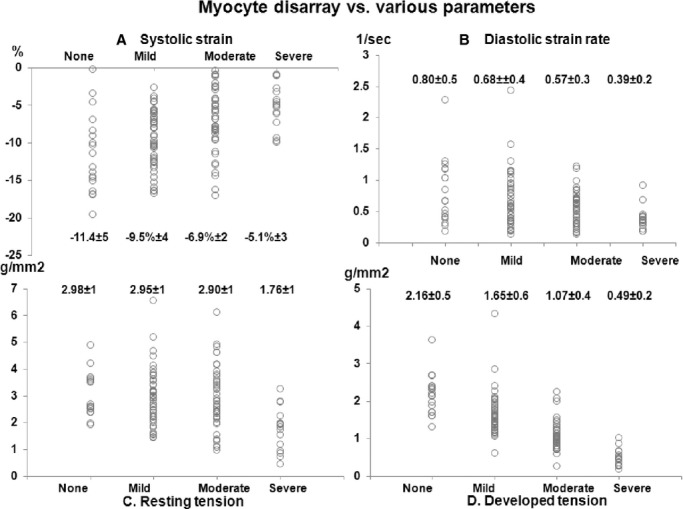
Associations between different grades of myocyte disarray on histopathology of the myectomy specimen and various parameters. A, Myocyte disarray vs longitudinal systolic strain. B, Myocyte disarray vs longitudinal diastolic strain rate. C, Myocyte disarray vs resting tension (RT). D, Myocyte disarray vs developed tension (DT). All P<0.001.
Figure 5.
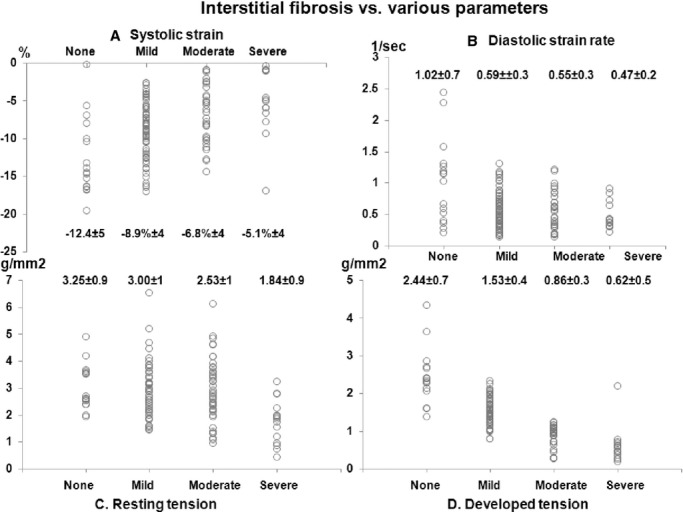
Associations between different grades of interstitial fibrosis on histopathology of the myectomy specimen and various parameters. A, Interstitial fibrosis vs longitudinal systolic strain. B, Interstitial fibrosis vs longitudinal diastolic strain rate. C, Interstitial fibrosis vs resting tension (RT). D, Interstitial fibrosis vs developed tension (DT). All P<0.001.
Table 4.
Association Between Various In Vitro Muscle Contractile Parameters (N=122)
| β | ||||||
|---|---|---|---|---|---|---|
| RT | DT | TPT | THR | +dT/dt | −dT/dt | |
| RT | — | 0.35* | 0.09 | −0.01 | 0.38* | 0.37* |
| DT | 0.35* | — | 0.50* | −0.02 | 0.95* | 0.95* |
| TPT | 0.09 | 0.50* | — | 0.1 | 0.30* | 0.41* |
| THR | −0.01 | −0.02 | 0.1 | — | 0.07 | 0.16 |
| +dT/dt | 0.38* | 0.95* | 0.41* | 0.16 | — | 0.96* |
| −dT/dt | 0.37* | 0.95* | 0.41* | 0.16 | 0.96* | — |
RT indicates resting tension; DT, developed tension; TPT, time to peak tension; THR, time to half relaxation; +dT/dt, maximum rate of tension rise; –dT/dt, maximum rate of tension fall.
P<0.05.
Finally, we tested the association between relevant baseline myocardial contractile parameters (DT and RT) and various potential clinical, echocardiographic, and histopathologic predictors, by using univariable and stepwise multivariable regression analyses. Septal systolic strain (β=−0.21) and septal diastolic strain rate (β=0.22), along with degree of myocyte disarray (β=−0.25) and interstitial fibrosis (β=−0.28), were significantly associated with RT, independent of other clinical and imaging parameters (all P<0.01, Table 5). Similarly, septal systolic strain (β=−0.19) and septal diastolic strain rate (β=0.20), along with degree of myocyte disarray (β=−0.33) and interstitial fibrosis (β=−0.40), were significantly associated with DT, independent of other clinical and imaging parameters (all P<0.01, Table 6).
Table 5.
Regression Analysis Demonstrating the Association Between Resting Tension and Various Potential Predictors (N=122)
| UnivariableAnalysis | Stepwise MultivariableAnalysis | |||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Age | −0.20 | 0.02 | ||
| Sex | −0.02 | 0.8 | ||
| Hypertension | −0.03 | 0.8 | ||
| Coronary artery disease | 0.07 | 0.4 | ||
| Betablockers | −0.02 | 0.8 | ||
| LV ejection fraction | 0.12 | 0.2 | ||
| Basal septal thickness | −0.15 | 0.11 | ||
| Resting LVOT gradient | 0.13 | 0.2 | ||
| Maximal LVOT gradient | 0.11 | 0.3 | ||
| Resting LV peak systolic pressure | 0.12 | 0.3 | ||
| Diastolic dysfunction | −0.14 | 0.15 | ||
| Mitral regurgitation | 0.08 | 0.4 | ||
| Left atrial dimension | 0.26 | 0.01 | ||
| LV end‐diastolic dimension | −0.05 | 0.6 | ||
| LV end‐systolic dimension | 0.009 | 0.9 | ||
| Basal septal strain | −0.29 | 0.001 | −0.21 | 0.01 |
| Basal septal diastolic strain rate | 0.27 | 0.003 | 0.22 | 0.01 |
| Degree of myocyte disarray on histologic specimen removed at myectomy | −0.32 | 0.001 | −0.25 | 0.001 |
| Degree of interstitial fibrosis on histologic specimen removed at myectomy | −0.37 | <0.001 | −0.28 | 0.001 |
| Degree of SICAD on histologic specimen removed at myectomy | −0.21 | 0.02 | ||
Degree of small intramural coronary arteriole dysplasia (SICAD) did not remain significant on multivariable analysis. LV indicates left ventricular; LVOT, left ventricular outflow tract.
Table 6.
Regression Analysis Demonstrating the Association Between Developed Tension and Various Potential Predictors (N=122)
| Univariable Analysis | Stepwise MultivariableAnalysis | |||
|---|---|---|---|---|
| β | P Value | β | P Value | |
| Age | −0.10 | 0.3 | ||
| Sex | −0.12 | 0.1 | ||
| Hypertension | −0.14 | 0.1 | ||
| Coronary artery disease | 0.06 | 0.5 | ||
| β‐Blockers | −0.04 | 0.6 | ||
| LV ejection fraction | 0.02 | 0.9 | ||
| Basal septal thickness | −0.05 | 0.6 | ||
| Resting LVOT gradient | 0.03 | 0.8 | ||
| Maximal LVOT gradient | 0.02 | 0.8 | ||
| Resting LV peak systolic pressure | 0.05 | 0.6 | ||
| Mitral regurgitation | −0.05 | 0.6 | ||
| Diastolic dysfunction | −0.13 | 0.2 | ||
| Left atrial dimension | 0.04 | 0.5 | ||
| LV end‐diastolic dimension | −0.07 | 0.5 | ||
| LV end‐systolic dimension | 0.14 | 0.14 | ||
| Basal septal systolic strain | −0.56 | <0.001 | −0.19 | 0.001 |
| Basal septal diastolic strain rate | 0.48 | <0.001 | 0.20 | 0.001 |
| Degree of myocyte disarray on histologic specimen removed at myectomy | −0.74 | <0.001 | −0.33 | <0.001 |
| Degree of interstitial fibrosis on histologic specimen removed at myectomy | −0.77 | <0.001 | −0.40 | <0.001 |
| Degree of SICAD on histologic specimen removed at myectomy | −0.44 | <0.001 | ||
Degree of small intramural coronary arteriole dysplasia (SICAD) did not remain significant on multivariable analysis. LV indicates left ventricular; LVOT, left ventricular outflow tract.
Discussion
In the current prospective study on HCM patients who underwent surgical myectomy, we demonstrate that echocardiographic basal septal longitudinal strain (both systolic and early diastolic) and various histopathologic abnormalities (characteristic of HCM) are associated with myocardial contractile parameters, measured in vitro on the myectomy specimen. This is one of the first studies, to the best of our knowledge in HCM patients, to report associations between in vivo regional myocardial mechanics of the same portion of the basal septum (measured noninvasively by using preoperative speckle tracking echocardiography), histology, and in vitro contractile performance of the septal muscle (removed at myectomy). Unlike previous studies, the current study did not test these associations in the hearts of sudden death patients but rather on myectomy specimens of patients who were alive postoperatively.6–8 To further strengthen the analyses, various histopathologic abnormalities were semiquantitatively graded, which has been demonstrated previously to be comparable to computer‐based quantification and reproducible.25
We did not find any significant associations of DT or RT with standard clinical and echocardiographic parameters of global systolic and diastolic LV function. The likely reason for this is that to demonstrate differences in gross systolic and diastolic parameters, the patient population would have to have a wider phenotypic spectrum (ranging from early asymptomatic to advanced symptomatic). On the other hand, because LV strain assessment is more precise for the determination of subtle changes of systolic and diastolic regional LV function, it would be more plausible to expect significant associations between in vitro muscle contractile performance and LV strain in this relatively homogeneous population. Indeed, we demonstrate that septal systolic strain and diastolic strain rate had a strong association with DT and a modest association with RT. On the other hand, while it makes intuitive sense that LV peak systolic pressure would be associated with LV strain, there was no association between the 2. This was most likely because, to demonstrate such an association, the patient population would have to have a wider spectrum of LV peak systolic pressure (ranging from none to severe LVOT obstruction).
Myocardial strain and strain rate imaging have become important techniques for quantitative assessment of regional LV function. These are typically measured in a semiautomated fashion using speckle tracking echocardiography, which uses frame‐by‐frame tracking of myocardial echodensities (speckles) on 2‐dimensional images.12,26–29 An important advantage of the technique is that these measurements are independent of the angle of image acquisition. Strain refers to change in the length of the tissue with respect to its original length (Figure 1B), while strain rate refers to the speed at which myocardial shortening or lengthening occurs (Figure 1C). We selected systolic strain and diastolic strain rate as functional correlates of regional LV function as previous studies have shown that systolic strain correlates with regional contractility30 while diastolic strain rate is a marker of regional diastolic function and reflects regional myocardial structure.31 We used velocity vector imaging to calculate segmental longitudinal systolic and early diastolic strain rates in 2‐ and 4‐chamber views, by using speckle tracking echocardiography, similar to prior reports.9–10 This technique has been demonstrated to be accurate with similar strain values for normal controls compared with other strain analysis techniques.12,27–29 For the purpose of comparative analysis with histopathology and muscle contractile data, we specifically used longitudinal strain values from the segment of the basal septum removed during myectomy. The mean longitudinal systolic and early diastolic septal strain values in our study population were similar to those published in the literature for HCM patients from the septum, with similar degrees of basal septal hypertrophy.9–10 As 1 would expect, the strain values in our study population were significantly worse than the values published in the literature for normal controls.12,27–29
In this study, we also report detailed histopathologic findings of the analyzed septal tissue. HCM, at a histologic level, is characterized by myocyte disarray, SICAD, and interstitial fibrosis. A previous report, albeit in a postmortem study, has suggested that myocyte disarray is a direct response to functional or structural abnormalities of the mutated sarcomeric protein.6–7,32 Myocyte disarray results in abnormal ventricular architecture that predisposes to worsening regional LV function, reflected by abnormal regional longitudinal LV strain as well as in vitro myocardial contractile performance, especially DT. Indeed, a previous report has also demonstrated an association between myocyte disarray and altered regional strain in transgenic mice.33 Our study is one of the first to translate such findings in humans and further extend to in vitro contractile performance. In the current study, we also observed that LV septal strain worsened with increasing degree of histopathologic fibrosis. Indeed, similar to the current study, previous smaller retrospective observational reports, including a cardiac magnetic resonance study, have demonstrated worse LV strain with increasing degree of myocardial fibrosis, even in the absence of overt systolic LV dysfunction.31,34–35
In isolated isometrically contracting muscle preparations, RT at Lmax is thought to reflect both the relative stiffness of the muscle and the level of diastolic calcium in the muscle cell. Previous reports have suggested that RT is increased in pathologic states; however, these observations were made in failing hearts with severely impaired LVEF and were compared with nonfailing, normal hearts (organ donors whose hearts were unsuitable for transplantation but who had no history of cardiac disease).14–16 Increased RT in such situations has been attributed to increased fibrosis and elevation of diastolic calcium, resulting in lack of complete dissociation of calcium from its myofilament binding sites following contraction. However, in the current study of HCM patients, we observe a paradoxical association between RT and interstitial fibrosis, where, despite the mean RT being higher than normal, there was a weak but significant inverse association between RT and interstitial fibrosis. It is conceivable that the positive correlation seen in failing hearts (based on traditional definition), between increasing fibrosis and RT, may not be seen in HCM hearts because the mechanism of fibrosis is potentially different. Unlike failing hearts (most commonly seen in patients with severe ischemic cardiomyopathy, where development of fibrosis is a result of replacement of normal myocytes with scar tissue due to prior ischemic damage), the potential mechanisms responsible for development of fibrosis in HCM patients are thought to be different. In addition to stress of intracavitary obstruction, small vessel disease, and ischemia,6,36–37 some studies, in mice both and humans, have suggested that there is early activation of pathways involved in fibrosis and collagen deposition (essentially a profibrotic state) in HCM hearts, even before the development of overt LVH.38 An important clinical observation also potentially corroborates our paradoxical finding. Fibrosis, seen in HCM, has different implications, in terms of systolic function, than what is seen in traditional failing hearts. It is well known, based on multiple prior reports, that, despite a high incidence of fibrosis in HCM patients (observed in as many as 60% to 70% patients on cardiac magnetic resonance39–40), the prevalence of LV systolic dysfunction is very low, except for a small proportion of patients who present with late‐stage “burnt out” HCM. In fact, a majority of HCM patients, including those in the current study, have preserved LV systolic function. Also, unlike patients with failing hearts, multiple prior reports have suggested that the long‐term outcomes of typical HCM patients, including symptomatic HCM patients (even those with severe LV hypertrophy, extensive fibrosis, and LVOT obstruction) undergoing surgical myectomy, are excellent.1,19 Also, another important consideration is that, in addition to the passive properties, there appear to be additional factors that affect RT. Indeed, in the current study, there was a significant correlation between DT and RT.
DT, in contrast, reflects the active process of contraction and can be increased by either an increase in calcium influx into the myocardial cell, an increase in release of calcium from the sarcoplasmic reticulum, or an increase in sensitivity of the myofilaments to calcium. Naturally, DT will be affected by myocyte disarray and, to an extent, by fibrosis. In the current study, DT also had an inverse relationship to myocyte disarray and fibrosis.
Limitations
The current study has the following limitations. There is a selection bias as these were symptomatic HCM patients undergoing myectomy, with lack of generalizability to nonobstructive or apical variants. The current study tests associations, not causality. The histopathologic features of HCM are distributed in a diffuse, often patchy manner, which is more likely to affect disease progression, as opposed to localized septal changes. Also, there was significant overlap of strain and resting/developed tension between varying histopathologic grades―hence, the inability to infer histopathologic grade of disarray and fibrosis in any individual patient would be difficult. While there could be multiple potential causes for the overlap, one possible cause is the individual response to peak systolic pressure in the form of LVOT gradient. It is conceivable that in some patients robust contractility may be preserved, while in others contractility may be overmatched by load.
Conclusions
In obstructive HCM patients who undergo surgical myectomy, reduced longitudinal systolic strain and diastolic strain rates, measured in the basal anterior septum, and greater myocyte disarray and interstitial fibrosis are associated with reduced myocardial performance measured in vitro as RT and DT.
Sources of Funding
This study was supported by endowment funds for hypertrophic cardiomyopathy research and research funds from the Kauffman Center for Heart Failure, both within the Heart and Vascular Institute, Cleveland Clinic.
Disclosures
None.
References
- 1.Maron BJ, Casey SA, Poliac LC, Gohman TE, Almquist AK, Aeppli DM. Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA. 1999; 281:650-655. [DOI] [PubMed] [Google Scholar]
- 2.Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, Seidman CE, Solomon SD. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002; 105:2992-2997. [DOI] [PubMed] [Google Scholar]
- 3.Nagueh SF, McFalls J, Meyer D, Hill R, Zoghbi WA, Tam JW, Quinones MA, Roberts R, Marian AJ. Tissue Doppler imaging predicts the development of hypertrophic cardiomyopathy in subjects with subclinical disease. Circulation. 2003; 108:395-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004; 44:412-427. [DOI] [PubMed] [Google Scholar]
- 5.St John Sutton MG, Lie JT, Anderson KR, O'Brien PC, Frye RL. Histopathological specificity of hypertrophic obstructive cardiomyopathy. Myocardial fibre disarray and myocardial fibrosis. Br Heart J. 1980; 44:433-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000; 84:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ. Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol. 2001; 88:275-279. [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986; 8:545-557. [DOI] [PubMed] [Google Scholar]
- 9.Carasso S, Yang H, Woo A, Jamorski M, Wigle ED, Rakowski H. Diastolic myocardial mechanics in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2010; 23:164-171. [DOI] [PubMed] [Google Scholar]
- 10.Carasso S, Yang H, Woo A, Vannan MA, Jamorski M, Wigle ED, Rakowski H. Systolic myocardial mechanics in hypertrophic cardiomyopathy: novel concepts and implications for clinical status. J Am Soc Echocardiogr. 2008; 21:675-683. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura Y, Elliott PM, Virdee MS, Sorajja P, Doi Y, McKenna WJ. Left ventricular diastolic function assessed using Doppler tissue imaging in patients with hypertrophic cardiomyopathy: relation to symptoms and exercise capacity. Heart. 2002; 87:247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marwick TH, Leano RL, Brown J, Sun JP, Hoffmann R, Lysyansky P, Becker M, Thomas JD. Myocardial strain measurement with 2‐dimensional speckle‐tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009; 2:80-84. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Popovic Z, Bhonsale A, Smedira NG, Tan C, Rodriguez ER, Thamilarasan M, Lytle BW, Lever HM, Desai MY. Association between septal strain rate and histopathology in symptomatic hypertrophic cardiomyopathy patients undergoing septal myectomy. Am Heart J. 2013; 166:503-511. [DOI] [PubMed] [Google Scholar]
- 14.Ogletree‐Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta‐adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation. 2001; 104:881-886. [DOI] [PubMed] [Google Scholar]
- 15.Ogletree ML, Sprung J, Moravec CS. Effects of remifentanil on the contractility of failing human heart muscle. J Cardiothorac Vasc Anesth. 2005; 19:763-767. [DOI] [PubMed] [Google Scholar]
- 16.Sprung J, Ogletree‐Hughes ML, McConnell BK, Zakhary DR, Smolsky SM, Moravec CS. The effects of propofol on the contractility of failing and nonfailing human heart muscles. Anesth Analg. 2001; 93:550-559. [DOI] [PubMed] [Google Scholar]
- 17.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011; 124:e783-e831. [DOI] [PubMed] [Google Scholar]
- 18.Smedira NG, Lytle BW, Lever HM, Rajeswaran J, Krishnaswamy G, Kaple RK, Dolney DO, Blackstone EH. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008; 85:127-133. [DOI] [PubMed] [Google Scholar]
- 19.Desai MY, Bhonsale A, Smedira NG, Naji P, Thamilarasan M, Lytle BW, Lever HM. Predictors of long‐term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013; 128:209-216. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440-1463. [DOI] [PubMed] [Google Scholar]
- 21.Nakatani S, Marwick TH, Lever HM, Thomas JD. Resting echocardiographic features of latent left ventricular outflow obstruction in hypertrophic cardiomyopathy. Am J Cardiol. 1996; 78:662-667. [DOI] [PubMed] [Google Scholar]
- 22.Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003; 16:777-802. [DOI] [PubMed] [Google Scholar]
- 23.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009; 10:165-193. [DOI] [PubMed] [Google Scholar]
- 24.Maron BJ, Wolfson JK, Epstein SE, Roberts WC. Morphologic evidence for “small vessel disease” in patients with hypertrophic cardiomyopathy. Z Kardiol. 1987; 76suppl 3:91-100. [PubMed] [Google Scholar]
- 25.Vasiljevic JD, Popovic ZB, Otasevic P, Popovic ZV, Vidakovic R, Miric M, Neskovic AN. Myocardial fibrosis assessment by semiquantitative, point‐counting and computer‐based methods in patients with heart muscle disease: a comparative study. Histopathology. 2001; 38:338-343. [DOI] [PubMed] [Google Scholar]
- 26.Mor‐Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011; 24:277-313. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Cao T, Duan Y, Yuan L, Wang Z. Velocity vector imaging in assessing myocardial systolic function of hypertensive patients with left ventricular hypertrophy. Can J Cardiol. 2007; 23:957-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Cao T, Duan Y, Yuan L, Yang Y. Velocity vector imaging in assessing the regional systolic function of patients with post myocardial infarction. Echocardiography. 2007; 24:940-945. [DOI] [PubMed] [Google Scholar]
- 29.Fine NM, Shah AA, Han IY, Yu Y, Hsiao JF, Koshino Y, Saleh HK, Miller FA, Jr, Oh JK, Pellikka PA, Villarraga HR. Left and right ventricular strain and strain rate measurement in normal adults using velocity vector imaging: an assessment of reference values and intersystem agreement. Int J Cardiovasc Imaging. 2013; 29:571-580. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ. Doppler‐derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation. 2002; 105:99-105. [DOI] [PubMed] [Google Scholar]
- 31.Park TH, Nagueh SF, Khoury DS, Kopelen HA, Akrivakis S, Nasser K, Ren G, Frangogiannis NG. Impact of myocardial structure and function postinfarction on diastolic strain measurements: implications for assessment of myocardial viability. Am J Physiol Heart Circ Physiol. 2006; 290:H724-H731. [DOI] [PubMed] [Google Scholar]
- 32.Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001; 104:1380-1384. [DOI] [PubMed] [Google Scholar]
- 33.Karlon WJ, McCulloch AD, Covell JW, Hunter JJ, Omens JH. Regional dysfunction correlates with myofiber disarray in transgenic mice with ventricular expression of ras. Am J Physiol Heart Circ Physiol. 2000; 278:H898-H906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popovic ZB, Benejam C, Bian J, Mal N, Drinko J, Lee K, Forudi F, Reeg R, Greenberg NL, Thomas JD, Penn MS. Speckle‐tracking echocardiography correctly identifies segmental left ventricular dysfunction induced by scarring in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2007; 292:H2809-H2816. [DOI] [PubMed] [Google Scholar]
- 35.Popovic ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, Flamm SD, Thomas JD, Lever HM, Desai MY. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr. 2008; 21:1299-1305. [DOI] [PubMed] [Google Scholar]
- 36.Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000; 35:36-44. [DOI] [PubMed] [Google Scholar]
- 37.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007; 356:830-840. [DOI] [PubMed] [Google Scholar]
- 38.Ho CY, Lopez B, Coelho‐Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, Gonzalez A, Colan SD, Seidman J, Dietz J, Seidman C. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010; 363:552-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, Nassenstein K, Schlosser T, Sabin GV, Sechtem U, Mahrholdt H. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56:875-887. [DOI] [PubMed] [Google Scholar]
- 40.O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli‐Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56:867-874. [DOI] [PubMed] [Google Scholar]


