Abstract
Background
Heart failure (HF) is common and is associated with high mortality. We aimed to determine associations of serum parathyroid hormone (PTH) and 25‐hydroxyvitamin D (25[OH]D) with incident HF and left ventricular mass.
Methods and Results
Among 6459 participants in the community‐based Multi‐Ethnic Study of Atherosclerosis, all of whom were free of prevalent clinical cardiovascular disease, we measured serum concentrations of PTH and 25(OH)D at the baseline examination. In longitudinal analyses, we tested associations of PTH and 25(OH)D with incident HF events, adjudicated by a panel of physicians. In cross‐sectional analyses of a subset of 4763 participants, we tested associations of PTH and 25(OH)D with left ventricular mass, measured by cardiac magnetic resonance imaging at baseline. Multivariable Cox proportional hazard and linear regression models were adjusted for demographics, physical examination measures, comorbidity, kidney function, and other mineral metabolism markers. Mean age was 62 years and 53% of participants were female. There were 180 incident HF events over a median (interquartile range) follow‐up time of 8.46 (7.67 to 8.63) years. Compared with participants with PTH <65 pg/mL, PTH ≥65 pg/mL was associated with a 50% greater risk of incident HF (95% CI: 3% to 210%) and a 5.3 g higher left ventricular mass (95% CI: 2.6, 7.9 g). In contrast, there was no association of 25(OH)D with risk of incident HF or elevated left ventricular mass.
Conclusions
In a racially/ethnically diverse population without prevalent cardiovascular disease, higher serum PTH concentration was associated with increased left ventricular mass and increased risk of incident HF. Further studies should be pursued to determine whether PTH excess may be a modifiable risk factor for HF.
Keywords: heart failure, hypertrophy, risk factors
Introduction
Heart failure (HF) is common and is associated with high morbidity and mortality.1 Thus, identification of modifiable risk factors may help prevent HF and its associated complications. Potential modifiable risk factors for HF include alterations in mineral metabolism.2–6 For example, parathyroid hormone (PTH) receptors may act directly on cardiomyocytes, vascular smooth muscle, and endothelial cells7–8 to induce hypertrophy9 or to increase afterload10–13 Similarly, 25‐hydroxyvitamin D [25(OH)D] deficiency may promote cardiovascular disease via negative regulation of the renin–angiotensin–aldosterone system,14 stimulation of inflammatory cytokines and oxidative stress,15 endothelial dysfunction,16 and induction of cardiac hypertrophy.17 Further study of alterations in PTH and 25(OH)D and risk of HF in humans may provide data to guide development of optimal therapies to prevent HF.
Several observational studies have reported associations of circulating 25(OH)D and PTH concentrations with HF risk. However, these studies have several limitations. Many prior studies have included participants with prevalent HF (rather than incident HF),18–20 which increases the concern for residual confounding, reverse causality, and survivor bias. Furthermore, several reports have been restricted to elderly adults3 or primarily white populations,3,21–22 with poor representation of racial/ethnic groups such as African Americans, who have a disproportionate burden of HF risk.23 Moreover, few have studied both 25(OH)D and PTH concentrations together or have accounted for other mineral metabolism markers, such as serum calcium, which has also been associated with cardiovascular disease. Therefore, it has been difficult to differentiate in prior studies whether the observed associations with HF are independent of other mineral metabolism markers.
Therefore, we studied associations of serum PTH and 25(OH)D concentrations with incident HF in a racially/ethnically diverse group of participants without cardiovascular disease at baseline in the Multi‐Ethnic Study of Atherosclerosis (MESA) study. We also examined the cross‐sectional associations of these mineral markers with left ventricular mass (LVM) to determine whether induction of cardiac hypertrophy was a plausible mechanism linking alterations in PTH and 25(OH)D with incident HF. We hypothesized that higher PTH and lower 25(OH)D concentrations would be associated with higher LVM and greater incidence of HF.
Methods
Study Population
The MESA is a community‐based prospective cohort study of clinical and subclinical cardiovascular disease.24 Between 2000 and 2002, MESA enrolled 6814 adults 45 to 84 years of age from 6 field centers (New York and Bronx counties, New York; Baltimore City and County, Maryland; Forsyth County, North Carolina; Chicago, Illinois; St. Paul, Minnesota; and Los Angeles, California). Only individuals without known prevalent clinical cardiovascular disease, defined as myocardial infarction, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, use of nitroglycerin, prior angioplasty, coronary artery bypass grafting, valve replacement, pacemaker or defibrillator implantation, or any surgery on the heart or arteries, were eligible to participate. Each site's institutional review board approved the study, and all participants provided informed consent.
For our analysis, we excluded participants with missing measures of PTH and/or 25(OH)D (N=350) and those without follow‐up data (N=5), leaving a final analytic population of 6459 participants.
Measurement of Serum PTH and 25(OH)D Concentrations
Serum samples were collected in the morning at the baseline MESA examination in 2000–2002, after an overnight fast. These samples were stored at −80°C until they were shipped to the University of Washington for analysis in 2011–2012. Intact serum PTH was quantified using a 2‐site immunoassay on a Beckman Access2 clinical analyzer (Beckman‐Coulter, Inc, Brea, CA).11 The reference range, determined from the central 95% of values from 43 normal laboratory personnel with normal 25(OH)D concentrations, is 17 to 65 pg/mL. The interassay coefficient of variation was 6.1% at 30.1 pg/mL and 3.4% at 94.5 pg/mL. Total 25(OH)D (25(OH)D2+25(OH)D3) was measured using high‐performance liquid chromatography–tandem mass spectrometry on a Waters Quattro Micro mass spectrometer (Waters, Milford, MA).25 Calibration was confirmed with National Institute of Standards and Technology control material SRM‐972.26 The interassay coefficient of variation was 4.4% at 10.4 ng/mL for 25(OH)D3 and 4.35% at 9.7 ng/mL for 25(OH)D2.
Incident HF
Cardiovascular disease outcomes were adjudicated by the MESA events committee, which included cardiologists, physician epidemiologists, and general clinicians,24,27 who review the reports gathered by a team of trained individuals who interview participants by phone and gather the appropriate records on each of the reported events. Details regarding the MESA processes and criteria for verifying, classifying, and adjudicating cardiovascular events have been previously reported.27–29 Briefly, in addition to the follow‐up MESA examinations, a telephone interviewer contacted each participant every 9 to 12 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. In order to verify self‐reported diagnoses, copies were requested of all death certificates and medical records for all hospitalizations and outpatient cardiovascular diagnoses. Next of kin interviews for out‐of‐hospital cardiovascular deaths were obtained. MESA was successful in getting medical records on an estimated 98% of hospitalized cardiovascular events and information on 95% of outpatient cardiovascular diagnostic encounters. Follow‐up telephone interviews were completed in 92% of living participants.
The end point evaluated in this study was a composite of probable and definite HF. An adjudicated diagnosis of probable HF required the following: clinical HF symptoms or signs, a physician diagnosis of HF, and medical treatment for HF. An adjudicated diagnosis of definite HF additionally required pulmonary edema/congestion by chest radiograph, evidence of a dilated left ventricle or reduced left ventricular ejection fraction by echocardiography or ventriculography, or evidence of left ventricular diastolic dysfunction by echocardiography.
Left Ventricular Mass
Cardiac magnetic resonance imaging was performed in 5098 participants at baseline and quantified centrally at a single reading center.27 Cardiac magnetic resonance imaging was performed with 1.5‐T magnets with determination of LVM and volumes as previously described.30 Briefly, a stack of short‐axis images covering the entire left ventricle was acquired with TR/TE 8 to 10 ms/3 to 5 ms, flip angle 20°, 6‐mm slice thickness, 4‐mm gap, flow compensation, in plane resolution 1.4 to 1.6 mm (frequency)×2.2 to 2.5 mm. The endocardial and epicardial myocardial borders were contoured using a semi‐automated method (MASS 2.4; Medis, the Netherlands). The difference between the epicardial and endocardial areas for all slices was multiplied by the slice thickness and section gap, and then multiplied by the specific gravity of myocardium (1.04 g/mL) to determine the LVM.27 For this analysis, of the 6459 participants included, 4763 had available LVM measures.
Covariates
All covariates were ascertained at the baseline MESA examination, concurrent with PTH and 25(OH)D measurements. Participants completed standardized interviews and extensive in‐person examinations, yielding demographic and lifestyle characteristics, medical history, anthropometric measurements, and laboratory data. Leisure‐time physical activity was estimated as the total amount of intentional exercise performed in a usual week, in metabolic equivalent task minutes. Diabetes was defined as a fasting glucose level ≥126 mg/dL or the use of insulin or oral hypoglycemic medications. Blood pressure was ascertained as the mean of the last 2 of 3 seated measurements. Glomerular filtration rate was estimated from serum creatinine using the Chronic Kidney Disease—Epidemiology Collaboration equation (eGFR CKD‐EPI).31 Urinary albumin concentration was determined by nephelometry, using the Array 360 CE Protein Analyzer (Beckman Instruments Inc). Urinary creatinine was measured by the rate Jaffé method using the Vitros 950IRC instrument (Johnson & Johnson Clinical Diagnostics Inc). Serum intact FGF23 was measured using the Kainos sandwich immunoassay (Kainos, Japan), which measures the full‐length (intact) FGF23 molecule by recognizing both midmolecule and distal epitopes.32 Serum calcium was measured by indirect ion selective electrode. Serum phosphorus was measured using a timed‐rate colorimetry reaction.
Statistical Analyses
High serum PTH concentration was defined as ≥65 pg/mL because 65 pg/mL is the upper limit of normal for our assay and because previous assessments of functional form have revealed threshold associations of PTH concentrations with adverse cardiovascular disease outcomes and 25(OH)D concentration near this level of PTH.3,33 Mean annual serum 25(OH)D concentration was estimated for each participant from baseline serum 25(OH)D concentration and month of blood draw using a cosinor model previously developed and validated in MESA.34 This annualized 25(OH)D concentration was evaluated using clinically relevant categories that have been previously published: ≥30 ng/mL (reference group‐ sufficient), 20 to 29 ng/mL (insufficient), and <20 ng/mL (deficient).6,35–36 We decided a priori to exclude participants missing either PTH or 25(OH)D rather than do multiple imputation, given the importance of these variables in our analyses.
Participants were considered at risk for incident HF from the date of their baseline MESA examination until the first occurrence of the outcome or until their data were censored due to death from non‐HF causes (n=422), loss to follow‐up, or the end of available follow‐up (July 2012). We explored differences in rates of incident HF by race/ethnic groups. Incidence rates were calculated by dividing number of events by person‐years of follow‐up. We performed a series of Kaplan–Meier survival analyses to examine the cumulative incidence rates of HF relative to PTH and 25(OH)D concentrations. We began by performing unadjusted Cox proportional hazard models with serum PTH and 25(OH)D as continuous variables using penalized smoothing splines with N0.2 evenly spaced knots among the inner 99% distribution of each mineral metabolism marker in the Cox models. We then performed multivariable Cox proportional hazards modeling serum PTH and 25(OH)D as in categories consistent with clinically meaningful cut‐offs, adjusting for covariates selected prior to analyses based on known or suspected biologic relationships. The first model included demographic data, including age, race/ethnicity, sex, and education attainment (high school, some college, completed college). The second model added likely potential confounding variables, including height, weight, smoking status (never, former, current), leisure‐time physical activity level (log‐transformed continuous variable), diabetes mellitus, eGFR, urine albumin to creatinine ratio (ACR) (log‐transformed continuous variable), systolic blood pressure, and use of antihypertensive medications. The third model added variables that may confound or mediate the associations of interest, including calcium, phosphorus, FGF23, and the alternative mineral measure (either PTH or 25(OH)D). We tested and confirmed that the assumption of proportional hazards was not violated in all Cox models.
In secondary analyses, we examined associations of PTH and 25(OH)D with left ventricular mass in cross‐sectional analyses. These analyses only included the subset of participants who had a cardiac magnetic resonance imaging performed to be consistent with prior MESA analyses that have examined LVM.27,30 We performed unadjusted linear regression models with serum PTH and 25(OH)D as continuous variables using penalized smoothing splines with N0.2 evenly spaced knots among the inner 99% distribution of each mineral metabolism marker. We then performed multivariable linear regression models with serum PTH and 25(OH)D modeled as categorical variables.
Approximately 5% or fewer of the study subjects were missing data on education, physical activity, smoking status, systolic blood pressure, or antihypertensive medication use. For the regression analyses, these subjects’ values were multiply imputed using chained equations.37 The multiple analyses over the imputations were combined using Rubin's rules to account for the variability in the imputation procedure.38
Subgroup‐specific hazard ratios (HRs) were calculated to evaluate whether associations were consistent across subgroups. The P values for interactions were evaluated with the Wald test. In exploratory analyses, we used log‐linear Poisson regression to determine the adjusted risk difference of high versus low PTH with incident HF by race/ethnic group. We calculated a P value for heterogeneity by bootstrapping.
We performed several sensitivity analyses. We chose a priori to do complete case analyses for all sensitivity analyses rather than use multiple imputations for missing covariates as was done for the primary analyses. In the first sensitivity analysis, we examined whether LVM may mediate any association between PTH and 25(OH)D by repeating our analyses with and without adjustment for baseline LVM among the N=4759 participants who had available LVM measurements. In the second sensitivity analysis, we examined whether the observed associations between elevated PTH and incident HF were consistent for preserved ejection fraction versus reduced ejection fraction HF among participants for whom LVEF was quantified at the time of HF hospitalization. Reduced ejection fraction HF was defined as LVEF <50% based on current guidelines.39 In the third sensitivity analysis, we examined whether interim nonfatal myocardial infarction (MI) may also potentially mediate the association between PTH and 25(OH)D and incident HF by adjusting for interim adjudicated nonfatal MI. In a final sensitivity analysis, we repeated our main analyses after excluding participants with elevated serum calcium (defined as >10.2 mg/dL) and elevated PTH to exclude those with possible primary hyperparathyroidism, which is less common and may have differing cardiovascular pathophysiology compared with secondary hyperparathyroidism.
All analyses were conducted with R Core Team (2013) R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, URL http://www.R-project.org/). The nominal level of significance was defined as P≤0.05 (2‐sided).
Results
Participant Characteristics
Among the 6459 participants in our analyses, mean age was 62 years, 53% were female, and mean systolic blood pressure was 127 mm Hg. The study population was diverse with 12.2% Chinese, 27.2% black, and 21.8% Hispanic participants. Compared with participants who had normal serum PTH concentration, those with elevated PTH were older, more likely to have diabetes, have higher blood pressure, have lower eGFR, and higher urine ACR (Table 1). Of participants with elevated PTH, 24% were white, 4.9% were Chinese, 43.1% were black, and 28% were Hispanic (Table 1). Compared with participants who had higher serum 25(OH)D concentration, those with lower 25(OH)D were more likely to be black, more likely to smoke, more likely to have diabetes and have higher eGFR (Table 1).
Table 1.
Baseline Characteristics of 6459 Participants in the Multi‐Ethnic Study of Atherosclerosis
| Covariate | All | PTH <65 pg/mL | PTH ≥65 pg/mL | 25(OH)D <20 ng/mL | 25(OH)D 20 to <30 ng/mL | 25(OH)D ≥30 ng/mL |
|---|---|---|---|---|---|---|
| N | 6459 | 5684 | 775 | 2112 | 2237 | 2110 |
| Age, y | 62.1 (10.3) | 61.9 (10.3) | 64.3 (10.1) | 60.6 (10.1) | 62.4 (10.3) | 63.4 (10.2) |
| Sex | ||||||
| Female | 3446 (53.4) | 2986 (52.5) | 460 (59.4) | 1175 (55.6) | 1112 (49.7) | 1159 (54.9) |
| Male | 3013 (46.6) | 2698 (47.5) | 315 (40.6) | 937 (44.4) | 1125 (50.3) | 951 (45.1) |
| Race/ethnicity | ||||||
| White | 2505 (38.8) | 2319 (40.8) | 186 (24.0) | 421 (19.9) | 862 (38.5) | 1222 (57.9) |
| Chinese American | 785 (12.2) | 747 (13.1) | 38 (4.9) | 160 (7.6) | 355 (15.9) | 270 (12.8) |
| Black | 1758 (27.2) | 1424 (25.1) | 334 (43.1) | 1068 (50.6) | 472 (21.1) | 218 (10.3) |
| Hispanic | 1411 (21.8) | 1194 (21.0) | 217 (28.0) | 463 (21.9) | 548 (24.5) | 400 (19.0) |
| Site | ||||||
| Wake Forest | 969 (15.0) | 843 (14.8) | 126 (16.3) | 342 (16.2) | 301 (13.5) | 326 (15.5) |
| Columbia | 1014 (15.7) | 828 (14.6) | 186 (24.0) | 416 (19.7) | 327 (14.6) | 271 (12.8) |
| Johns Hopkins | 1036 (16.0) | 908 (16.0) | 128 (16.5) | 417 (19.7) | 330 (14.8) | 289 (13.7) |
| Minnesota | 1029 (15.9) | 933 (16.4) | 96 (12.4) | 305 (14.4) | 337 (15.1) | 387 (18.3) |
| Northwestern | 1138 (17.6) | 1034 (18.2) | 104 (13.4) | 381 (18.0) | 390 (17.4) | 367 (17.4) |
| UCLA | 1273 (19.7) | 1138 (20.0) | 135 (17.4) | 251 (11.9) | 552 (24.7) | 470 (22.3) |
| Height, cm | 166.3 (10.0) | 166.4 (10.0) | 165.5 (10.0) | 166.4 (9.8) | 166.4 (10.1) | 166 (10.1) |
| Weight, lb | 172.9 (38.1) | 171.1 (37.1) | 185.6 (42.5) | 183.8 (39.7) | 172 (36.9) | 162.8 (34.6) |
| Highest level of education completed | ||||||
| High school | 2317 (35.9) | 2008 (35.3) | 309 (39.9) | 777 (36.8) | 812 (36.3) | 728 (34.5) |
| Some college/technical school | 1836 (28.4) | 1609 (28.3) | 227 (29.3) | 660 (31.2) | 619 (27.7) | 557 (26.4) |
| College graduate | 2287 (35.4) | 2053 (36.1) | 234 (30.2) | 667 (31.6) | 799 (35.7) | 821 (38.9) |
| Total gross family income, $ | ||||||
| <20 000 | 1491 (23.1) | 1289 (22.7) | 202 (26.1) | 513 (24.3) | 539 (24.1) | 439 (20.8) |
| 20 000 to 49 999 | 2260 (35.0) | 1953 (34.4) | 307 (39.6) | 764 (36.2) | 778 (34.8) | 718 (34.0) |
| ≥50 000 | 2463 (38.1) | 2237 (39.4) | 226 (29.2) | 719 (34.0) | 845 (37.8) | 899 (42.6) |
| Smoking status | ||||||
| Never | 3263 (50.5) | 2846 (50.1) | 417 (53.8) | 1025 (48.5) | 1186 (53.0) | 1052 (49.9) |
| Former | 2343 (36.3) | 2069 (36.4) | 274 (35.4) | 709 (33.6) | 794 (35.5) | 840 (39.8) |
| Current | 835 (12.9) | 756 (13.3) | 79 (10.2) | 370 (17.5) | 251 (11.2) | 214 (10.1) |
| Median physical activity, met min/wk | 840 (105.0 to 2032.5) | 840 (157.5 to 2100.0) | 630 (0.0 to 1582.5) | 630 (0.0 to 1680.0) | 787.5 (105.0 to 1888.1) | 1125 (330.0 to 2435.6) |
| Nutritional vitamin D intake, µg/day | 4.4 (3.8) | 4.4 (3.8) | 4.2 (4.0) | 4.0 (3.6) | 4.5 (3.9) | 4.7 (3.8) |
| Diabetes | ||||||
| Normal | 4755 (73.6) | 4240 (74.6) | 515 (66.5) | 1440 (68.2) | 1598 (71.4) | 1717 (81.4) |
| Impaired fasting glucose | 897 (13.9) | 754 (13.3) | 143 (18.5) | 352 (16.7) | 320 (14.3) | 225 (10.7) |
| Untreated diabetes | 170 (2.6) | 145 (2.6) | 25 (3.2) | 63 (3.0) | 76 (3.4) | 31 (1.5) |
| Treated diabetes | 631 (9.8) | 539 (9.5) | 92 (11.9) | 254 (12.0) | 242 (10.8) | 135 (6.4) |
| LDL, mg/dL | 117.0 (31.4) | 117.3 (31.0) | 114.4 (33.8) | 117.5 (33.0) | 117.5 (31.2) | 115.9 (29.9) |
| HDL, mg/dL | 51.1 (14.9) | 51.1 (14.9) | 50.9 (14.9) | 50.0 (14.3) | 49.7 (14.3) | 53.7 (15.7) |
| Mean triglycerides, mg/dL | 131.7 (89.4) | 132.2 (89.6) | 127.6 (88.0) | 124.7 (82.8) | 139.7 (105.3) | 130.0 (75.9) |
| Median triglycerides, mg/dL | 111 (77.0 to 161.0) | 112 (78.0 to 162.0) | 107 (77.0 to 153.8) | 104 (74.0 to 152.0) | 117 (83.0 to 170.0) | 112 (78.0 to 160.0) |
| Any lipid‐lowering medication | ||||||
| No | 5410 (83.8) | 4790 (84.3) | 620 (80.0) | 1812 (85.8) | 1859 (83.1) | 1739 (82.4) |
| Yes | 1046 (16.2) | 892 (15.7) | 154 (19.9) | 298 (14.1) | 378 (16.9) | 370 (17.5) |
| eGFR mL/min per 1.73 m2 | 78.2 (16.3) | 78.7 (15.6) | 74.2 (20.0) | 81.8 (17.1) | 78.3 (15.7) | 74.3 (15.1) |
| Mean albumin/creatinine ratio | 25.5 (150.8) | 21.2 (109.4) | 57.5 (318.3) | 28.7 (175.9) | 29.0 (173.5) | 18.7 (81.9) |
| Median albumin/creatinine ratio | 5.3 (3.3 to 11.0) | 5.1 (3.3 to 10.3) | 7.7 (4.3 to 18.8) | 5.8 (3.5 to 12.4) | 5.4 (3.3 to 11.6) | 4.9 (3.2 to 9.5) |
| Systolic blood pressure, mm Hg | 126.5 (21.5) | 125.4 (21.0) | 134.8 (23.4) | 128.5 (22.0) | 126.2 (21.5) | 124.8 (20.9) |
| Any hypertensive medication | ||||||
| No | 4081 (63.2) | 3690 (64.9) | 391 (50.5) | 1284 (60.8) | 1410 (63.0) | 1387 (65.7) |
| Yes | 2375 (36.8) | 1992 (35.0) | 383 (49.4) | 826 (39.1) | 827 (37.0) | 722 (34.2) |
| Serum calcium, mg/dL | 9.6 (0.4) | 9.7 (0.4) | 9.6 (0.5) | 9.6 (0.4) | 9.6 (0.4) | 9.7 (0.4) |
| Serum phosphorus, mg/dL | 3.7 (0.5) | 3.7 (0.5) | 3.5 (0.6) | 3.7 (0.5) | 3.6 (0.5) | 3.7 (0.5) |
| Mean FGF‐23, pg/mL | 40.3 (18.5) | 39.9 (17.4) | 43.3 (24.9) | 38.4 (17.5) | 40.6 (18.0) | 41.9 (19.7) |
| Median FGF‐23, pg/mL | 37.7 (30.5 to 46.4) | 37.4 (30.3 to 46.2) | 39.4 (31.4 to 49.0) | 36.5 (29.0 to 44.7) | 37.6 (30.7 to 46.5) | 38.8 (31.7 to 48.6) |
| Parathyroid hormone, pg/mL | 44.8 (21.8) | 39.1 (12.0) | 86.2 (31.1) | 52.7 (24.1) | 44.1 (22.8) | 37.5 (14.4) |
| Annualized 25(OH)D, ng/mL | 25.7 (11.2) | 26.6 (11.2) | 19.5 (9.5) | 14.3 (3.8) | 25.0 (2.9) | 38.0 (9.1) |
Mean (SD) for continuous covariates; N (%) for categorical covariates; median (IQR), where noted. CKD indicates chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; PTH, parathyroid hormone.
Among white participants, 7.4% had elevated PTH and 16.8% had low 25(OH)D; among black participants, 19% had elevated PTH and 60.8% had low 25(OH)D; among Chinese participants, 4.8% had elevated PTH and 20.4% had low 25(OH)D; and among Hispanic participants, 15.4% had elevated PTH and 32.8% had low 25(OH)D.
PTH and Incident HF
There were 180 incident HF events over a median (interquartile range) follow‐up time of 8.46 (7.67 to 8.63) years. Participants with higher PTH had higher crude rates of incident HF (Table 2). The crude rate of incident HF was highest among black and Hispanic participants with high PTH (Figure 1). Kaplan–Meier analysis demonstrated that the association of high PTH with incident HF was similar throughout follow‐up (Figure 2A).
Table 2.
Associations of Parathyroid Hormone (PTH) and 25‐Hydroxyvitamin D (25[OH]D) With Incident Heart Failure in the Multi‐Ethnic Study of Atherosclerosis (N=6459)
| Covariate | Number at Risk | Number of Events | Unadjusted Incidence Rate (per 1000 pys) | HR (95% CI) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| PTH | ||||||
| <65 pg/mL | 5684 | 138 | 3.1 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| ≥65 pg/mL | 775 | 42 | 7.4 | 1.96 (1.39, 2.77) | 1.44 (1.00, 2.06) | 1.50 (1.03, 2.19) |
| 25(OH)D | ||||||
| ≥30 ng/mL | 2110 | 53 | 3.2 | Ref | Ref | Ref |
| 20 to 29 ng/mL | 2112 | 62 | 3.8 | 1.19 (0.82, 1.72) | 1.02 (0.69, 1.51) | 0.95 (0.64, 1.41) |
| <20 ng/mL | 2237 | 65 | 3.9 | 1.29 (0.84, 1.98) | 1.04 (0.66, 1.63) | 0.97 (0.61, 1.55) |
Model 1: adjusted for age, sex, race/ethnicity, education. Model 2: Model 1+height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, systolic blood pressure, antihypertensive medications. Model 3: Model 2+ calcium, phosphorus, FGF‐23, PTH/25(OH)D (alternative measure to the predictor). ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; HR, hazard ratio; pys, person years.
Figure 1.

Unadjusted incidence rates of heart failure by parathyroid hormone concentration in various race/ethnic groups in the Multi‐Ethnic Study of Atherosclerosis. IR indicates incidence rates; PTH, parathyroid hormone.
Figure 2.

Cumulative incidence of heart failure by baseline serum concentrations of (A) parathyroid hormone (PTH) and (B) 25‐hydroxyvitamin D (25[OH]D) among 6459 participants in the Multi‐Ethnic Study of Atherosclerosis. CHF indicates congestive heart failure.
In unadjusted models, the functional form of the association of PTH with incident HF appeared linear (Figure 3, top left panel). In models adjusted for demographic characteristics, participants with higher PTH had a nearly 2‐fold risk of incident HF (Table 2). With additional adjustment for patient characteristics, comorbid diseases, medication use, and measures of kidney function, this association was attenuated but remained statistically significant with a 44% greater risk of incident HF. Adjustment for height and weight in these models was responsible for much of the attenuation (HR 1.96 [1.39, 2.77] to 1.71 [1.2, 2.44] with adjustment for height and weight). Further adjustment for other mineral metabolism markers did not alter this association (Table 2).
Figure 3.
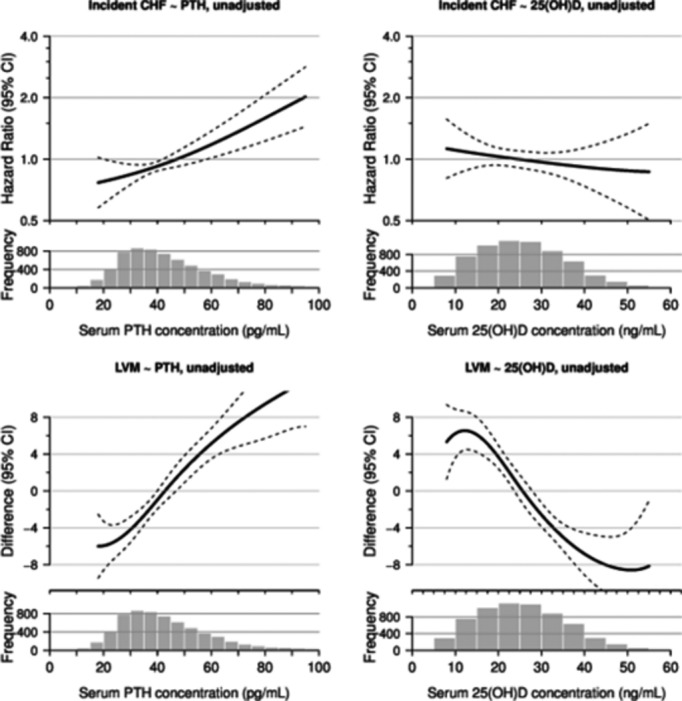
The smooth spline estimates the hazard ratio of incident heart failure by concentration of serum PTH (top left panel) and 25(OH)D (top right panel) and estimates the difference in left ventricular mass (LVM) by concentration of serum PTH (bottom left panel) and 25(OH)D (bottom right panel). CHF indicates congestive heart failure; PTH, parathyroid hormone.
In additional multivariable models (model 3) stratified by important participant characteristics, the association between PTH with incident HF was largely consistent across subgroups. The association of PTH ≥65 pg/mL with incident HF was stronger in blacks, those with eGFR <60 mL/min per 1.73 m2, and those in the highest quartile of FGF23, although the interactions for these characteristics were not statistically significant (P>0.05) (Figure 4).
Figure 4.

Adjusted association* of high parathyroid hormone (≥65 pg/mL vs <65 pg/mL) with incident heart failure by important subgroups in the Multi‐Ethnic Study of Atherosclerosis (N=6459). *Adjusted for age, sex, race/ethnicity, education, height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, SBP, antihypertensive medications, calcium, phosphorus, FGF‐23, and 25(OH)D. ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SBP, systolic blood pressure.
The adjusted risk difference of high versus low PTH for incident HF by race was highest in blacks (1.3 [0.2, 2.7] events per 1000 person‐years for blacks). The adjusted risk difference of high versus low PTH for incident HF was not statistically significant in other race/ethnic groups: −0.1 (−1.2, 1.2) events per 1000 person‐years for whites, 0.3 (−1.4, 3.6) events per 1000 person‐years for Chinese, and 0.4 (−1.0, 2.0) per 1000 person‐years for Hispanics. The P value for heterogeneity was <0.0001.
PTH and LVM
The mean LVM was 145 (39) g among the 4763 participants included in this study. In cross‐sectional analyses, participants with high PTH had higher mean LVM (Table 3). In unadjusted models, the association of PTH with LVM appeared linear (Figure 3, bottom left panel). In models adjusted for demographic characteristics, LVM was 11.1 g higher in participants with higher PTH. With additional adjustment for patient characteristics, comorbid diseases, medication use, and measures of kidney function, this association was attenuated but remained statistically significant. Adjustment for height and weight in these models was responsible for much of the attenuation (difference in LVM 11.1[8, 14.2] to 6.9 [4.1, 9.7] with adjustment for height and weight). Further adjustment for other mineral metabolism markers did not alter this association (Table 3).
Table 3.
Associations of Parathyroid Hormone (PTH) and 25‐Hydroxyvitamin D (25[OH]D) With Left Ventricular Mass (LVM) in the Multi‐Ethnic Study of Atherosclerosis (N=4763)
| Covariate | N | Unadjusted Mean (SD), g | Difference in LVM (95% CI), g | ||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||
| PTH | |||||
| <65 pg/mL | 4244 | 144 (38.8) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| ≥65 pg/mL | 519 | 155 (42.7) | 11.1 (8, 14.2) | 4.1 (1.5, 6.7) | 5.3 (2.6, 7.9) |
| 25(OH)D | |||||
| ≥30 ng/mL | 1631 | 139.5 (37.7) | Ref | Ref | Ref |
| 20 to 29 ng/mL | 1663 | 145.7 (39.1) | 2.8 (0.8, 4.8) | −0.8 (−2.4, 0.9) | −1.3 (−3, 0.4) |
| <20 ng/mL | 1469 | 151 (40.7) | 4.2 (1.9, 6.5) | −1.8 (−3.7, 0.1) | −2.8 (−4.9, −0.8) |
Model 1: adjusted for age, sex, race/ethnicity, education. Model 2: Model 1+height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, systolic blood pressure, antihypertensive medications. Model 3: Model 2 +calcium, phosphorus, FGF‐23, PTH/25(OH)D (alternative measure to the predictor). ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate.
In multivariable models (model 3) stratified by important participant characteristics, the difference in LVM in participants with high PTH was stronger in Chinese participants, and those with eGFR <60 mL/min per 1.73 m2, although the interactions for these characteristics were not statistically significant (P>0.05). However, the difference in LVM in participants with high PTH was significantly stronger in men versus women (Figure 5).
Figure 5.

Association* of high parathyroid hormone (≥65 pg/mL vs <65 pg/mL) with left ventricular mass in important subgroups in the Multi‐Ethnic Study of Atherosclerosis (N=4763). *Adjusted for age, sex, race/ethnicity, education, height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, SBP, antihypertensive medications, calcium, phosphorus, and 25(OH)D. ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
25(OH)D and Incident HF
Crude rates of incident HF were similar across categories of 25(OH)D (Table 2). However, the association of lower 25(OH)D appeared to be stronger after almost 5 years of follow‐up (Figure 2B). In unadjusted models, the functional form of the association between 25(OH)D with incident HF appeared linear (Figure 3, top right panel). In multivariable models, there was no association between low 25(OH)D and incident HF (Table 2). Interactions of categorical 25(OH)D with continuous PTH concentration were not statistically significant (P=0.4). There was no difference in the association of low 25(OH)D with incident HF in specific subgroups (Figure 6).
Figure 6.

Association* of low 25‐hydroxyvitamin D (<20 ng/mL vs ≥20 ng/mL) with incident heart failure in important subgroups in the Multi‐Ethnic Study of Atherosclerosis (N=6459). *Adjusted for age, sex, race/ethnicity, education, height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, SBP, antihypertensive medications, calcium, phosphorus, and PTH. ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; HR, hazard ratio; PTH, parathyroid hormone; SBP, systolic blood pressure.
25(OH)D and LVM
In cross‐sectional unadjusted models, the association between 25(OH)D and LVM appeared linear (Figure 3, bottom right panel). In multivariable models, there was no association between 25(OH)D with LVM (Table 3). Interactions by PTH concentration was not statistically significant (P=0.3). The association of 25(OH)D with LVM was fairly consistent across all subgroups (data not shown). However, there was a statistically significant interaction by gender where women had lower LVM with low 25(OH)D concentrations (P=0.01).
Sensitivity Analyses
In the first sensitivity analysis, we adjusted for baseline LVM in the models examining the association of PTH with incident HF in the subgroup of participants that had measures of LVM available. In this analysis adjusted for patient characteristics, comorbid diseases, medication use, measures of kidney function, and mineral metabolism markers, the association of PTH≥65 pg/mL was similar to the primary analysis (HR 1.67 [95% CI 1.01, 2.75]) (Table 4). This association was moderately attenuated with adjustment for LVM (HR 1.34 [95% CI 0.78, 2.3]) (Table 4). Similar to the primary analysis, there was no association of insufficient or deficient 25(OH)D with incident HF, which did not change with adjustment for LVM (Table 4).
Table 4.
Associations of Parathyroid Hormone (PTH) and 25‐Hydroxyvitamin D (25[OH]D) With Incident Heart Failure in the Multi‐Ethnic Study of Atherosclerosis, Adjusted for Baseline Left Ventricular Mass (LVM) (N=4759)**
| Covariate | Number at Risk | Number of Events | Unadjusted Incidence Rate (per 1000 pys) | HR (95% CI) | |
|---|---|---|---|---|---|
| Model 3a | Model 3b | ||||
| PTH | |||||
| <65 pg/mL | 4240 | 89 | 2.7 | Ref | Ref |
| ≥65 pg/mL | 519 | 25 | 6.6 | 1.67 (1.01, 2.75) | 1.34 (0.78, 2.3) |
| 25(OH)D | |||||
| ≥30 ng/mL | 1631 | 34 | 2.6 | Ref | Ref |
| 20 to 29 ng/mL | 1661 | 42 | 3.3 | 1.08 (0.66, 1.77) | 1.17 (0.71, 1.91) |
| <20 ng/mL | 1467 | 38 | 3.5 | 1.17 (0.65, 2.10) | 1.19 (0.64, 2.20) |
Model 3a: adjusted for age, sex, race/ethnicity education, height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, systolic blood pressure, antihypertensive medications, calcium, phosphorus, FGF‐23, PTH/25(OH)D (alternative measure to the predictor). Model 3b: Model 1+baseline LVM. ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; pys, person years.
These analyses were based on a complete case approach in which participants with missing covariate data were excluded (rather than using multiple imputation for missing covariates as was done for the primary analyses).
This analysis excluded 4 participants who did not have time‐to‐event data available.
In the second sensitivity analysis, we examined whether the association of elevated PTH with incident HF was similar in preserved ejection fraction versus reduced ejection fraction HF (Table 5). In these analyses, 37 participants with a HF hospitalization did not have LVEF information available. However, among the remaining participants, we found that the association of elevated PTH was significantly associated with risk of reduced ejection fraction HF (Table 5). While the association of elevated PTH with preserved ejection fraction HF was not statistically significantly, the HR was similar to that in the main analysis (Table 5).
Table 5.
Associations of Parathyroid Hormone (PTH) With Incident Preserved and Reduced Ejection Fraction (EF) Heart Failure in the Multi‐Ethnic Study of Atherosclerosis (N=6458)**
| Number at Risk | Number of Events | Unadjusted Incidence Rate (per 1000 pys) | Model 1 | HR (95% CI) | ||
|---|---|---|---|---|---|---|
| Model 2 | Model 3 | |||||
| Preserved EF | ||||||
| PTH <65 pg/mL | 5683 | 47 | 1.1 | Ref | Ref | Ref |
| PTH ≥65 pg/mL | 775 | 12 | 2.1 | 1.84 (1.00, 3.39) | 1.43 (0.76, 2.71) | 1.41 (0.74, 2.69) |
| Reduced EF | ||||||
| PTH <65 pg/mL | 5683 | 59 | 1.3 | Ref | Ref | Ref |
| PTH ≥65 pg/mL | 775 | 24 | 4.2 | 2.66 (1.65, 4.29) | 1.92 (1.14, 3.25) | 1.90 (1.10, 3.30) |
Model 1: adjusted for age, sex, race/ethnicity, education. Model 2: Model 1+height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, systolic blood pressure, antihypertensive medications. Model 3: Model 2+calcium, phosphorus, FGF‐23, 25(OH)D. ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; pys, person years.
These analyses were based on a complete case approach in which participants with missing covariate data were excluded (rather than using multiple imputation for missing covariates as was done for the primary analyses).
One participant did not have LVEF information at the time of hospitalization and so was excluded from this analysis.
In the third sensitivity analysis, we examined whether adjustment for interim adjudicated nonfatal MI attenuated the associations of PTH and 25(OH)D with incident HF. There were 175 nonfatal MI events among study participants during the follow‐up period. Adjustment for nonfatal MI very minimally attenuated the association of elevated PTH with incident HF (Table 6). Similar to the main analysis, there remained no association of 25(OH)D with incident HF (Table 6).
Table 6.
Associations of Parathyroid Hormone (PTH) and 25‐Hydroxyvitamin D (25[OH]D) With Incident Heart Failure in the Multi‐Ethnic Study of Atherosclerosis (N=6347), Adjusted for Non‐Fatal MI*
| Covariate | Number at Risk | Number of Events | Unadjusted Incidence Rate (per 1000 pys) | HR (95% CI) | |
|---|---|---|---|---|---|
| Model 3a | Model 3b | ||||
| PTH | |||||
| <65 pg/mL | 5594 | 136 | 3.1 | 1.00 (Ref) | 1.00 (Ref) |
| ≥65 pg/mL | 753 | 39 | 7.0 | 1.46 (0.99, 2.14) | 1.42 (0.95, 2.11) |
| 25(OH)D | |||||
| ≥30 ng/mL | 2084 | 51 | 3.1 | Ref | Ref |
| 20 to 29 ng/mL | 2197 | 65 | 3.8 | 0.99 (0.67, 1.48) | 1.02 (0.68, 1.52) |
| <20 ng/mL | 2066 | 59 | 3.8 | 0.97 (0.60, 1.56) | 1.01 (0.61, 1.67) |
Model 3a: adjusted for age, sex, race/ethnicity, education, height, weight, smoking, physical activity level, diabetes, eGFR, urine ACR, systolic blood pressure, antihypertensive medications, calcium, phosphorus, FGF‐23, PTH/25(OH)D (alternative measure to the predictor). Model 3b: Model 3a +additionally adjusted for non‐fatal MI as a time‐varying covariate. ACR indicates albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; HR, hazard ratio; MI, myocardial infarction; pys, person years.
These analyses were based on a complete case approach in which participants with missing covariate data were excluded (rather than using multiple imputation for missing covariates as was done for the primary analyses).
In the final sensitivity analysis, we repeated the analyses examining the associations of PTH with incident HF and LVM, excluding participants with elevated serum calcium (defined as >10.2 mg/dL). Excluding 75 participants (of whom 2 had incident HF events), the multivariable association between elevated PTH and incident HF was unchanged (HR 1.5, 95% CI: 1.02, 2.21). Excluding 55 participants with elevated serum calcium, the difference in LVM among participants with elevated PTH was also unchanged in multivariable models (5.3 g, 95% CI: 2.5, 8.1).
Discussion
Among a large, racially and ethnically diverse population without prevalent cardiovascular disease at baseline, we found that elevated serum PTH concentration was significantly associated with higher risk of incident HF, independent of known HF risk factors. Furthermore, PTH was also associated with greater LVM, and adjustment for LVM partially attenuated the association of PTH with HF. These associations were consistent after adjustment for a comprehensive panel of mineral metabolism markers, including FGF23. In contrast, there was no independent association of 25(OH)D with incident HF or LVM. These results suggest that PTH excess may be associated with incident HF, independent of other mineral metabolism markers, and in part by promoting LVH.
Associations of PTH with incident HF have been reported previously. In a study of older adults in the Cardiovascular Health Study, participants with high serum PTH concentrations had a 30% (95% CI: 6% to 61%) higher risk of incident HF.3 In a study of older, Swedish men, each SD increment in PTH was associated with 41% greater risk (95% CI: 12% to 77%) of HF hospitalization.40 Among men 60 to 79 years of age, another study reported that elevated PTH was associated with a 66% (95% CI: 1.30, 2.13) increased risk of incident HF.41 In contrast, in a European study of middle aged men and women, higher PTH was only associated with incident HF among those who were obese.42 Contrary to our findings, a recent analysis from The Artherosclerosis Risk in Communities Study did not find an association of elevated PTH with incident HF after adjustment for cardiovascular risk factors.43 The main difference between our study and this one was that we included slightly older participants as well as Chinese and Hispanic participants, which may explain in part the discrepant findings.43 Overall, our study extends prior investigations by studying a relatively large, racially/ethnically diverse population with a broad age range and free of clinical cardiovascular disease at baseline.
Black race, in particular, is a known risk factor for 25(OH)D deficiency and elevated PTH concentration as well as HF, particularly at younger ages.23 In our study population, while heterogeneity of the PTH‐HF association by race/ethnicity was not statistically significant, it is interesting that the prevalence of elevated PTH concentration and the rate of incident HF were highest among black participants and that the adjusted risk difference of high versus low PTH was statistically significant in blacks and not in other race/ethnic groups. A previous study also noted important race/ethnic differences in the association of mineral metabolism markers with risk of coronary heart disease.34 Our results suggest that there may be heterogeneity in the association of elevated PTH with HF by race/ethnic group. This warrants further investigation, particularly among black participants.
Prior studies have also noted associations of elevated PTH concentrations with greater LVM. In a study of middle‐aged men and women from Norway, those with the highest concentrations of serum PTH had 12% to 17% higher adjusted LVM.9 A study of Cardiovascular Health Study participants as well as a study of Dutch participants found associations of PTH with higher LVM only in those with reduced eGFR.44–45 In our study, associations of elevated PTH with LVM was not stronger among participants with eGFR <60 mL/min per 1.73 m2. Another study of men and women with primary hyperparathyroidism noted no difference in LVM compared with healthy controls.46 In our study, we excluded the few participants with possible primary hyperparathyroidism and focused on possible secondary hyperparathyroidism, which may explain the difference between our study and this prior study.
Our study provides evidence that PTH may be an important contributor to the development of subclinical and clinical HF. While observational studies cannot demonstrate causality, our results combined with prior studies do demonstrate consistent temporal associations of elevated PTH with subsequent HF risk and point to elevated LVM as a plausible partial mediator of this relationship. While the primary role of PTH is to maintain and regulate calcium and phosphorus homeostasis, PTH receptors have been identified in cardiomyocytes, vascular smooth muscle, and endothelial cells.7–8 A study of adult rat ventricular cardiomyocytes found that low concentrations of PTH improved cell shortening of cardiomyocytes,47 suggesting that elevated PTH interferes with normal and adaptive myocyte contractility. In rats, PTH exerts a trophic effect on cardiomyocytes, with an increase in total cellular mass.48 Furthermore, clinical studies have shown associations between PTH and hypertension, impaired brachial artery flow‐mediated dilation and aortic pulse pressure,10–12 all of which may lead to increased afterload, thereby increasing the risk of HF. Further studies are needed to determine whether targeting PTH can decrease the risk of HF.
We did not find an association of 25(OH)D with incident HF. These results are consistent with some, but not all, prior studies of 25(OH)D. In the Framingham Offspring Study, 25(OH)D <15 ng/mL was associated with increased risk of a composite cardiovascular outcome.22 However, HF as an individual outcome was not evaluated, and relationships of 25(OH)D with specific cardiovascular diseases may vary. For example, lower serum 25(OH)D concentration was associated with increased risk of coronary heart disease in white and Chinese MESA participants,34 but not with HF in the current study. A study of over 3299 white patients referred to coronary angiography found that baseline 25(OH)D was associated with impaired left ventricular function at baseline and with >2.84‐fold risk (95% CI: 1.20 to 6.74) of death from HF.18 The inclusion of subjects with symptomatic cardiovascular disease (raising concerns for reverse causality and residual confounding) and racial/ethnic homogeneity are limitations to this study. Similar to our results, in the Cardiovascular Health Study no association between low 25(OH)D concentrations and incident HF was observed.3 Also, among older men, another investigation reported no association of low 25(OH)D with incident HF after adjustment for age.41
We also did not observe an association of 25(OH)D with LVM. Results of prior investigations are conflicting. A study of 1800 participants from Italy did find an association between low 25(OH)D and elevated LVM.49 However, similar to our results, the studies from the Cardiovascular Health Study and the Netherlands that demonstrated associations of PTH with LVM found no association between 25(OH)D and LVM.45,50 Furthermore, the short‐term clinical trials completed to date have demonstrated no improvement of LVM with vitamin D treatments.51–53 One possible explanation for the lack of improvement in HF outcomes with vitamin D supplementation may be because vitamin D supplementation may not be very effective in reducing PTH concentrations. For example, some trials of vitamin D supplementation have demonstrated that although there was a significant increase in 25(OH)D levels, PTH does not significantly decrease.54–55 Overall, mixed results of human studies evaluating 25(OH)D, HF, and LVM temper enthusiasm that 25(OH)D may be a powerful intervention to prevent and treat HF.
Adjustment for other mineral metabolism markers including serum calcium, phosphorus, and FGF23 did not attenuate the association of higher PTH with incident HF and higher LVM, suggesting that excess PTH may have an independent effect on cardiomyocytes. FGF23 is considered to be causal in inducing cardiac hypertrophy.56 However, we observed that PTH excess may be associated with HF and increased LVM, independent of FGF23 and other mineral metabolism markers. We did, however, note that the association of higher PTH with incident HF was stronger for participants in the highest quartile of FGF23, although the interaction by FGF23 concentration was not statistically significant. Adjustment for a comprehensive panel of simultaneously measured mineral metabolism markers in our study is an advance over prior investigations.
Our study had several strengths. We studied a large, racially/ethnically diverse community‐based population, which extends the external validity of our findings. Incident HF was defined by rigorous adjudication processes. Cardiac magnetic resonance imaging, a “gold standard” technique, was used to quantify LVM and was centrally read. Thus, our ascertainment of our outcomes was rigorous. We were able to reduce residual confounding because MESA excluded people with prevalent clinical cardiovascular disease and because we adjusted for a comprehensive panel of important mineral markers. We do recognize a few limitations as well. We had single baseline measures of PTH and 25(OH)D. We were not able to capture use of vitamin D supplements. It is possible that cases of incident HF were missed as the adjudication process for HF relied on initial participant reporting of a hospitalization. This was an observational study, so we cannot determine causality.
In conclusion, in a racially/ethnically diverse population without prevalent cardiovascular disease, elevated serum PTH concentration was associated with increased LVM and increased risk of incident HF. These results are consistent with prior investigations and extend these findings to a more generalizable patient population. Our study may augment a body of growing literature that supports the need for further intervention studies to determine whether targeting elevated PTH may help prevent or treat HF.
Sources of Funding
This work was supported by the following grants from the National Institutes of Health: K23DK088865 (Bansal) and R01HL096875 (de Boer). This research was also supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐RR‐025005 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd‐Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MBAmerican Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012; 125:e2-e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta‐analysis. JAMA. 2011; 305:1119-1127. [DOI] [PubMed] [Google Scholar]
- 3.Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol. 2011; 58:1433-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagstrom E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundstrom J, Melhus H, Held C, Lind L, Michaelsson K, Arnlov J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009; 119:2765-2771. [DOI] [PubMed] [Google Scholar]
- 5.Zhao G, Ford ES, Li C, Croft JB. Serum 25‐hydroxyvitamin D levels and all‐cause and cardiovascular disease mortality among US adults with hypertension: the NHANES linked mortality study. J Hypertens. 2012; 30:284-289. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Chen M, Hankins SR, Nunez AE, Watson RA, Weinstock PJ, Newschaffer CJ, Eisen HJDrexel Cardiovascular Health Collaborative Education, Research, and Evaluation Group. Serum 25‐hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all‐cause in United States adults. Am J Cardiol. 2012; 110:834-839. [DOI] [PubMed] [Google Scholar]
- 7.Monego G, Arena V, Pasquini S, Stigliano E, Fiaccavento R, Leone O, Arpesella G, Potena L, Ranelletti FO, Di Nardo P, Capelli A. Ischemic injury activates PTHrP and PTH1R expression in human ventricular cardiomyocytes. Basic Res Cardiol. 2009; 104:427-434. [DOI] [PubMed] [Google Scholar]
- 8.Potthoff SA, Janus A, Hoch H, Frahnert M, Tossios P, Reber D, Giessing M, Klein HM, Schwertfeger E, Quack I, Rump LC, Vonend O. PTH‐receptors regulate norepinephrine release in human heart and kidney. Regul Pept. 2011; 171:35-42. [DOI] [PubMed] [Google Scholar]
- 9.Saleh FN, Schirmer H, Sundsfjord J, Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003; 24:2054-2060. [DOI] [PubMed] [Google Scholar]
- 10.van Ballegooijen AJ, Kestenbaum B, Sachs MC, de Boer IH, Siscovick DS, Hoofnagle AN, Ix JH, Visser M, Brouwer IA. Association of 25‐hydroxyvitamin D and parathyroid hormone with incident hypertension: The Multi‐Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2014; 63:1214-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosworth C, Sachs MC, Duprez D, Hoofnagle AN, Ix JH, Jacobs DR, Jr, Peralta CA, Siscovick DS, Kestenbaum B, de Boer IH. Parathyroid hormone and arterial dysfunction in the multi‐ethnic study of atherosclerosis. Clin Endocrinol (Oxf). 2013; 79:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mateus‐Hamdan L, Beauchet O, Bouvard B, Legrand E, Fantino B, Annweiler C. High parathyroid hormone, but not low vitamin D concentrations, expose elderly inpatients to hypertension. Geriatr Gerontol Int. 2013; 13:783-791. [DOI] [PubMed] [Google Scholar]
- 13.van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: a systematic review and meta‐analysis of prospective studies. Am Heart J. 2013; 165:655-664. [DOI] [PubMed] [Google Scholar]
- 14.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin‐angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004; 89–90:387-392. [DOI] [PubMed] [Google Scholar]
- 15.Assalin HB, Rafacho BP, dos Santos PP, Ardisson LP, Roscani MG, Chiuso‐Minicucci F, Barbisan LF, Fernandes AA, Azevedo PS, Minicucci MF, Zornoff LA, de Paiva SA. Impact of the length of vitamin D deficiency on cardiac remodeling. Circ Heart Fail. 2013; 6:809-816. [DOI] [PubMed] [Google Scholar]
- 16.Caprio M, Mammi C, Rosano GM. Vitamin D: a novel player in endothelial function and dysfunction. Arch Med Sci. 2012; 8:4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta GK, Agrawal T, DelCore MG, Mohiuddin SM, Agrawal DK. Vitamin D deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swine. Exp Mol Pathol. 2012; 93:82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilz S, Marz W, Wellnitz B, Seelhorst U, Fahrleitner‐Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross‐sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008; 93:3927-3935. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol. 2008; 102:1540-1544. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto T, Tanigawa T, Onishi K, Fujimoto N, Matsuda A, Nakamori S, Matsuoka K, Nakamura T, Koji T, Ito M. Serum intact parathyroid hormone levels predict hospitalisation for heart failure. Heart. 2009; 95:395-398. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JL, Vanwoerkom RC, Horne BD, Bair TL, May HT, Lappe DL, Muhlestein JB. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? Am Heart J. 2011; 162:331-339. [DOI] [PubMed] [Google Scholar]
- 22.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008; 117:503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009; 360:1179-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002; 156:871-881. [DOI] [PubMed] [Google Scholar]
- 25.Sachs MC, Shoben A, Levin GP, Robinson‐Cohen C, Hoofnagle AN, Swords‐Jenny N, Ix JH, Budoff M, Lutsey PL, Siscovick DS, Kestenbaum B, de Boer IH. Estimating mean annual 25‐hydroxyvitamin D concentrations from single measurements: the Multi‐Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013; 97:1243-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008; 88:511S-512S. [DOI] [PubMed] [Google Scholar]
- 27.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008; 52:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the multi‐ethnic study of atherosclerosis. Arch Intern Med. 2008; 168:2138-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan RT, Bluemke D, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, McClelland R, Lima JA. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events MESA (multi‐ethnic study of atherosclerosis). J Am Coll Cardiol. 2011; 57:1735-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi‐ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006; 186:S357-S365. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD‐EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010; 55:622-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson‐Cohen C, Lima JA, Polak JF, Blondon M, Ruzinski J, Rock D, de Boer IH. Fibroblast growth factor‐23 and cardiovascular disease in the general population: the Multi‐Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014; 7:409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson‐Cohen C, Katz R, Hoofnagle AN, Cauley JA, Furberg CD, Robbins JA, Chen Z, Siscovick DS, de Boer IH, Kestenbaum B. Mineral metabolism markers and the long‐term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab. 2011; 96:2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson‐Cohen C, Hoofnagle AN, Ix JH, Sachs MC, Tracy RP, Siscovick DS, Kestenbaum BR, de Boer IH. Racial differences in the association of serum 25‐hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013; 310:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. Vitamin D parathyroid hormone, and sudden cardiac death: results from the Cardiovascular Health Study. Hypertension. 2011; 58:1021-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.http://www.ncbi.nlm.nih.gov/books/NKD56070/ [Google Scholar]
- 37.Royston P. Multiple imputation of missing values. The STATA Journal. 2004; 4:227-241. [Google Scholar]
- 38.Rubin DB. Multiple Imputation for Nonresponse in Surveys. 1987Hoboken: Wiley Publishing [Google Scholar]
- 39.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 62:e147-e239. [DOI] [PubMed] [Google Scholar]
- 40.Hagstrom E, Ingelsson E, Sundstrom J, Hellman P, Larsson TE, Berglund L, Melhus H, Held C, Michaelsson K, Lind L, Arnlov J. Plasma parathyroid hormone and risk of congestive heart failure in the community. Eur J Heart Fail. 2010; 12:1186-1192. [DOI] [PubMed] [Google Scholar]
- 41.Wannamethee SG, Welsh P, Papacosta O, Lennon L, Whincup PH, Sattar N. Elevated parathyroid hormone, but not vitamin D deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circ Heart Fail. 2014; 7:732-739. [DOI] [PubMed] [Google Scholar]
- 42.di Giuseppe R, Buijsse B, Hirche F, Wirth J, Arregui M, Westphal S, Isermann B, Hense HW, Dierkes J, Boeing H, Stangl GI, Weikert C. Plasma fibroblast growth factor 23, parathyroid hormone, 25‐hydroxyvitamin D3, and risk of heart failure: a prospective, case‐cohort study. J Clin Endocrinol Metab. 2014; 99:947-955. [DOI] [PubMed] [Google Scholar]
- 43.Folsom AR, Alonso A, Misialek JR, Michos ED, Selvin E, Eckfeldt JH, Coresh J, Pankow JS, Lutsey PL. Parathyroid hormone concentration and risk of cardiovascular diseases: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2014; 168:296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Ballegooijen AJ, Visser M, Kestenbaum B, Siscovick DS, de Boer IH, Gottdiener JS, deFilippi CR, Brouwer IA. Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the Cardiovascular Health Study). Am J Cardiol. 2013; 111:418-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Ballegooijen AJ, Snijder MB, Visser M, van den Hurk K, Kamp O, Dekker JM, Nijpels G, Stehouwer CD, Henry RM, Paulus WJ, Brouwer IA. Vitamin D in relation to myocardial structure and function after eight years of follow‐up: the Hoorn study. Ann Nutr Metab. 2012; 60:69-77. [DOI] [PubMed] [Google Scholar]
- 46.Walker MD, Fleischer JB, Di Tullio MR, Homma S, Rundek T, Stein EM, Zhang C, Taggart T, McMahon DJ, Silverberg SJ. Cardiac structure and diastolic function in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2010; 95:2172-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tastan I, Schreckenberg R, Mufti S, Abdallah Y, Piper HM, Schluter KD. Parathyroid hormone improves contractile performance of adult rat ventricular cardiomyocytes at low concentrations in a non‐acute way. Cardiovasc Res. 2009; 82:77-83. [DOI] [PubMed] [Google Scholar]
- 48.Schluter KD, Piper HM. Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol. 1992; 263:H1739-H1746. [DOI] [PubMed] [Google Scholar]
- 49.Masson S, Agabiti N, Vago T, Miceli M, Mayer F, Letizia T, Wienhues‐Thelen UH, Mureddu GF, Davoli M, Boccanelli A, Latini R. The fibroblast growth factor‐23 and vitamin D emerge as non‐traditional risk factors and may affect cardiovascular risk. J Intern Med. 2014 [DOI] [PubMed] [Google Scholar]
- 50.van Ballegooijen AJ, Visser M, Cotch MF, Arai AE, Garcia M, Harris TB, Launer LJ, Eiriksdottir G, Gudnason V, Brouwer IA. Serum vitamin D and parathyroid hormone in relation to cardiac structure and function: the ICELAND‐MI substudy of AGES‐Reykjavik. J Clin Endocrinol Metab. 2013; 98:2544-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witham MD, Ireland S, Houston JG, Gandy SJ, Waugh S, Macdonald TM, Mackenzie IS, Struthers AD. Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: randomized, controlled trial. Hypertension. 2014; 63:706-712. [DOI] [PubMed] [Google Scholar]
- 52.Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, McMurdo ME. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013; 173:1672-1679. [DOI] [PubMed] [Google Scholar]
- 53.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd‐Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012; 307:674-684. [DOI] [PubMed] [Google Scholar]
- 54.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010; 3:195-201. [DOI] [PubMed] [Google Scholar]
- 55.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Combined calcium and vitamin D supplementation is not superior to calcium supplementation alone in improving disturbed bone metabolism in patients with congestive heart failure. Eur J Clin Nutr. 2008; 62:1388-1394. [DOI] [PubMed] [Google Scholar]
- 56.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon‐Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011; 121:4393-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]


