Abstract
Background
A significant number of implantable cardioverter‐defibrillator (ICD) patients do not experience shocks after ICD implant. Elective generator exchange (GE) has been associated with increased risk of infection and ICD lead failure. There is a paucity of contemporary data reporting on shock incidence with replacement devices.
Methods and Results
Patients undergoing elective GE (n=24 203) who transmit data remotely via a remote monitoring system were analyzed to determine the incidence of ICD shocks after GE. A total of 16 230 patients (67%) did not experience a shock with the first ICD (group A), and 7973 (33%) received at least 1 shock (group B). Patients in group A were older (71.3 versus 68.8 years, P<0.001) and more often female (71% versus 77% male, P<0.001). Over an average follow‐up of 1.9±1.2 years after GE, the proportion of patients with shocks and risk of ICD shocks was lower for those who did not receive a shock during the first battery life (group A: 9.9% versus 27.7%, hazard ratio 0.36, 95% CI 0.34 to 0.38, P<0.001). The cumulative rate of ICD shocks at 5 years after GE was 25.7% in group A and 51.1% in group B.
Conclusions
In this large cohort of ICD patients implanted across the United States, two thirds did not receive ICD shock therapy prior to GE. The occurrence of ICD shocks prior to GE is an important predictor of shocks after GE; however, even among those without shocks during first battery life, the incidence of shocks at 5 years following GE is >25%. These data should support informed decision making for patients and physicians at the time of ICD generator end of service.
Keywords: defibrillation, electrophysiology, sudden death
Introduction
Implantable cardioverter‐defibrillator (ICD) therapy is associated with significant reductions in all‐cause mortality among patients with a history of cardiac arrest (secondary prevention) or patients with cardiomyopathy but without a documented history of ventricular arrhythmias (primary prevention). In the United States, >80% of new ICD implants are performed for primary prevention,1 and long‐term follow‐up from clinical trials suggests that the annual rate of appropriate ICD therapy in these patients ranges between 5% and 12% per year.2–4 Data from the National Cardiovascular Data Registry (NCDR) demonstrate that the median ICD pulse generator service life is between 4 and 5 years, suggesting that a large percentage of patients implanted with ICDs will reach the end of battery life without receiving ICD shocks during the first battery service life.1 ICD therapy is often viewed as a life‐long commitment for which patients are scheduled for pulse generator exchange (GE) as a routine matter of course at the end of service life; however, 2 important considerations should prompt a more detailed risk–benefit analysis prior to elective GE. First, elective ICD GE is associated with a major complication rate of ≈4%,5 and the occurrence of major complications in this setting may be associated with an increased risk of mortality.6 Second, compared with patients undergoing initial ICD implantation, patients receiving replacement devices are older, have more comorbidities, and have shorter life expectancy.1 Consequently, the risk–benefit ratio of elective GE may be different than that at the time of initial ICD implant. Informed decision making regarding ICD GE has been limited by a paucity of contemporary data on prognosis and incidence of ICD therapies following GE. We analyzed the incidence of ICD shocks among all patients using the Latitude remote monitoring system (Boston Scientific Corp) who underwent at least 1 elective ICD pulse GE.
Methods
Study Design
The ALTITUDE project is a clinical science initiative formed to prospectively analyze data from ICD and cardiac resynchronization therapy‐defibrillator (CRT‐D) devices followed on a remote monitoring system (Latitude). Deidentified patient data from the remote monitoring system were analyzed for this study. Participating centers elected to engage in a data use agreement that allows for the use of the data for research purposes in accordance with the US Health Insurance Portability and Accountability Act of 1996. At the time of this analysis, 173 957 patients with ICDs at 1875 centers were being followed on the Latitude system, of whom 31 195 died prior to reaching the end of battery service life or device explant. A total of 35 954 patients underwent explant of at least 1 ICD pulse generator for any indication, but only patients undergoing elective GE at end of battery service life (n=24 203) were included in this cohort. ICD explants for other indications (ie, system upgrade, hardware recall, or infection) were excluded from this analysis. The data set included follow‐up through November 2012, and patients undergoing elective GE at end of battery service life were implanted from November 2000 to September 2009.
The initial decision to implant an ICD, the decision to proceed with elective GE, and all device programming were performed at the discretion of the treating physician. Patients were stratified based on whether they had received ICD shocks during the first pulse GE service life. For the purpose of this study, only ICD shocks were included in the analysis. Other forms of ICD therapy, such as antitachycardia pacing, were not included. Intracardiac electrogram adjudication for determining appropriate versus inappropriate ICD shocks was not available for this analysis; therefore, all delivered shocks were included. Given the reduced likelihood of inappropriate ICD shocks with an initial detection rate of ≥200 beats per minute (bpm),7 we included a subgroup analysis of ICD shocks occurring after GE for detection rates above this cutoff.
Statistical Analysis
Continuous variables are presented as mean±SD, and categorical data are summarized as frequencies and percentages. The primary end point was incidence of ICD shocks following elective GE. The incidence of the primary end point, stratified by whether or not ICD shocks occurred during the first battery life, was estimated by Kaplan–Meier curves for time to first event. The log‐rank test was used to calculate P values, and hazard ratios (HRs) and confidence intervals (CIs) were generated from a univariate Cox proportional hazards model. For all Cox proportional hazards models, the proportional hazards assumption was assessed visually via plots of log(−log[survival]) versus log(time).
For all comparisons, P<0.05 was considered to be statistically significant. Analysis was performed using the SAS System, release 8.2 software or higher (SAS Institute Inc).
Results
The study cohort included 24 203 patients who underwent elective pulse GE while being followed on the Latitude system. Of those, 5505 (22.8%) had single‐chamber ICDs, 8507 (35.1%) had dual‐chamber ICDs, and 10 191 (42.1%) had CRT‐D devices. Of these patients, 16 230 (67%) did not receive any ICD shocks with the first battery life (group A), and the remaining 7973 (33%) received at least 1 ICD shock (group B). Across the entire cohort, mean age at the time of GE was 70.5±11.9 years, 72.8% were male, and mean longevity of the first pulse generator was 5.4±1.3 years. As highlighted in Table 1, patients in group A were significantly older and more often female, and a larger percentage had CRT‐D devices. Among patients with atrial leads (group A: 78.6%; group B: 74.5%), the frequency of patients with a device‐detected atrial fibrillation burden of >5% was lower in group A (24.5% versus 31.9%, P<0.001), and among patients with CRT devices, those in group A were more likely to have a percentage of left ventricular pacing >95%. Patients in group A were more frequently programmed with a single zone for tachytherapy detection, whereas patients in group B were more likely to be programmed with 2 or 3 zones. For patients in both groups A and B, antitachycardia pacing therapy was programmed ON in the ventricular tachycardia zones in >99% of patients who had a ventricular tachycardia therapy zone programmed.
Table 1.
Baseline Characteristics
| Group A (n=16 230) | Group B (n=7973) | P Value | |
|---|---|---|---|
| Age at GE, y | 71.3±11.6 | 68.8±12.5 | <0.001 |
| Male sex, % | 70.9 | 76.9 | <0.001 |
| Time of first battery implant, y | 5.4±1.3 | 5.3±1.2 | <0.001 |
| Device type, % | <0.001 | ||
| Single‐chamber ICD | 21.4 | 25.5 | |
| Dual‐chamber ICD | 34.0 | 37.4 | |
| Biventricular ICD | 44.6 | 37.1 | |
| Tachytherapy device programming, % | <0.001 | ||
| 1 zone | 27.7 | 20.6 | |
| 2 zones | 51.5 | 53.4 | |
| 3 zones | 20.8 | 26.0 | |
| VT dectection rate, bpm | 174±14 | 172±16 | <0.001 |
| VF detection rate, bpm | 202±17 | 203±18 | <0.001 |
| Afib burden >5%*, % | 24.5 | 31.9 | <0.001 |
| RV pacing >5%, % | 42.9 | 47.0 | <0.001 |
| LV pacing >95%*, % | 75.9 | 61.8 | <0.001 |
| DFT performed at GE, % | 69.7 | 68.3 | 0.025 |
Afib indicates atrial fibrillation; bpm, beats per minute; DFT, defibrillation threshold testing; GE, generator exchange; ICD, implantable cardioverter‐defibrillator; LV, left ventricle; RV, right ventricle; VF, ventricular fibrillation; VT, ventricular tachycardia.
Among patients with atrial leads.
Among patients with cardiac resynchronization devices.
During an average follow‐up of 1.9±1.2 years after GE, the percentage of patients with shocks and the risk of ICD shocks were lower in group A (9.9% versus 27.7%, HR 0.36, 95% CI 0.34 to 0.38, P<0.001). The cumulative rate of ICD shocks at 5 years after GE was 25.7% in group A and 51.1% in group B (Figure 1). As shown in Figure 1, the frequency of ICD shocks after GE in both groups was relatively constant, beginning within the first year after GE and without evidence of reaching a plateau by 5 years.
Figure 1.
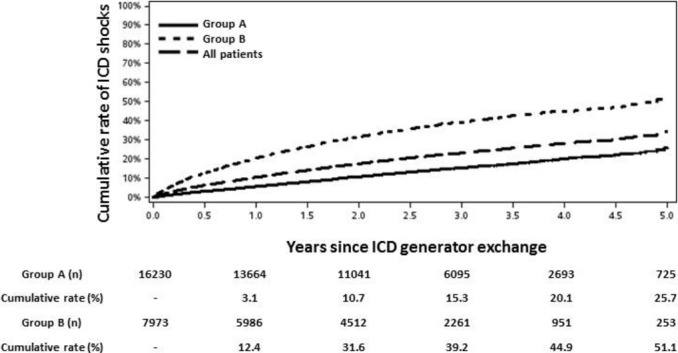
Cumulative rate of ICD shocks following generator exchange in groups A and B. Cumulative rate and number at risk are specified beneath each time point. ICD indicates implantable cardioverter‐defibrillator.
Because intracardiac electrogram adjudication was not available for all shocks in this analysis, ICD shocks delivered for onset detection rates >200 bpm were analyzed separately, given the reduced likelihood of inappropriate therapies above this cutoff.7 At the mean follow‐up after GE of 1.9±1.2 years, the percentage of patients with shocks for detection rates >200 bpm was also significantly lower in group A (6.0% versus 18.6%, HR 0.32, 95% CI 0.30 to 0.35, P<0.001). The cumulative rate of ICD shocks for detection rates >200 bpm after GE is presented in Figure 2. Among patients in group A, the cumulative rate was 9.2% at 3 years and 17.1% at 5 years; among patients in group B, the corresponding rates were 24.3% and 33.3% (Figure 2). Consistent with the findings shown in Figure 1, the cumulative rate of shocks for detection rates >200 bpm began to accrue soon after GE and continued to increase at a relatively constant rate for 5 years of follow‐up in both groups.
Figure 2.
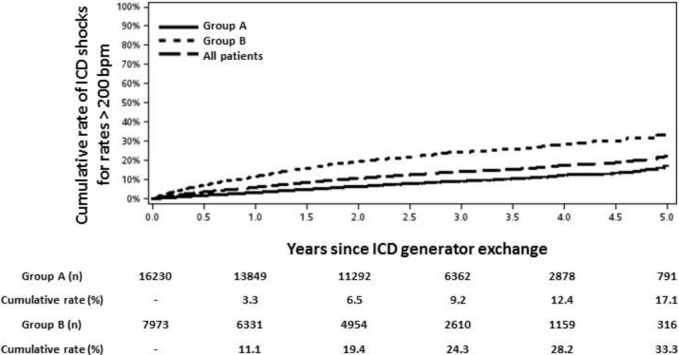
Cumulative rate of ICD shocks for detection rates >200 bpm following generator exchange in groups A and B. Cumulative rate and number at risk are specified beneath each time point. bpm indicates beats per minute; ICD, implantable cardioverter‐defibrillator.
At the mean follow‐up (1.9±1.2 years) after GE, patients in group A with single‐chamber, dual‐chamber, and CRT‐D devices had cumulative rates of ICD shocks of 11%, 10%, and 10%, respectively. Although numerically similar, the cumulative rate of ICD shocks was significantly higher in patients with single‐chamber versus CRT‐D devices (HR 1.14, 95% CI 1.01 to 1.30, P=0.04). In group B, the corresponding cumulative rates of ICD shocks at the mean follow‐up were 31%, 28%, and 32%, with a significant difference between patients with CRT‐D devices compared with dual‐chamber ICDs (HR 1.18, 95% CI 1.06 to 1.30, P=0.001).
Given the presence significant baseline differences between groups A and B (Table 1), we performed a multivariate Cox proportional hazards model to predict the time to first shock after GE (Table 2). Baseline covariates included in the model were absence of shocks during the first battery life (group A versus group B), age, male sex, dual‐chamber or CRT‐D device type (relative to single‐chamber ICD), and decision to perform a defibrillation threshold test at the time of GE. Although the decision to perform a defibrillation threshold test is predicated on several variables, it may also be a marker of overall baseline health8 and thus was included in the model to further account for baseline differences between groups A and B. The results of the multivariate model are presented in Table 2. In the multivariate model, the presence of shocks during the first battery life (group B), younger patients, male sex, and the decision not to perform an implant defibrillation threshold test were all associated with an increased HR, suggesting an increased arrhythmic risk following GE. Importantly, the HR in the multivariate model for group A versus group B (0.31, 95% CI 0.29 to 0.33) was similar to the HR in unadjusted analyses (0.30, 95% CI 0.22 to 0.38), suggesting that the presence of shocks during the first battery life remains an important predictor of risk after GE, even when accounting for other baseline covariates. The results of the multivariate Cox model for time to first shock for detection rate >200 bpm after GE were similar (Table 2, lower panel).
Table 2.
Multivariate Cox Proportional Hazards Model of Time to First ICD Shock After Generator Exchange (Top Panel) and Time to First ICD Shock For Detection Rate >200 bpm (Bottom Panel)
| Covariate | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Group A (relative to group B) | 0.31 (0.29 to 0.33) | <0.001 |
| Age | 0.99 (0.99 to 1.00) | 0.001 |
| Male sex | 1.43 (1.32 to 1.55) | <0.001 |
| Dual‐chamber ICD (relative to single chamber) | 0.93 (0.86 to 1.01) | 0.088 |
| CRT‐D (relative to single chamber) | 1.01 (0.93 to 1.10) | 0.743 |
| Implant DFT performed | 0.31 (0.29 to 0.33) | 0.020 |
| Group A (relative to group B) | 0.33 (0.31 to 0.36) | <0.001 |
| Age | 0.99 (0.99 to 1.00) | <0.001 |
| Male sex | 1.55 (1.40 to 1.73) | <0.001 |
| Dual‐chamber ICD (relative to single chamber) | 0.84 (0.75 to 0.93) | 0.001 |
| CRT‐D (relative to single chamber) | 0.94 (0.85 to 1.05) | 0.282 |
| Implant DFT performed | 0.88 (0.80 to 0.96) | 0.006 |
bpm indicates beats per minute; CRT‐D, cardiac resynchronization therapy‐defibrillator; DFT, defibrillation threshold test; ICD, implantable cardioverter‐defibrillator.
Discussion
In this large cohort of patients implanted with ICDs in the United States, two thirds did not receive ICD shocks during the first battery life. The occurrence of ICD shocks during first battery life is an important predictor of shocks after GE; however, even among those who did not experience shocks prior to GE, the cumulative rate of shocks at 5 years after GE is >25%. Among those patients who did receive ICD shocks during first battery life, the cumulative rate of shocks at 5 years after GE is approximately twice as high as it is for those without shocks. Notably, the rate of ICD shocks after GE increases at a relatively consistent rate in both groups without evidence of reaching a plateau by 5 years.
Supported by data from seminal clinical trials,3–4 >100 000 ICDs are implanted annually in the United States,9 the vast majority of which are implanted for primary prevention. Given the expected annual rate of ICD therapy of ≈5% to 12% per year among recipients of ICDs for primary prevention,2–4 coupled with an average ICD battery life of 4 to 5 years,1 it is evident that a large percentage of patients implanted with ICDs will reach the end of first battery life without ever receiving ICD shocks. Our data from a large, real‐world cohort in the United States support these findings by demonstrating that about two thirds of patients did not receive ICD shocks during first battery life, the mean longevity of which was ≈5.4 years. Although data‐supported guidelines exist for guiding the initial implantation of ICDs for primary and secondary prevention,10 there is a relative paucity of data to support decision making at the time of GE.
Prior studies have provided some data on the incidence of ICD therapies after GE. Van Welsenes et al reported outcomes of 154 primary prevention ICD recipients undergoing GE, of whom 114 (74%) had not received appropriate ICD therapy during first battery life.11 Among the 114 patients without ICD therapy during first battery life, the cumulative rate of ICD therapy (antitachycardia pacing and shocks) was 14% at 3 years after GE. The corresponding 3‐year event rate in group A in our study was very comparable at 16.7%. In the European Incidence Free Survival after ICD Replacement (INSURE) registry of 510 patients undergoing first ICD GE, 265 (52%) had not received ICD therapy during first battery life, and in this group, the cumulative rate of ICD therapy (antitachycardia pacing and shocks) at 3 years after GE was 21.4%.12 Notably, ≈87% of patients in the INSURE registry underwent initial ICD implant for a secondary prevention indication. This suggests an overall higher risk cohort and likely accounts for the lower percentage of patients reaching end of first battery life without having received ICD therapy than in our study and for the higher cumulative 3‐year event rate following GE.
The decision to implant an ICD is predicated on several assumptions about risk and benefit, most notably that the risk of arrhythmic death following ICD implantation is sufficiently high and the risk of nonarrhythmic death is sufficiently low that the ICD is likely to be beneficial. This balance of competing risks, coupled with the expectation of reasonable overall life expectancy and sufficiently low risk of periprocedural complications associated with ICD implant, is used to guide patients and physicians in making an informed decision about initial ICD implant. Several important data points, however, suggest that the assumptions regarding risk and benefit associated with ICD GE may be significantly different from those at the time of initial implant. First, recent data from the NCDR demonstrate that, compared with patients receiving new ICDs, those receiving replacement devices are significantly older (70.7 versus 67.5 years) and significantly more likely to have comorbidities such as atrial fibrillation.1 The mean age of patients receiving replacement devices in our cohort (70.5 years) is quite similar to that reported in the NCDR. Furthermore, all‐cause mortality, both unadjusted and propensity score matched, in the NCDR was significantly higher over 6 years for patients receiving replacement devices compared with those receiving new ICD implants. These data clearly demonstrate that overall survival after ICD GE is significantly worse than after initial ICD implant. A recent study of 231 patients from a US Department of Veterans Affairs population further highlighted the differences in risk profiles between initial ICD implant and GE by demonstrating that more than a quarter of patients undergoing GE may no longer meet primary prevention ICD indications due to improvement in left ventricular function and the absence of any appropriate ICD therapy delivered during the first battery life.13 Finally, the risk of major complications (predominantly hematoma requiring evacuation, infection requiring device extraction, or lead malfunction requiring reoperation) associated with ICD GE has been estimated at ≈4% from the REPLACE registry.5 In a registry of patients undergoing ICD GE from Ontario, Canada, the occurrence of a major complication associated with GE was associated with an increased risk of mortality at up to 6 months following generator replacement.6 In aggregate, these data suggest that patients undergoing ICD GE may have a higher risk of nonarrhythmic death than those undergoing initial ICD implant and that ICD GE is associated with a significant procedural risk. Both of these issues may change the overall risk–benefit analysis at the time of GE.
The risks of GE may be mitigated by longer lasting device technology. The devices studied in this analysis, using older battery technology, had an average battery life of 5.4 years, which is in line with recent data from the NCDR.1 A computational analysis from a US Department of Veterans Affairs database of implants from 1992 to 2007 suggested that the number of device replacements would have decreased by 57% and 75% if the batteries had lasted 7 or 9 years, respectively.14 Compared with the devices analyzed in this study, the latest generations of devices and battery technology have demonstrated improved longevity,15 which may reduce the risk of GE‐related complications for patients who survive long enough to require multiple replacements.
To assist in informed decision making, our data provide an additional important piece of information regarding the rate of ICD shocks following GE. In our cohort, among patients without a history of ICD shocks, approximately one quarter received an ICD shock during the 5 years after GE. In comparison, in the ICD arm of the Sudden Cardiac Death in Heart Failure (SCD‐HeFT) trial, the cumulative rate of shocks (appropriate and inappropriate) at 5 years was 31%.3 Although direct comparisons between a clinical trial and a real‐world cohort should be performed with caution, particularly due to the limited baseline clinical data available for patients in the ALTITUDE cohort, our data from a large number of patients implanted with ICDs suggest that among patients who do not receive shocks during first battery life, the rate of shocks at 5 years following GE (25.7%) is lower than the comparable benchmark rate following initial implant. With regard to differences in prognosis between patients with and without cardiac resynchronization devices, previous data from the ALTITUDE database have demonstrated that the incidence of shocks, both appropriate and inappropriate, is similar between ICD and CRT‐D recipients in the first 5 years after device implant.16 Our data corroborate these findings and extend them to the second battery life by demonstrating similar rates of shocks after GE in both groups A and B for patients with single‐ or dual‐chamber ICDs versus CRT‐D devices.
Given the shorter life expectancy, higher prevalence of comorbidities, and significant risk of periprocedural complications among patients undergoing GE, it is conceivable that for many patients who reach the end of first battery life without having received ICD shocks, the risk–benefit analysis of proceeding with GE may no longer favor continued ICD therapy for primary prevention. For patients with cardiac resynchronization devices, replacement with a cardiac resynchronization therapy‐pacemaker, instead of a CRT‐D device, may allow patients to benefit from the long‐term reductions in mortality associated with cardiac resynchronization17 without exposure to the physiological18 and psychological19 adverse effects associated with ICD shocks. In aggregate, although our data do not allow us to directly comment on risk–benefit analysis at the time of GE, in the absence of more direct evidence, our data should facilitate an informed discussion between patient and provider and, as has been recently highlighted, provide an opportunity for addressing advance care planning and goals of care at the end of ICD battery life.9
Limitations
Several important limitations of our study should be noted. First, we do not have data regarding whether ICDs were initially implanted for primary or secondary indications. Given that our cohort is from a large database in the United States, it can be presumed that the vast majority of implants were for primary prevention, consistent with the rate of overall new ICD implants in this country. Similarly, although the ALTITUDE database provides a large cross‐section of patients with ICDs, the baseline demographic data available are limited; therefore, we are unable to assess for other differences between patients who did and did not receive a shock with the first battery life. In addition, we were unable to adjudicate whether ICD shocks were appropriate or inappropriate. Although the analysis of ICD shocks for detection rates >200 bpm may provide some selection for appropriate therapies, this remains a major limitation of our study. Finally, we are unable to comment on survival and can report only on frequency of ICD shocks after GE, which are known to overestimate the true rate of aborted sudden death.20
Conclusions
In a large cohort of patients implanted with ICDs, two thirds did not receive ICD shocks during the first battery life. Among patients who received shocks during first battery life, the cumulative rate of shocks at 5 years after GE is >50%, and in the absence of significant comorbidities or changes in goals of care, these patients should be offered GE. Among patients who did not experience shocks during first battery life, the cumulative rate of shocks at 5 years following GE is >25%. Although our data are limited by the lack of more detailed baseline clinical information to gauge the potential arrhythmic risk after GE, in light of recent data that patients receiving replacement ICDs may be at lower arrhythmic risk and higher procedural risk than those undergoing initial ICD implant, our data should facilitate an informed decision‐making process between patients and providers about the risks and benefits of proceeding with GE and the long‐term implications of ongoing ICD therapy.
Disclosures
Paul Jones receives salary from and has equity interest in Boston Scientific Corporation. Scott Wehrenberg receives salary from Boston Scientific Corporation. Leslie Saxon has received research support and honoraria from Boston Scientific Corporation.
References
- 1.Kramer DB, Kennedy KF, Noseworthy PA, Buxton AE, Josephson ME, Normand SL, Spertus JA, Zimetbaum PJ, Reynolds MR, Mitchell SL. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter‐defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes. 2013; 6:488-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alsheikh‐Ali AA, Homer M, Maddukuri PV, Kalsmith B, Estes NA, III, Link MS. Time‐dependence of appropriate implantable defibrillator therapy in patients with ischemic cardiomyopathy. J Cardiovasc Electrophysiol. 2008; 19:784-789. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005; 352:225-237. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002; 346:877-883. [DOI] [PubMed] [Google Scholar]
- 5.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R. Complication rates associated with pacemaker or implantable cardioverter‐defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010; 122:1553-1561. [DOI] [PubMed] [Google Scholar]
- 6.Krahn AD, Lee DS, Birnie D, Healey JS, Crystal E, Dorian P, Simpson CS, Khaykin Y, Cameron D, Janmohamed A, Yee R, Austin PC, Chen Z, Hardy J, Tu JV. Predictors of short‐term complications after implantable cardioverter‐defibrillator replacement: results from the Ontario ICD Database. Circ Arrhythm Electrophysiol. 2011; 4:136-142. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NA, III, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012; 367:2275-2283. [DOI] [PubMed] [Google Scholar]
- 8.Russo AM, Wang Y, Al‐Khatib SM, Curtis JP, Lampert R. Patient, physician, and procedural factors influencing the use of defibrillation testing during initial implantable cardioverter defibrillator insertion: findings from the NCDR(R). Pacing Clin Electrophysiol. 2013; 36:1522-1531. [DOI] [PubMed] [Google Scholar]
- 9.Kramer DB, Buxton AE, Zimetbaum PJ. Time for a change—a new approach to ICD replacement. N Engl J Med. 2012; 366:291-293. [DOI] [PubMed] [Google Scholar]
- 10.Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA, III, Ferguson TB, Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD, Ellenbogen KA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hayes DL, Page RL, Stevenson LW, Sweeney MO. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. [corrected]. Circulation. 2012; 126:1784-1800. [DOI] [PubMed] [Google Scholar]
- 11.Van Welsenes GH, Van Rees JB, Thijssen J, Trines SA, Van Erven L, Schalij MJ, Borleffs CJ. Primary prevention implantable cardioverter defibrillator recipients: the need for defibrillator back‐up after an event‐free first battery service‐life. J Cardiovasc Electrophysiol. 2011; 22:1346-1350. [DOI] [PubMed] [Google Scholar]
- 12.Erkapic D, Sperzel J, Stiller S, Meltendorf U, Mermi J, Wegscheider K, Hugl B. Long‐term benefit of implantable cardioverter/defibrillator therapy after elective device replacement: results of the INcidence free SUrvival after ICD REplacement (INSURE) trial—a prospective multicentre study. Eur Heart J. 2013; 34:130-137. [DOI] [PubMed] [Google Scholar]
- 13.Kini V, Soufi MK, Deo R, Epstein AE, Bala R, Riley M, Groeneveld PW, Shalaby A, Dixit S. Appropriateness of primary prevention implantable cardioverter‐defibrillators at the time of generator replacement: are indications still met? J Am Coll Cardiol. 2014; 63:2388-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandra I. Impact of ICD battery longevity on need for device replacements‐insights from a Veterans Affairs database. Pacing Clin Electrophysiol. 2010; 33:314-319. [DOI] [PubMed] [Google Scholar]
- 15.Alam MB, Munir MB, Rattan R, Flanigan S, Adelstein E, Jain S, Saba S. Battery longevity in cardiac resynchronization therapy implantable cardioverter defibrillators. Europace. 2014; 16:246-251. [DOI] [PubMed] [Google Scholar]
- 16.Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP. Long‐term outcome after ICD and CRT implantation and influence of remote device follow‐up: the ALTITUDE survival study. Circulation. 2010; 122:2359-2367. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JG, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, Daubert JC. Long‐term mortality with cardiac resynchronization therapy in the Cardiac Resynchronization‐Heart Failure (CARE‐HF) trial. Eur J Heart Fail. 2012; 14:628-634. [DOI] [PubMed] [Google Scholar]
- 18.Powell BD, Saxon LA, Boehmer JP, Day JD, Gilliam FR, III, Heidenreich PA, Jones PW, Rousseau MJ, Hayes DL. Survival after shock therapy in implantable cardioverter‐defibrillator and cardiac resynchronization therapy‐defibrillator recipients according to rhythm shocked. The ALTITUDE survival by rhythm study. J Am Coll Cardiol. 2013; 62:1674-1679. [DOI] [PubMed] [Google Scholar]
- 19.Freedenberg V, Thomas SA, Friedmann E. Anxiety and depression in implanted cardioverter‐defibrillator recipients and heart failure: a review. Heart Failure Clin. 2011; 7:59-68. [DOI] [PubMed] [Google Scholar]
- 20.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006; 113:776-782. [DOI] [PubMed] [Google Scholar]


