Abstract
Background
Peripheral arterial disease (PAD) shares several risk factors with atrial fibrillation (AF), and persons with PAD have an increased risk of stroke. It is unclear if PAD is associated with an increased risk for AF and whether this potential association explains the increased risk of stroke observed in those with PAD.
Methods and Results
We examined the association between PAD, measured by ankle‐brachial index (ABI), and incident AF and incident stroke, separately, in 6568 participants (mean age 62±10 years, 53% women, 62% nonwhite) from the Multi‐Ethnic Study of Atherosclerosis (MESA). ABI values <1.0 or >1.4 defined PAD. AF was ascertained through review of hospital discharge records and from Medicare claims data until December 31, 2010. An independent adjudication committee ascertained stroke events. Cox regression was used to estimate hazard ratios and 95% CIs for the association between PAD and AF and stroke. Over a median follow‐up of 8.5 years, 301 (4.6%) participants developed AF and 140 (2.1%) developed stroke. In a model adjusted for sociodemographics, cardiovascular risk factors, and potential confounders, PAD was associated with an increased risk of AF (hazard ratio 1.5, 95% CI 1.1 to 2.0). In a similar model, PAD was associated with incident stroke (hazard ratio 1.7, 95% CI 1.1 to 2.5), and the magnitude of risk was not different after inclusion of AF as a time‐dependent covariate (hazard ratio 1.7, 95% CI 1.1 to 2.5).
Conclusions
PAD is associated with an increased risk of AF and stroke in MESA. Potentially, the relationship between PAD and stroke is not mediated by AF.
Keywords: ankle‐brachial index, atrial fibrillation, peripheral arterial disease, stroke
Introduction
Peripheral arterial disease (PAD) and atrial fibrillation (AF) share several common risk factors, including diabetes and smoking.1–3 In the Women's Health Initiative Study, PAD was independently associated with the development of AF among postmenopausal women.4 However, the association of PAD defined by the ankle‐brachial index (ABI) and incident AF has not been examined in men or in middle‐aged women of diverse racial and ethnic backgrounds. Similarly, PAD and stroke share common risk factors. An increased risk of PAD has been reported in patients with stroke and an increased risk of stroke has been shown in patients with PAD.5–10 It is well established that PAD independently predicts stroke in patients with AF and consequently has been included as 1 of the components of the CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, and prior stroke or transient ischemic attack–vascular disease/sex category) score.11–13 Furthermore, ischemic stroke triples the mortality rate among patients with AF compared with AF patients who do not experience stroke.14
Potentially, these commonalities among PAD, AF, and stroke reflect shared pathophysiologic states in which each condition influences the development of the other. Therefore, we examined the association between PAD and incident AF and stroke in the Multi‐Ethnic Study of Atherosclerosis (MESA). We also investigated whether the association between PAD and stroke is mediated by AF.
Methods
Study Population
Details of MESA have been reported previously.15 Briefly, between July 2000 and September 2002, 6814 participants were recruited at 6 field centers (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN) in the United States. The institutional review board at each site approved the study and each participant gave informed consent at the time of enrollment. Requirement for study participation included age between 45 and 84 years and no history of clinical cardiovascular disease. For the purpose of this analysis, participants were excluded if they did not undergo baseline ABI measurement, baseline characteristics were not recorded, and/or follow‐up data regarding AF or stroke were missing. Additionally, baseline cases of AF were excluded to examine incident AF.
Of the 6814 participants enrolled in MESA, we excluded 58 participants who had a diagnosis of AF before enrollment. The majority of these cases were detected by Medicare linkage, and only 1 case was present in the baseline electrocardiogram. Of those who remained, 6 participants with missing follow‐up data, 77 participants with missing ABI data, and 105 participants with either missing baseline characteristics or missing medication data also were excluded. After all exclusions (n=246), 6568 participants (mean age 62±10 years, 53% women, 38% whites, 27% blacks, 22% Hispanics, and 12% Chinese Americans) remained and were included in the final analysis.
Baseline Characteristics
Participant characteristics recorded during the initial MESA visit were used in this analysis. Age, sex, race/ethnicity, income, and education were self‐reported. Annual income was categorized into 3 levels (<$20 000, $20 000 to $49 999, and ≥$50 000). Similarly, education was categorized into “high school or less,” “some college,” and “college or more.” Smoking was defined as current or former smoker. Blood samples were obtained after a 12‐hour fast. Serum measurements of total cholesterol, high‐density lipoprotein (HDL) cholesterol, plasma glucose, and high‐sensitivity C‐reactive protein (hs‐CRP) were used in this analysis. Diabetes was defined as a fasting glucose value ≥126 mg/dL or a history of diabetes medication use. After the participant rested for 5 minutes in a seated position, blood pressure was measured 3 separate times and the mean of the last 2 values was used. The use of aspirin, statins, and antihypertensive and lipid‐lowering medications was self‐reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Left ventricular hypertrophy was defined by the Cornell criteria (R‐wave amplitude AVL plus S‐wave amplitude V3 ≥2800 mm for men and ≥2000 mm for women) using electrocardiographic data.16
Peripheral Arterial Disease
Systolic blood pressures were measured in both the right and left brachial, posterior tibial, and dorsalis pedis arteries with Doppler instruments. The average of the measurements was used to calculate ABI for each side. Consistent with current guidelines, a value of <1.0 or >1.4 defined PAD.10,17 In a secondary analysis, we examined the association of low (<1.0) and high (>1.4) ABI values (referent group 1.0≤ABI≤1.4) separately with AF. Additionally, the association of ABI as a continuous variable with AF was examined and is presented as the risk of AF with decreasing ABI values by decrements of 0.1 after excluding participants with ABI values >1.4.
Atrial Fibrillation
Follow‐up phone calls to study participants every 9 to 12 months were used to identify hospitalizations and medical records, including discharge diagnoses. Additionally, for participants 65 years or older enrolled in fee‐for‐service Medicare, Medicare claims data were used to identify inpatient AF cases. Incident AF was defined by International Classification of Disease, Ninth Revision codes 427.31 or 427.32.
Stroke
Trained personnel abstracted medical records to identify possible stroke events. Two physicians from the MESA Events Committee independently reviewed medical records for end point classification and assignment of event dates. If the reviewing physicians disagreed on the event classification, they adjudicated differences. If disagreements persisted, the full Events Committee made the final classification. Stroke was classified as a dichotomous variable (present or absent) and was defined by the rapid onset of documented focal neurologic deficits lasting ≥24 hours or until death.15 If symptoms were present for <24 hours, a clinically relevant lesion on brain imaging must have been present for stroke to be documented as present. Due to the small number of documented cases of stroke (n=140), we grouped hemorrhages (eg, subarachnoid, intraparenchymal) and infarcts into 1 category.
Statistical Analysis
Categorical variables were reported as frequency and percentage, while continuous variables were recorded as mean±standard deviation (SD). Statistical significance for categorical variables was tested using the χ2 method and the Wilcoxon rank‐sum procedure for continuous variables. Kaplan–Meier estimates were used to compute cumulative incidence of AF by PAD, and the difference in estimates was compared by using the log‐rank procedure.18 Follow‐up time was defined as the time between the initial study visit until the diagnosis of AF, loss to follow‐up, death, or end of follow‐up, which was December 31, 2010. Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CI) for the association between PAD and AF. Multivariable models were constructed with incremental adjustments as follows: Model 1 adjusted for age, sex, race/ethnicity, income, and education; Model 2 adjusted for Model 1 covariates plus smoking status, systolic blood pressure, diabetes, body mass index, total cholesterol, HDL cholesterol, aspirin, antihypertensive and lipid‐lowering medications, hs‐CRP levels, and left ventricular hypertrophy. Using similar models, we also examined the association between PAD and stroke with and without AF to explore the attenuating effect of AF on stroke among participants with PAD by adjusting for AF as a time‐dependent covariate. Although there were a small number of stroke events (n=140), ischemic strokes represented the majority (n=116, 83%) and a sensitivity analysis was performed examining the mediation of AF on the association of PAD with ischemic strokes. We tested for interactions between our main effect variable and age, sex, and race/ethnicity. We also constructed a restricted cubic spline model to examine the graphical relationship between ABI and the risk for AF and stroke, and incorporated knots at the 5th, 50th, and 95th percentiles.19 The proportional hazards assumption was not violated in our analysis. Statistical significance was defined as P<0.05. SAS Version 9.3 (SAS Institute Inc) was used for all analyses.
Results
A total of 774 (12%) study participants had baseline PAD. Baseline characteristics for study participants stratified by PAD status are shown in Table 1. Participants with PAD were more likely to be older and female, to have a high school diploma or less, and to have an annual income <$20 000 than participants without PAD. A higher percentage of black participants (17%) had PAD than other races (11% whites, 7.8% Hispanics, 8.1% Chinese Americans; P<0.0001). PAD participants also were more likely to smoke, to have diabetes, and to have higher values for systolic blood pressure, HDL cholesterol, and hs‐CRP. Additionally, participants with PAD were more likely to report using statins and antihypertensive and lipid‐lowering medications.
Table 1.
Baseline Characteristics of Study Participants Stratified by PAD (N=6568)
| Characteristic | PAD (n=774) | No PAD (n=5794) | P Value* |
|---|---|---|---|
| Age, mean (SD), years | 67 (10) | 61 (10) | <0.0001 |
| Male sex, % | 282 (36) | 2832 (49) | <0.0001 |
| Race/ethnicity, % | |||
| White | 282 (36) | 2237 (39) | |
| Black | 314 (41) | 1487 (26) | <0.0001 |
| Chinese American | 64 (8.3) | 725 (13) | |
| Hispanic | 114 (15) | 1345 (23) | |
| Education, % | |||
| High school or less | 337 (44) | 2051 (35) | |
| Some college | 248 (32) | 1623 (28) | <0.0001 |
| College or more | 189 (24) | 2120 (37) | |
| Annual income, % | |||
| <$20 000 | 280 (36) | 1471 (25) | |
| $20 000 to $49 999 | 275 (36) | 2028 (35) | <0.0001 |
| ≥$50 000 | 219 (28) | 2295 (40) | |
| Body mass index, mean (SD) kg/m2 | 28 (5.9) | 28 (5.4) | 0.41 |
| Current or former smoker, % | 445 (57) | 2803 (48) | <0.0001 |
| Diabetes, % | 157 (20) | 756 (13) | <0.0001 |
| Systolic blood pressure, mean (SD), mm Hg | 136 (25) | 125 (21) | <0.0001 |
| Total cholesterol, mean (SD), mg/dL | 196 (38) | 194 (35) | 0.21 |
| HDL cholesterol, mean (SD), mg/dL | 52 (16) | 51 (15) | 0.043 |
| Antihypertensive medications, % | 386 (50) | 2012 (35) | <0.0001 |
| Statins, % | 166 (21) | 793 (14) | <0.0001 |
| Aspirin, % | 197 (25) | 1349 (23) | 0.18 |
| Lipid‐lowering medications, % | 181 (23) | 865 (15) | <0.0001 |
| hs‐CRP, mean (SD), mg/L | 5.0 (7.3) | 3.6 (5.7) | <0.0001 |
| Left ventricular hypertrophy, % | 31 (4.0) | 216 (3.7) | 0.70 |
HDL indicates high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; PAD, peripheral arterial disease; SD, standard deviation.
Statistical significance for continuous data was tested using Wilcoxon rank‐sum procedure and for categorical data using the χ2 test.
During a median follow‐up of 8.5 years, 301 (4.6%) participants developed AF. AF incidence rates per 1000 person‐years were 12 (95% CI 9.5 to 15.4) for participants with PAD compared with 5.3 (95% CI 4.6 to 6.0) for participants without PAD. Figure 1 shows the unadjusted cumulative incidence curves for AF by PAD (log‐rank P<0.0001).
Figure 1.
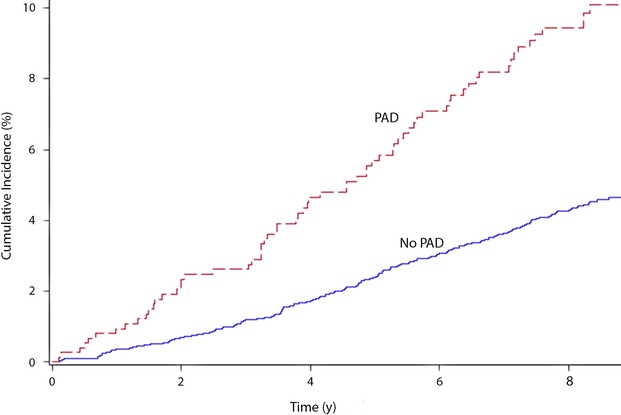
Unadjusted cumulative incidence of AF by PAD (N=6568). Cumulative incidence curves are different (log‐rank P<0.0001). Ankle‐brachial index values of <1.0 or >1.4 defined PAD. AF indicates atrial fibrillation; PAD, peripheral arterial disease; y, years.
Participants with PAD had twice the risk of AF in an unadjusted Cox regression model (HR 2.3, 95% CI 1.8 to 3.0). The HR remained significant after adjustment for sociodemographics, cardiovascular risk factors, and potential confounders, and the results were consistent across subgroups stratified by age, sex, and race/ethnicity (Table 2). Expanding the demographic model to include field center and expanding the smoking variable to include pack‐years did not change the result (HR 1.5, 95% CI 1.1 to 2.0).
Table 2.
Risk of AF With PAD
| Subgroup | Events/No. at Risk | Model 1* HR (95% CI) | P Value | Model 2* HR (95% CI) | P Value | Interaction P Value* |
|---|---|---|---|---|---|---|
| All | 301/6568 | 1.7 (1.3 to 2.2) | 0.0004 | 1.5 (1.1 to 2.0) | 0.0041 | — |
| Age, years* | ||||||
| <62 | 43/3218 | 2.2 (0.93 to 5.4) | 0.071 | 1.8 (0.74 to 4.4) | 0.20 | 0.79 |
| ≥62 | 258/3350 | 1.9 (1.4 to 2.5) | <0.0001 | 1.7 (1.3 to 2.3) | 0.0003 | |
| Sex | ||||||
| Female | 115/3454 | 1.7 (1.1 to 2.6) | 0.0098 | 1.6 (1.1 to 2.5) | 0.028 | 0.64 |
| Male | 186/3114 | 1.6 (1.1 to 2.4) | 0.012 | 1.5 (1.01 to 2.2) | 0.043 | |
| Race/ethnicity | ||||||
| White | 166/2519 | 1.6 (1.1 to 2.3) | 0.022 | 1.5 (0.98 to 2.2) | 0.064 | 0.71 |
| Nonwhite | 135/4049 | 1.8 (1.2 to 2.7) | 0.0038 | 1.6 (1.04 to 2.4) | 0.034 | |
AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio; PAD, peripheral arterial disease.
Adjusted for Model 1 covariates plus smoking status, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive and lipid‐lowering medications, high‐sensitivity C‐reactive protein levels, and left ventricular hypertrophy.
Interactions tested using Model 2.
Dichotomized at the median age for study participants.
The associations between AF and high (>1.4) and low (<1.0) ABI values were examined separately and were in the same direction as the main result for PAD (ABI <1.0, adjusted HR 1.5, 95% CI 1.1 to 2.0; ABI >1.4, adjusted HR 1.8, 95% CI 0.65 to 4.8). The result for ABI values >1.4 was not significant due to the small number of participants in this group (n=40) and consequently a small number of AF cases (n=4). The HR for ABI as a continuous variable, decreasing by 0.1, was 1.1 (95% CI 1.04 to 1.2) after excluding participants with ABI values >1.4.
Incident stroke occurred in 140 (2.1%) participants. Only 36 (26%) cases of stroke had PAD, and of these, only 1 (2.8%) had a diagnosis of AF before stroke. Table 3 shows the HR for stroke among participants with PAD compared with those without PAD. Additionally, HRs are shown with and without AF as a time‐dependent covariate. In a sociodemographically adjusted model, PAD was associated with twice the risk of stroke compared with no PAD. The magnitude of the association did not substantively change with the addition of AF. A similar result was observed when the association between PAD and stroke was examined in a model further adjusted for cardiovascular risk factors and potential confounders (Table 3). A sensitivity analysis including only ischemic strokes showed a trend for the association between PAD and stroke (HR 1.5, P=0.071). The addition of AF to this model did not substantively alter our findings (HR 1.5, P=0.060).
Table 3.
Association of PAD With Risk of Stroke With and Without Adjustment for AF
| Events/No. at Risk | Model 1* HR (95% CI) | P Value | Model 1** With AF HR (95% CI) | P Value | Model 2* HR (95% CI) | P Value | Model 2** With AF HR (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| No PAD | 104/5794 | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| PAD | 36/774 | 2.0 (1.4 to 3.0) | 0.0004 | 2.1 (1.4 to 3.0) | 0.0003 | 1.7 (1.1 to 2.5) | 0.015 | 1.7 (1.1 to 2.5) | 0.011 |
AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio; PAD, peripheral arterial disease.
Adjusted for age, sex, race/ethnicity, income, and education.
AF adjusted as a time‐dependent covariate.
Adjusted for Model 1 covariates plus smoking status, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive and lipid‐lowering medications, high‐sensitivity C‐reactive protein levels, and left ventricular hypertrophy.
Figure 2 shows the association between ABI values and incident AF and stroke using a restricted cubic spline model. As shown, there was an increased risk for AF and stroke with decreasing ABI values. A similar trend was observed for increasing ABI values.
Figure 2.
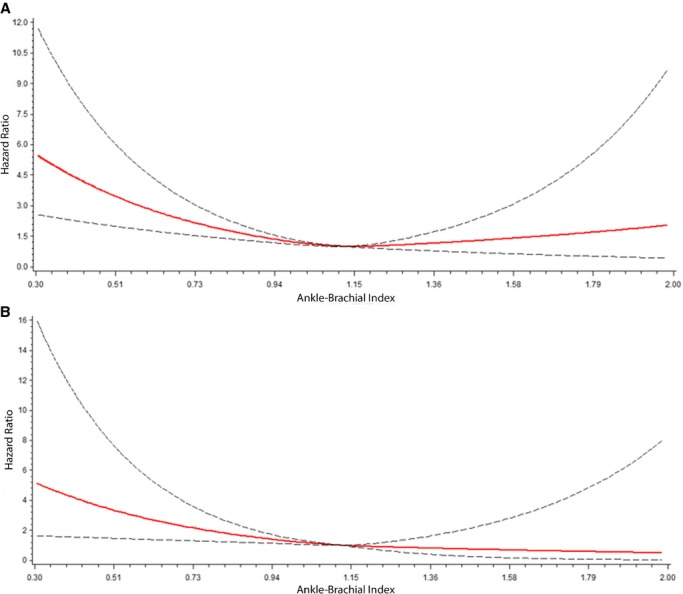
Risk of AF (A) and stroke (B) with ABI. Each hazard ratio was computed with the median ABI value of 1.12 as the referent group and was adjusted for age, sex, and race/ethnicity. Dotted lines represent the 95% CI. The hazard ratio for AF is depicted in (A). The hazard ratio for stroke is depicted in (B). ABI indicates ankle‐brachial index; AF, atrial fibrillation; CI, confidence interval.
Discussion
In this analysis from MESA, we examined the association between PAD and incident AF and stroke, separately. We also examined whether a potential association between PAD and AF explained the known association between PAD and stroke. Our study revealed 2 key findings. First, PAD is associated with incident AF regardless of age, sex, race/ethnicity, and cardiovascular risk factors. Second, our results suggest that the known association between PAD and stroke, which also was confirmed in our study, is not mediated by AF.
A recent examination of data from the Women's Health Initiative Study has shown that PAD, which was ascertained by self‐reported history, is independently associated with incident AF among postmenopausal women (HR 1.53, 95% CI 1.37 to 1.72).4 This result is similar to that observed in the current study. Additionally, the racially and ethnically diverse population of men and women in MESA increases the generalizability of our results compared with the Women's Health Initiative Study. Also, we used ABI to define PAD instead of self‐reported history, which was used in the Women's Health Initiative Study. Considering that a small number of patients with abnormal ABI values have symptoms of PAD, our results provide a more accurate estimate of the risk for AF associated with PAD.20
Several reports have described the association of PAD with stroke.7–10 Our results confirm these findings in MESA by using ABI measurements. However, to our knowledge, no studies have examined the effect of incident AF on the association between PAD and stroke. Due to many shared risk factors, we hypothesized that the risk of stroke among those with PAD was mediated by AF. Our results do not support this claim. This provides useful information to better understand the underlying mechanisms which are needed to appropriately target preventative interventions to reduce the burden of stroke.
The association between PAD and AF is not completely understood. Both conditions share several risk factors and are associated with increased levels of inflammation and platelet‐mediated thrombosis.1–3,1–22 It is plausible that the association of PAD with AF reflects an underlying state (eg, increased levels of inflammation, thrombosis, and coagulation) with a predisposition for both conditions to the influence the development of the other. Further research is needed to elucidate these underlying mechanisms.
Patients with a high burden of medial arterial calcification have high ABI values, which represents a different pathological state compared with low ABI values.23 In our study, the association of both low and high ABI values with AF was in the same direction as the main model for PAD. This indicates that PAD, regardless of the pathophysiological basis or pattern, is associated with an increased risk for the development of AF. Among diabetic patients, arterial stenosis and incompressibility may have resulted in the misclassification of PAD.24–25 Diabetes is present in ≈41% of patients with PAD, and the risk for AF is increased by 40% in these patients.26–27 Diabetes also has been associated with a 10% higher risk of subclavian stenosis and a greater prevalence of subclinical episodic AF.28–29 While a differential bias for AF cannot be ruled out, the direction likely is toward the null. Furthermore, our multivariable results were not substantively affected when controlling for diabetes.
Several limitations of the current analysis should be addressed. Incident AF cases were ascertained from hospitalization discharge records and inpatient Medicare claims data using International Classification of Disease, Ninth Revision codes, which possibly resulted in misclassification. However, this method has been shown to have adequate positive predictive value for the identification of AF events.30–31 Additionally, paroxysmal cases of AF potentially were missed due to its time‐dependent nature. This would only make our results conservative because these missed AF cases would be included in the non‐AF group. Also, the actual onset of AF cannot be precisely known, and cases may have occurred before hospital admission in which the diagnosis was confirmed. Routine monitoring for AF was not performed in MESA and therefore asymptomatic cases possibly were missed. However, we do not know of any reason to suggest that the resulting bias, if any, would have been differential in nature, rather than merely reducing effect estimates toward the null. PAD was defined by ABI values <1.0 and >1.4 according to current guidelines.17 The association between PAD and ABI may vary depending on the definition of abnormal ABI (eg, <0.9).32 Hence, comparing our results with other studies that use different definitions of abnormal ABI should be done with caution. ABI values used in this analysis were obtained during a single visit, and results may vary with interval measurements.9 Differences in the measurement of ABI may have posed another source of variability.33 However, the measurements of ABI in the United States and in MESA are standardized. Furthermore, the small number of AF cases that occurred before stroke may have limited the power to detect the mediating effect of AF on stroke. Similar to other observational studies, we also acknowledge that residual confounding remains a possibility.
In conclusion, we have shown that PAD, as defined by abnormal ABI values, is associated with an increased risk of AF in MESA. Additionally, our results suggest that the relationship between PAD and stroke is not mediated by AF. Further research is needed to examine the usefulness of ABI to identify patients who have an increased risk for the development of AF.
Disclosures
Dr Nazarian is a consultant and principal investigator for research funding awarded to Johns Hopkins University from Biosence Webster Inc.
Acknowledgments
This research was supported by contracts N01‐HC‐95159 through N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐RR‐024156 and UL1‐RR‐025005 from the National Center for Research Resources. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
References
- 1.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994; 271:840-844. [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol. 1998; 82:2N-9N. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the united states: results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004; 110:738-743. [DOI] [PubMed] [Google Scholar]
- 4.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, Connelly S, Hlatky M, Wassertheil‐Smoller S, Stefanick ML. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women's Health Initiative Observational Study. Heart. 2013; 99:1173-1178. [DOI] [PubMed] [Google Scholar]
- 5.Leng GC, Lee AJ, Fowkes FG, Whiteman M, Dunbar J, Housley E, Ruckley CV. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996; 25:1172-1181. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee A, Fowkes FG, Rothwell PM. Associations between peripheral artery disease and ischemic stroke: implications for primary and secondary prevention. Stroke. 2010; 41:2102-2107. [DOI] [PubMed] [Google Scholar]
- 7.Meves SH, Diehm C, Berger K, Pittrow D, Trampisch HJ, Burghaus I, Tepohl G, Allenberg JR, Endres HG, Schwertfeger M, Darius H, Haberl RLgetABI Study Group. Peripheral arterial disease as an independent predictor for excess stroke morbidity and mortality in primary‐care patients: 5‐year results of the getabi study. Cerebrovasc Dis. 2010; 29::546-:554. [DOI] [PubMed] [Google Scholar]
- 8.Uchiyama S, Goto S, Matsumoto M, Nagai R, Origasa H, Yamazaki T, Shigematsu H, Shimada K, Yamada N, Bhatt DL, Steg PG, Ikeda YREduction of Atherothrombosis for Continued Health Registry Investigators. Cardiovascular event rates in patients with cerebrovascular disease and atherothrombosis at other vascular locations: results from 1‐year outcomes in the japanese reach registry. J Neurol Sci. 2009; 287:45-51. [DOI] [PubMed] [Google Scholar]
- 9.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008; 52:1736-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle‐brachial index and incident cardiovascular events in the MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010; 56:1506-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen JB, Lip GY, Lane DA, Kober L, Hansen ML, Karasoy D, Hansen CM, Gislason GH, Torp‐Pedersen C. Vascular disease and stroke risk in atrial fibrillation: a nationwide cohort study. Am J Med. 2012; 125:e813-e823. [DOI] [PubMed] [Google Scholar]
- 12.Olesen JB, Torp‐Pedersen C, Hansen ML, Lip GY. The value of the cha2ds2‐vasc score for refining stroke risk stratification in patients with atrial fibrillation with a chads2 score 0‐1: a nationwide cohort study. Thromb Haemost. 2012; 107:1172-1179. [DOI] [PubMed] [Google Scholar]
- 13.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864-2870. [DOI] [PubMed] [Google Scholar]
- 14.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. Long‐term survival after ischemic stroke in patients with atrial fibrillation. Neurology. 2014; 82:1033-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871-881. [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB, Casale PN, Eisenberg RR, Miller DH, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. J Am Coll Cardiol. 1984; 3:82-87. [DOI] [PubMed] [Google Scholar]
- 17.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 127:1425-1443. [DOI] [PubMed] [Google Scholar]
- 18.Gray RJ, Tsiatis AA. A linear rank test for use when the main interest is in differences in cure rates. Biometrics. 1989; 45:899-904. [PubMed] [Google Scholar]
- 19.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009; 62:e511. [DOI] [PubMed] [Google Scholar]
- 20.Wang JC, Criqui MH, Denenberg JO, McDermott MM, Golomb BA, Fronek A. Exertional leg pain in patients with and without peripheral arterial disease. Circulation. 2005; 112:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high‐risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005; 46:937-954. [DOI] [PubMed] [Google Scholar]
- 22.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009; 373:155-166. [DOI] [PubMed] [Google Scholar]
- 23.Suominen V, Rantanen T, Venermo M, Saarinen J, Salenius J. Prevalence and risk factors of PAD among patients with elevated ABI. Eur J Vasc Endovasc Surg. 2008; 35:709-714. [DOI] [PubMed] [Google Scholar]
- 24.Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle‐brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008; 51:1292-1298. [DOI] [PubMed] [Google Scholar]
- 25.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008; 48:1197-1203. [DOI] [PubMed] [Google Scholar]
- 26.Novo S. Classification, epidemiology, risk factors, and natural history of peripheral arterial disease. Diabetes Obes Metab. 2002; 4suppl 2:S1-S6. [DOI] [PubMed] [Google Scholar]
- 27.Huxley RR, Filion KB, Konety S, Alonso A. Meta‐analysis of cohort and case‐control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011; 108:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, McDermott MM. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004; 44:618-623. [DOI] [PubMed] [Google Scholar]
- 29.Marfella R, Sasso FC, Siniscalchi M, Cirillo M, Paolisso P, Sardu C, Barbieri M, Rizzo MR, Mauro C, Paolisso G. Brief episodes of silent atrial fibrillation predict clinical vascular brain disease in type 2 diabetic patients. J Am Coll Cardiol. 2013; 62:525-530. [DOI] [PubMed] [Google Scholar]
- 30.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and african‐americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012; 21:141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan TH, Farooqui FA, Niazi K. Critical review of the ankle brachial index. Curr Cardiol Rev. 2008; 4:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeelani NU, Braithwaite BD, Tomlin C, MacSweeney ST. Variation of method for measurement of brachial artery pressure significantly affects ankle‐brachial pressure index values. Eur J Vasc Endovasc Surg. 2000; 20:25-28. [DOI] [PubMed] [Google Scholar]


