Abstract
Background
Hypertension is a frequent risk factor for the development of heart failure with preserved ejection fraction (HFPEF). Progressive extracellular matrix accumulation has been presumed to be the fundamental pathophysiologic mechanism that leads to the transition to impaired diastolic reserve. However, the contribution of other mechanisms affecting active and passive components of diastolic function has not been comprehensively assessed. In this study, we investigated the potential role of impaired myocardial oxygen delivery in the pathophysiology of HFPEF.
Methods and Results
Patients with HFPEF, those with controlled hypertension, and healthy controls underwent simultaneous right‐heart catheterization, echocardiography, and paired arterial and coronary sinus blood gas sampling at rest and during supine‐cycle ergometry. Despite a lower workload (HFPEF vs control, hypertension: 43±8 versus 114±12, 87±14 W; P<0.001 and P<0.05, respectively), peak exercise pulmonary capillary wedge pressure was markedly higher in HFPEF patients compared with healthy and hypertensive controls (32±2 versus 16±1 and 17±1 mm Hg, both P<0.001). During exercise, the transcardiac oxygen gradient increased significantly in all groups; however, the peak transcardiac oxygen gradient was significantly lower in HFPEF patients (P<0.05). In addition, the left ventricular–work corrected transcardiac oxygen gradient remained significantly lower in HFPEF patients compared with controls (P<0.001).
Conclusion
The current study provides unique data suggesting that the abnormal diastolic reserve observed during exertion in HFPEF patients may, in part, be explained by impaired myocardial oxygen delivery due possibly to microvascular dysfunction. Further studies are required to confirm the structural and functional basis of these findings and to investigate the influence of potential therapies on this abnormality.
Keywords: exercise, heart failure, hemodynamics, myocardial oxygenation, preserved ejection fraction
Introduction
While heart failure with reduced ejection fraction (HFREF) and heart failure with preserved ejection fraction (HFPEF) share many symptomatic and clinical features, HFPEF has emerged over the past decade as a particularly challenging disorder to treat. For patients with HFREF, pharmacologic intervention with angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, mineralocortcoid antagonists, and β‐adrenoceptor blockers have clearly been shown to improve symptoms, ventricular performance, and prognosis. In contrast, HFPEF, a disorder characterized by normal or nearly normal left ventricular (LV) systolic function and evidence of diastolic dysfunction,1–2 has yet to be convincingly affected by any of these therapies and other, more recently investigated agents including phosphodiesterase type 5 inhibitors.3–4
Patients with HFPEF are typically older than patients with HFREF and have a higher incidence of underlying diseases such as hypertension, diabetes, and atrial fibrillation.3 HFPEF currently accounts for approximately half of all hospital admissions for heart failure,5 and given that the average population age is increasing, it is likely that hospitalizations due to HFPEF will increase in the near term. Taken together, given the lack of therapeutic options for HFPEF, there is a major need to better understand the pathogenesis of HFPEF to be able to develop more effective therapies.
The lack of effective therapies for HFPEF suggests that current interventions may not effectively target key contributory mechanisms or that they are unable to sufficiently modify their targets. For HFPEF patients, exertional dyspnea and corresponding exercise intolerance are the key symptoms6–8; this has been ascribed to impaired diastolic reserve.9–10 The specific mechanism responsible for the impairment of diastolic reserve, remains a source of considerable conjecture. While the passive effects of interstitial fibrosis are commonly proposed to underlie the abnormal diastology in HFPEF, it is also possible that impaired active relaxation of the heart plays a contributory role.11–12
In the present study, we considered the possibility that the impairment in diastolic reserve, particularly in the context of exertion, may reflect abnormalities of the active relaxation phase of diastole and, further, that this is a process initiated by underlying factors such as hypertension. We therefore investigated the relationship between resting and exercise hemodynamics and measures of myocardial oxygen delivery in healthy control volunteers, hypertensive subjects, and patients with HFPEF.
Methods
Patient Population
Healthy volunteers were recruited from the general community and had no history of significant comorbidities including cardiovascular, pulmonary, or other systemic diseases. Hypertensive controls were included if they had a reported blood pressure >140/90 mm Hg, were treated with antihypertensive medication, and were free from a history of limiting exertional dyspnea. HFPEF patients were recruited from the Alfred Hospital Heart Centre clinics or at the time of evaluation for suspected HFPEF. HFPEF patients were included on the basis of a history of exertional dyspnea, LV ejection fraction >45%, and either resting echocardiographic features of definite diastolic dysfunction (E/e′ >15) or an exercise pulmonary capillary wedge pressure (PCWP) >25 mm Hg as suggested by us13 and others. Patients were excluded if they had a body mass index (BMI) >35 kg/m2, unstable coronary artery disease, or history of stress‐induced syncope or ventricular tachycardia during exercise or were unable to perform an exercise test. The study was approved by the Alfred Hospital Research and Ethics Committee, and all participants provided written informed consent.
Cardiac Catheterization
A 7Fr balloon‐tipped pulmonary artery catheter (Edwards Lifesciences) was inserted through an introducer sheath placed in the right internal jugular or a brachial vein for measurement of right atrial pressure, pulmonary artery pressure, and PCWP. The wedge position was confirmed by the use of fluoroscopy and pressure waveform, and the mean PCWP was measured at end‐expiration. Cardiac output was measured by using thermodilution with measurements taken in triplicate or from 5 readings for patients in atrial fibrillation. A 3Fr radial or brachial artery cannula was inserted for blood pressure recording and blood sampling. A second catheter, inserted through an introducer sheath in a brachial vein, was placed in the coronary sinus for blood sampling. After baseline hemodynamic measurements, simultaneous arterial and coronary sinus blood samples were obtained for measurement of blood gases, glucose, and lactate.
Subjects then exercised in the supine position on a cycle ergometer mounted onto the catheter table. Subjects started at a work rate of 0.3 W/kg body weight for 3 minutes. The workload was increased to 0.6, 1.0, and 1.5 W/kg, respectively, every 3 minutes or to maximum tolerated levels, as we previously described.8 Hemodynamic indices were obtained again at peak exercise, and when a stable coronary sinus catheter position was maintained, repeat arterial and coronary sinus blood gas samples were obtained. Transcardiac oxygen content and carbon dioxide gradients were calculated as the difference between the relevant contents in the coronary sinus and arterial blood samples. Whole blood oxygen content was calculated according to standard formulas, and the carbon dioxide content was calculated according to the methods of Sun et al.14 Left ventricular work was calculated as (mean arterial pressure−PCWP)×cardiac output×0.0136.15
Rest and Exercise Transthoracic Echocardiography
Standard M‐mode, 2‐dimensional, and Doppler blood flow recordings were performed by using standardized instruments.16–17 Measurements were performed offline. All parameters were measured in triplicate and averaged. Tissue Doppler images of the mitral annulus movement were obtained from the apical 4‐chamber view. A sample volume was placed at the lateral and septal annular sites. Analysis was performed for the early (e′) and late (a′) diastolic peak velocities. The E/e′ ratio was calculated using the mean from the lateral and septal E/e′. Pulsed‐wave Doppler echocardiography was used to assess peak early (E) and late (A) wave flow velocity. During exercise, apical 4‐chamber views were captured for LV volume analysis.
Statistical Analysis
Continuous normally distributed data are presented as mean±SEM. Non‐normal data are represented as the median and interquartile range. Within‐subject comparisons were performed with use of a paired Student t test. Between‐group analyses were conducted by ANOVA with Bonferroni post‐hoc testing or Kruskal–Wallis test with Mann–Whitney post‐hoc testing as appropriate. ANCOVA was used to determine the contribution of between‐group differences in covariates including age and workload where indicated. Association between variables was tested by using Pearson's correlation coefficient. A value of P<0.05 was considered to be statistically significant. Statistical analysis was performed using a commercially available software package (IBM SPSS Statistics version 19; SPSS Inc).
Results
Baseline Characteristics
The present study included HFPEF patients, hypertensive patients, and healthy control subjects. As demonstrated in Table 1, HFPEF patients were older and hypertensive and more likely to be receiving antihypertensive and heart failure medication.
Table 1.
Baseline Demographics and Echocardiography
| Control Group (n=12) | Hypertensive Group (n=7) | HFPEF Group (n=9) | |
|---|---|---|---|
| Characteristics | |||
| Age, y | 54±2 | 62±1 | 74±2***,## |
| BMI, kg/m2 | 24 (21 to 26) | 32 (28 to 33)* | 29 (27 to 30)* |
| Comorbidities | |||
| Diabetes | 0 | 0 | 1 (11%) |
| Hypertension | 0 | 7 (100%) | 6 (67%) |
| CAD | 0 | 0 | 0 |
| Echocardiography | |||
| LVEDD, mm | 51±1 | 52±2 | 46±2 |
| LVEF, % | 62±2 | 61±3 | 66±2 |
| LV mass, g/m2 | 72±4 | 73±4 | 92±10* |
| LA volume index, mL/m2 | 29±2 | 25±2 | 41±8* |
| E/A ratio | 1.4 (1.2 to 1.8) | 0.9 (0.6 to 1.5)* | 0.8 (0.6 to 1.6)* |
| E/e′ | 7.3 (6.2 to 7.6) | 8.9 (6.7 to 9.7) | 12.7 (10.7 to 17.0)* |
Data are mean±SEM or median (interquartile range) as appropriate. BMI indicates body mass index; CAD, coronary artery disease; HFPEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction. *P<0.05, ***P<0.001 vs controls. ##P<0.01 vs hypertension.
Cardiac Structure and Function
As illustrated in Table 1, LV ejection fraction (LVEF) was similar for all 3 groups. HFPEF patients were characterized by echocardiographic features of LV hypertrophy compared with controls, and there was a concomitant, significant increase in left atrial volume index compared with healthy subjects. The hypertensive subjects were asymptomatic and were well treated for hypertension.
Resting and Exercise Hemodynamics and Echocardiography
At rest, mean arterial blood pressure was similar across the 3 study groups; systolic blood pressure was higher in HFPEF patients compared with controls (153±4 versus 130±4 mm Hg, P<0.01), whereas hypertensive subjects had an average systolic blood pressure of 143±6 mm Hg. Consistent with their diagnosis, the mean pulmonary artery and mean PCWP at rest were significantly higher in HFPEF patients (Table 2), while cardiac index was similar across the 3 groups.
Table 2.
Within‐Group Rest Versus Exercise Hemodynamics
| Control (n=12) | Hypertension (n=7) | HFPEF (n=9) | ||||
|---|---|---|---|---|---|---|
| Rest | Exercise | Rest | Exercise | Rest | Exercise | |
| HR, bpm | 60±3 | 118±5*** | 68±4 | 119±8*** | 70±4 | 111±5*** |
| MAP, mm Hg | 92±3 | 119±4*** | 98±3 | 124±5** | 99±5 | 120±5** |
| SBP, mm Hg | 130±4 | 186±7*** | 143±6 | 196±13** | 149±5 | 185±7** |
| DBP, mm Hg | 72±2 | 85±4* | 76±3 | 88±3** | 74±6 | 88±6** |
| mPAP, mm Hg | 13±1 | 29±2*** | 15±1 | 30±1** | 25±3 | 45±2*** |
| sPAP, mm Hg | 22±1 | 45±2*** | 24±2 | 50±2*** | 37±5 | 65±4*** |
| dPAP, mm Hg | 8±1 | 17±2** | 9±1 | 19±2** | 15±3 | 32±3*** |
| PCWP, mm Hg | 8±1 | 16±2*** | 9±1 | 17±1** | 14±2 | 32±2*** |
| CI, L/min per m2 | 2.9±0.2 | 7.2±0.3*** | 2.8±0.2 | 6.7±0.6*** | 2.6±0.2 | 4.3±0.5** |
| LVWI, kg‐m min−1 m−2 | 4.8±0.3 | 16.6±0.7*** | 5.1±0.4 | 16.1±1.6*** | 4.8±0.3 | 9.1±0.9*** |
Data are mean±SEM. CI indicates cardiac index; DBP, diastolic blood pressure; dPAP, diastolic pulmonary artery pressure; HFPEF, heart failure with preserved ejection fraction; HR, heart rate; LVWI, left ventricular work index; MAP, mean arterial pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure. *P<0.05, **P<0.01, ***P<0.001.
During a symptom‐limited exercise hemodynamic study, patients with HFPEF displayed a significantly diminished peak exercise capacity compared with healthy controls (43±8 versus 114 ±12 W, P<0.001). Exercise capacity in hypertensive subjects (87±14 W) was greater than that in HFPEF (P<0.05), and although it was lower than that of controls, the difference was not statistically significant. As shown in Table 2, the exercise time was also significantly shorter in HFPEF patients. During exercise, all groups significantly increased cardiac output (control and hypertensive subjects both P<0.001; HFPEF subjects, P<0.01); however, the peak cardiac index was significantly lower in HFPEF subjects (Table 2). Consistent with these data, HFPEF patients displayed a decreased peak exercise LVEF (59±3%) compared with hypertensive subjects (74±2%, P=0.003) and healthy controls (74±2%, P<0.001). As shown in Figure 1, the LV end‐systolic and end‐systolic volume indexes were significantly smaller in the HFPEF group at baseline. During exercise, there was a significant fall in the LV end‐systolic volume index in both control and hypertensive subjects in contrast to that observed in HFPEF subjects. The heart rate responses to exertion were similar across groups, while the stroke volume response to exercise was significantly lower in HFPEF patients (P<0.05) compared with healthy subjects.
Figure 1.
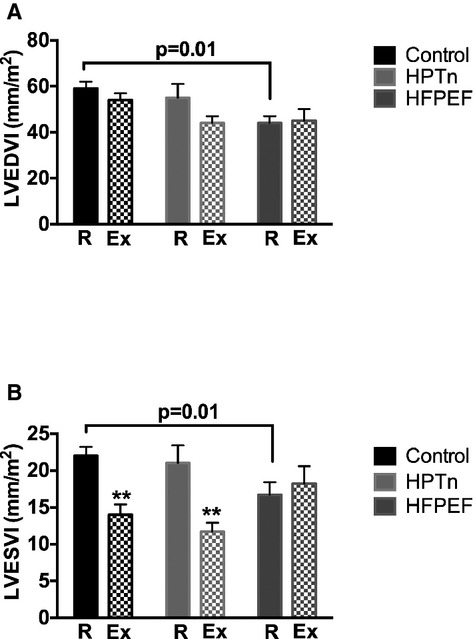
Bar graphs representing the left ventricular end‐diastolic volume index (LVEDVI) (A) and the left ventricular end‐systolic volume index (LVESVI) at rest (R) and during exercise (Ex) in control, hypertensive, and HFPEF subjects. **P<0.01 rest vs exercise. HFPEF indicates heart failure with preserved ejection fraction; HPTn, hypertension.
As expected exercise resulted in significant within‐group increases in heart rate, systemic and pulmonary pressures, and cardiac index (Table 2). Consistent with prior studies, the PCWP at peak exercise in HFPEF patients was markedly higher than that in controls or hypertensives (both P<0.001, Table 3). Furthermore, after accounting for baseline differences in resting PCWP, the magnitude of the rise in PCWP during exercise continued to be greater in HFPEF compared with either control or hypertensive subjects (both P<0.05). The peak cardiac index and LV work were significantly lower in HFPEF patients (Table 3). Double product was higher in HFPEF patients at rest compared with controls but did not differ during exercise as shown in Table 3.
Table 3.
Between‐Group Rest and Exercise Hemodynamics
| Control (n=12) | Hypertension (n=7) | HFPEF (n=9) | |
|---|---|---|---|
| Rest | |||
| HR, bpm | 60±3 | 68±4 | 70±4 |
| MAP, mm Hg | 92±3 | 98±3 | 99±5 |
| SBP, mm Hg | 130±4 | 143±6 | 149±5** |
| DBP, mm Hg | 72±2 | 76±3 | 74±6 |
| mPAP, mm Hg | 13±1 | 15±1 | 25±3***,## |
| sPAP, mm Hg | 22±1 | 24±2 | 37±5**,# |
| dPAP, mm Hg | 8±1 | 9±1 | 15±3** |
| PCWP, mm Hg | 8±1 | 9±1 | 14±2** |
| CI, L/min per m2 | 2.9±0.2 | 2.8±0.2 | 2.6±0.2 |
| RPP, mm Hg/min | 7885±586 | 9765±718 | 10604±727* |
| LVWI, kg‐m min−1 m−2 | 4.8±0.3 | 5.1±0.4 | 4.8±0.3 |
| LVSWI, g‐m m−2 | 82±6 | 77±7 | 70±7 |
| Peak exercise | |||
| Ex. time, minutes | 13±1 | 11±1 | 7±1** |
| Workload, W | 114±12 | 87±14 | 43±8***,# |
| HR | 118±5 | 119±8 | 111±5 |
| MAP | 119±4 | 124±5 | 120±5 |
| SBP, mm Hg | 186±7 | 196±13 | 185±7 |
| DBP, mm Hg | 85±4 | 88±3 | 88±6 |
| mPAP, mm Hg | 29±2 | 30±1 | 45±2***,## |
| sPAP, mm Hg | 45±2 | 50±2 | 65±4***,# |
| dPAP, mm Hg | 17±2 | 19±2 | 32±3***,## |
| PCWP | 16±2 | 17±1 | 32±2***,### |
| CI, L/min per m2 | 7.2±0.3 | 6.7±0.6 | 4.3±0.5***,## |
| RPP, mm Hg/min | 22158±1361 | 23670±2594 | 20757±1541 |
| LVWI, kg‐m min−1 m−2 | 16.6±0.7 | 16.1±1.6 | 9.1±0.9***,### |
| LVSWI, g‐m m−2 | 143±8 | 139±16 | 84±9***,## |
CI indicates cardiac index; DBP, diastolic blood pressure; dPAP, diastolic pulmonary artery pressure; HFPEF indicates heart failure with preserved ejection fraction; HR, heart rate; LVSWI, left ventricular stroke work index; LVWI, left ventricular work index; MAP, mean arterial pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RPP, rate pressure product; SBP, systolic blood pressure; sPAP, systolic pulmonary artery pressure. *P<0.05, **P<0.01, ***P<0.001 vs controls. #P<0.05, ##P<0.01, ###P<0.001 vs hypertension.
Given that exercise capacity in HFPEF was limited due to symptoms, we explored the relationship between workload and hemodynamic performance. Peak LV work was significantly correlated with peak workload (r=0.46, P=0.01). The peak PCWP normalized to work was significantly higher in HFPEF patients compared with control or hypertensive subjects (HFPEF 76±12 mm Hg/W per kg versus control 11±1 mmHg/W per kg and hypertension 15±1 mm Hg/W per kg, both P<0.001).
Rest and Exercise Arterial and Coronary Sinus Oxygen Content
Complete transcardiac blood sampling data were obtained in 10 controls, 7 hypertensives, and 8 HFPEF patients. In particular, patients were included in this analysis only if the coronary sinus catheter remained stable during exercise as demonstrated by intermittent fluoroscopy during exercise and by repeat contrast venography at the conclusion of exercise. At baseline, biochemical parameters were generally similar across groups, although at baseline the arterial oxygen content was slightly lower in HFPEF compared with controls (16.8±0.3 versus 18.9±0.4 mL O2/100 mL, P<0.05) The transcardiac gradient oxygen content (CaO2–CcsO2) values for controls, hypertensives, and HFPEF patients were similar at baseline as shown in Figure 2A, as was the transcardiac release gradient for the carbon dioxide content (CcsCO2–CaCO2) for controls, hypertensives, and HFPEF patients: 11.1±0.8, 9.8±0.8, and 8.1±1.0 mLCO2/100 mL. During exercise, there was a significant within‐group increase in the transcardiac gradient for oxygen in all groups; however, the magnitude was smaller in HFPEF patients. Control subjects (rest versus exercise: 112±3 versus 149±4 mL O2/100 mL, P<0.001), hypertensives (rest versus exercise: 120±4 versus 136±5 mL O2/100 mL, P<0.001), and HFPEF (rest versus exercise: 112±7 versus 125±6 mL O2/100 mL, P=0.01). The peak transcardiac oxygen gradient was significantly less in HFPEF patients as shown in Figure 2B. When comparing the capacity for enhanced myocardial extraction of oxygen during exercise, the increase in the transcardiac oxygen gradient during exercise was significantly blunted in both hypertensives and HFPEF patients (Figure 2C). Furthermore, on ANCOVA, the between‐group differences in exercise stimulated transcardiac oxygen gradient were not explained by differences in either age (P=0.76) or workload (P=0.43).
Figure 2.
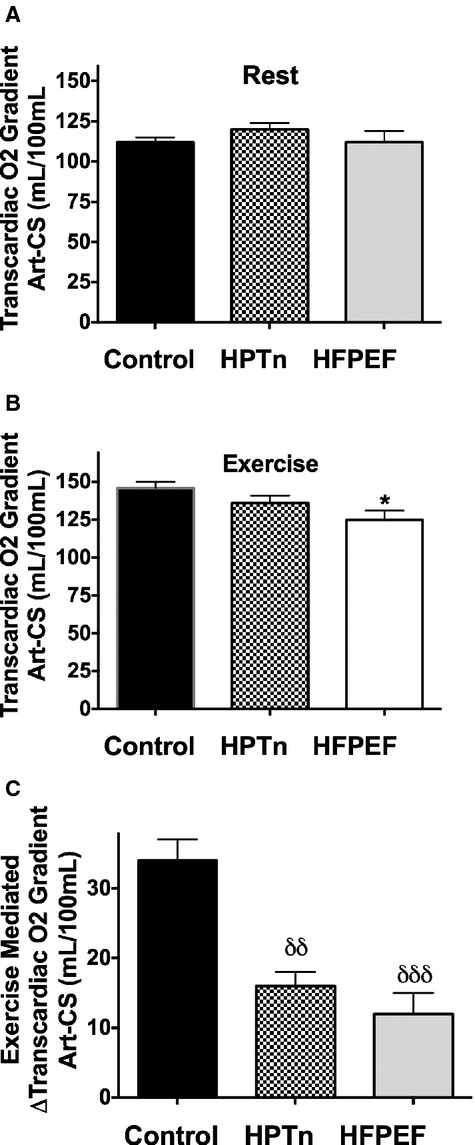
Bar graphs showing the transcardiac oxygen gradient at rest (A) and during exercise (B) and the exercise‐induced augmentation in transcardiac oxygen gradient (C). δδP<0.01, δδδP<0.001. HFPEF indicates heart failure with preserved ejection fraction; HPTn, hypertension.
We next determined whether the capacity for augmented myocardial oxygen extraction during exercise was associated with the exercise‐induced change in PCWP. As shown in Figure 3, there was a significant negative correlation between the change in the transcardiac oxygen gradient and the exercise‐induced peak PCWP, suggesting that limitation of oxygen extraction capacity could be associated with elevated exercise PCWP. Across the entire cohort, there was a significant correlation between LV work and the transcardiac oxygen gradient (r=0.55, P<0.001). Therefore, to exclude the possibility that the reduction in transcardiac oxygen gradient in HFPEF and hypertensive patients was due to lower LV work, we performed an ANCOVA. In this analysis, the transcardiac oxygen gradient remained significantly lower in HFPEF patients compared with that in control subjects (P<0.001).
Figure 3.
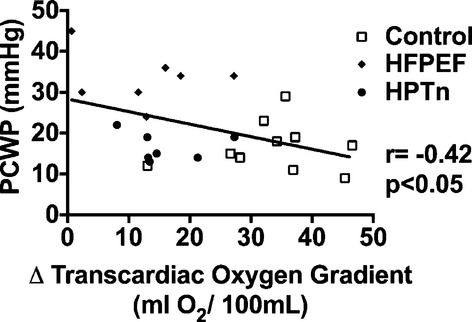
Scatterplot representing the relationship between transcardiac oxygen gradient and peak pulmonary capillary wedge pressure (PCWP). HFPEF indicates heart failure with preserved ejection fraction; HPTn, hypertension.
In an attempt to ascertain whether there was evidence of a change in myocardial substrate metabolism during the progressive stage of hypertension to HFPEF, we measured the transcardiac gradients for glucose and lactate at rest and exercise in the 3 study cohorts. At rest and during exercise, significant transcardiac extraction of glucose occurred across the entire cohort (rest, arterial vs coronary sinus: 5.88±0.14 versus 5.57±0.12 mmol/L P=0.001; exercise, arterial vs coronary sinus: 5.93±0.13 versus 5.56±0.08 mmol/L P<0.001). However, there was no significant difference between groups, and exercise did not significantly alter the transcardiac gradient. As shown in Figure 4, net transcardiac uptake gradients were also evident for lactate. Exercise increased the uptake of lactate significantly across all groups, with no significant difference in the magnitude of uptake between the groups.
Figure 4.
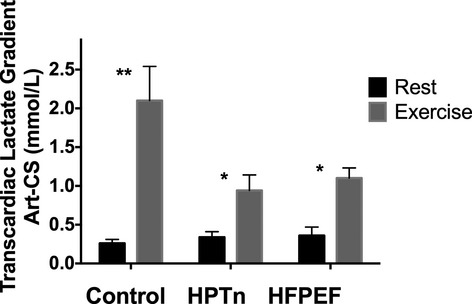
Bar graphs representing transmyocardial lactate net uptake at rest (open bars) and during exercise (solid bar) in control, hypertensive, and HFPEF subjects. **P<0.01, *P<0.05 vs rest. HFPEF indicates heart failure with preserved ejection fraction; HPTn, hypertension.
Discussion
In contrast to HFREF, there has been limited progress in regard to the development of effective therapies that improve functional capacity and outcome for patients with HFPEF. To some extent, this impasse may represent the complex pathophysiology of HFPEF and the fact that the predisposing risk factors, such as hypertension, appear to be more prevalent in HFPEF than in HFREF.3 Accumulating data suggest that HFPEF is a syndrome that reflects contributions from several mechanisms, including myocardial, vascular, and noncardiovascular elements. In particular, altered diastolic reserve is the key mechanism, together with increased vascular stiffness, reduced endothelial function, abnormal ventriculovascular coupling, and pulmonary vascular remodeling. In the present study, we showed that HFPEF patients exhibited a rapid rise in PCWP at low workload consistent with altered diastolic reserve as previously reported.8 Our study also examined the hemodynamic response in a group of subjects with well‐controlled hypertension, without significant LV hypertrophy. This subject group demonstrated a similar change in PCWP during exercise, albeit at a somewhat lower tolerated workload. In the setting of symptomatic limitation, HFPEF patients were unable to achieve workloads of a similar magnitude to that in control and hypertensive subjects.
Recent clinical trials of angiotensin‐converting enzyme inhibition, angiotensin II receptor blockade, and aldosterone antagonism18–20 have, in part, been predicated on the assumption that the increase in ventricular stiffness due to extracellular matrix accumulation is a key determinant of the pathophysiology and outcome of HFPEF.21 The lack of effective outcomes with these interventions raises the possibility that the interventions do not sufficiently reverse cardiac fibrosis or, alternatively, that they do not target the appropriate mechanism underpinning altered diastology. As such, the precise role of interstitial fibrosis alone, however, is somewhat unclear. For example, a recent study of volume loading in HFPEF patients showed a similar magnitude of increase in LV filling pressures to that observed in healthy controls, suggesting passive diastolic stiffness may be less important.22 Diastolic performance is determined by both active energy requiring and passive components that contribute to the rate of relaxation and the stiffness of the ventricle. We found that patients with HFPEF had smaller baseline LV end‐systolic and end‐systolic volume indexes, as previously observed.23 During exercise, the LV end‐systolic volume index did not change significantly in any group consistent with previous studies24; however, the LV end‐systolic volume index fell significantly in control and hypertensive subjects consistent with preserved contractile reserve.
The relationship between apparently reduced contractile reserve and the inability of the left ventricle to relax rapidly and to a sufficient extent to maintain a low filling pressure may be explained in several ways. As reviewed in detail by Tschope and Paulus,25 diastolic lengthening velocity is influenced by systolic shortening, reflecting the contribution of restorative forces mediated by the bidirectional “spring” protein, titin. In the present study, we found that contractile reserve was reduced, albeit at a lower workload due to symptom limitation, thus possibly contributing to impaired diastolic reserve. Moreover, in the context of HFPEF it has been proposed that a switch to the less‐compliant N2BA isoform of titin and the relative hypophosphorylation are responsible.
Beyond the potential contribution of altered structural protein expression and function, we considered the possibility that abnormalities of myocardial energetics might also contribute to the physiological defect that characterize HFPEF. In particular, given that coronary flow reserve has been previously shown to be impaired in hypertensives,26–27 we aimed to test the hypothesis that impaired myocardial oxygen delivery might be an important contributor to impaired exercise diastolic reserve in HFPEF via its influence of diastolic performance. We performed simultaneous hemodynamic studies and transcardiac blood sampling at rest and during exercise and compared these observations with those in healthy control subjects and asymptomatic hypertensive individuals. In each group, during exercise there was a significant increase in the transcardiac oxygen gradient, consistent with increased extraction of oxygen by the myocardium. Importantly, we found that the LV–work corrected transcardiac oxygen gradient was significantly lower in HFPEF patients compared with controls. Moreover, we observed that the coronary sinus oxygen content was lowest in individuals with the highest peak PCWP during exercise. Under normal circumstances, myocardial oxygen consumption occurring during increased cardiac work is achieved by an increase in myocardial blood flow together with an increase in myocardial oxygen extraction.28 As such, the present data are highly suggestive of an impairment of myocardial oxygen delivery due to microvascular dysfunction, and such an abnormality could potentially contribute to the exercise induced diastolic dysfunction observed in HFPEF. The notion of impaired oxygen delivery is consistent with recent studies in skeletal muscle demonstrating reduced capillary density.29 In the present study, we were not able to calculate total myocardial oxygen consumption, given that the simultaneous measurement of myocardial blood flow during coronary venous blood sampling with exercise is not presently possible due to the lack of clinically approved coronary sinus thermodilution catheters.
From a mechanistic perspective, impaired myocardial blood flow reserve during exertion could be explained by several potential mechanisms. The increment in blood flow that accompanies increased ventricular work is currently thought to reflect the actions of locally generated metabolites such as adenosine, together with shear stress–induced release of nitric oxide by the endothelium.30 In this context, we and others have shown that the systemic and local vascular responses to exercise and metabolic stress are reduced in HFPEF patients, consistent with endothelial dysfunction.8,24 Alternatively, impaired vasodilator capacity may also reflect a reduction in the absolute cross‐sectional area (ie, number of resistance vessels) of the microcirculation, given that this limitation to flow may only become evident at maximal vasodilatation.30–31 Consistent with this possibility, recent studies in myocardial biopsy samples from patients undergoing cardiac surgery showed reduced microvascular density in obesity,32 the latter being associated with HFPEF.
Our studies, therefore, suggest that myocardial oxygen delivery may be limiting in HFPEF patients. Myocardial oxygen is closely coupled with ATP production and external work,33 although previous studies indicate that in healthy subjects, only 25% of the oxygen consumed ultimately participates in external work.34 Phan and colleagues35 demonstrated the presence of a reduced phosphocreatine‐to‐ATP ratio in HFPEF patients consistent with the possibility that myocardial oxygen availability may be limiting in HFPEF. In particular, ATP is required during diastole for the detachment of the myosin head from actin and for the removal of Ca2+ from the contractile apparatus back to the sarcoplasmic reticulum. It is also possible that Ca2+ leak from the sarcoplasmic reticulum leading to elevated diastolic Ca2+ levels may lead to increased ATP use together with incomplete diastolic relaxation due to active resting tone.36 This process was also demonstrated to be more apparent at higher heart rates, including the range observed during exercise in the current study. However, previous studies using rapid pacing suggest that elevated heart rate in the absence of a change in afterload may not be sufficient to induce diastolic dysfunction.37
In addition to our new findings in relation to the myocardial biology of HFPEF, it is evident that the clinical features of HFPEF represent the integrated effects of multisystem alterations. These include impaired endothelial function, impaired skeletal muscle oxygen delivery, ventriculovascular mismatch, elevated arterial stiffness, renal impairment, pulmonary dysfunction, and obesity among others.
Our study has several limitations. By its invasive nature, the sample size was small; however, clear between‐group differences were evident. It is also possible that smaller between‐group differences may not have been detected. There were some differences in the demographic features of the control and HFPEF groups, notably in regard to age and BMI. In a recent study of healthy subjects, we demonstrated that although a higher PCWP and lower cardiac output during exercise are associated with aging, the magnitude of the influence of age is far less than that observed in HFPEF, indicating that age per se was not a major confounding factor in the present study.38 In addition, on ANCOVA, we found that age was not a significant contributor to the observed between‐group differences in key hemodynamic variables such as the exercise‐induced PCWP. The hypertensive group and HFPEF patients had a higher BMI than controls, but the hemodynamic responses in hypertensives were similar to that in controls, indicating that BMI per se was not responsible for the findings in our study. Accordingly, we believe the relevance of between‐group differences in age and BMI in our study to be limited.
Taken together, the present study suggests that patients with HFPEF may have an intrinsic inability to increase myocardial oxygen delivery during exertion due to microvascular dysfunction, which would contribute to defective active relaxation, particularly during physical activity. The application of noninvasive methodology including cardiac magnetic resonance imaging– and positron emission tomography–based assessment of myocardial metabolism during exertion in larger numbers of HFPEF patients may also yield insights into the frequency and extent of microvascular abnormalities in this difficult‐to‐treat patient group, which may lead to the evaluation of targeted therapeutic modalities.
Sources of Funding
Dr Empel was supported by a grant from the Interuniversity Cardiology Institute Netherlands, Utrecht, Netherlands. Dr Kaye is supported by funding from the National Health and Medical Research Council of Australia and the Victorian Government OIS Program.
Disclosures
None.
Acknowledgments
The authors thank Donna Vizi, Jenny Starr, Sofie Karapanagiotidis, and Liz Dewar for their excellent assistance.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012; 14:803-869. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007; 28:2539-2550. [DOI] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006; 355:251-259. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011; 32:670-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitzman DW, Daniel KR. Diastolic heart failure in the elderly. Heart Fail Clin. 2007; 3:437-453. [DOI] [PubMed] [Google Scholar]
- 6.Brutsaert DL. Cardiac dysfunction in heart failure: the cardiologist's love affair with time. Prog Cardiovasc Dis. 2006; 49:157-181. [DOI] [PubMed] [Google Scholar]
- 7.Ingle L, Cleland JG, Clark AL. Perception of symptoms is out of proportion to cardiac pathology in patients with “diastolic heart failure”. Heart. 2008; 94:748-753. [DOI] [PubMed] [Google Scholar]
- 8.Maeder MT, Thompson BR, Brunner‐La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010; 56:855-863. [DOI] [PubMed] [Google Scholar]
- 9.Brutsaert T, Sys SU, Gillbert TC. Diastolic dysfunction and congestive heart failure. J.Am.Coll.Cardiol. 1993; 22:318-325. [DOI] [PubMed] [Google Scholar]
- 10.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013; 15:776-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II. Causal mechanisms and treatment. Circulation. 2002; 105:1503-1508. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013; 62:263-271. [DOI] [PubMed] [Google Scholar]
- 13.van Empel VP, Kaye DM. Integration of exercise evaluation into the algorithm for evaluation of patients with suspected heart failure with preserved ejection fraction. Int J Cardiol. 2013; 168:716-722. [DOI] [PubMed] [Google Scholar]
- 14.Sun XG, Hansen JE, Stringer WW, Ting H, Wasserman K. Carbon dioxide pressure‐concentration relationship in arterial and mixed venous blood during exercise. J Appl Physiol. 2001; 90:1798-1810. [DOI] [PubMed] [Google Scholar]
- 15.Nichols AB, Pearson MH, Sciacca RR, Cannon PJ. Left ventricular mechanical efficiency in coronary artery disease. J Am Coll Cardiol. 1986; 7:270-279. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440-1463. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009; 10:165-193. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐preserved trial. Lancet. 2003; 362:777-781. [DOI] [PubMed] [Google Scholar]
- 19.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008; 359:2456-2467. [DOI] [PubMed] [Google Scholar]
- 20.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370:1383-1392. [DOI] [PubMed] [Google Scholar]
- 21.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006; 113:1966-1973. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, Carrick‐Ranson G, Levine BD. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013; 127:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011; 58:265-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010; 56:845-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tschope C, Paulus WJ. Is echocardiographic evaluation of diastolic function useful in determining clinical care? Doppler echocardiography yields dubious estimates of left ventricular diastolic pressures. Circulation. 2009; 120:810-820. [DOI] [PubMed] [Google Scholar]
- 26.Bozbas H, Pirat B, Yildirir A, Eroglu S, Simsek V, Sade E, Atar I, Aydinalp A, Ozin B, Muderrisoglu H. Coronary microvascular function in patients with isolated systolic and combined systolic/diastolic hypertension. J Clin Hypertens (Greenwich). 2012; 14:871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozakova M, Palombo C, Pratali L, Pittella G, Galetta F, L'Abbate A. Mechanisms of coronary flow reserve impairment in human hypertension. an integrated approach by transthoracic and transesophageal echocardiography. Hypertension. 1997; 29:551-559. [DOI] [PubMed] [Google Scholar]
- 28.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol (1985). 2004; 97:404-415. [DOI] [PubMed] [Google Scholar]
- 29.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014; 306:H1364-H1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schelbert HR. Anatomy and physiology of coronary blood flow. J Nucl Cardiol. 2010; 17:545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye DM, Jennings G, Angus JA. Evidence for impaired endothelium dependent vasodilation in experimental left ventricular dysfunction. Clin Exp Pharmacol Physiol. 1994; 21:709-719. [DOI] [PubMed] [Google Scholar]
- 32.Campbell DJ, Somaratne JB, Prior DL, Yii M, Kenny JF, Newcomb AE, Kelly DJ, Black MJ. Obesity is associated with lower coronary microvascular density. PLoS One. 2013; 8:e81798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanoverschelde JL, Wijns W, Essamri B, Bol A, Robert A, Labar D, Cogneau M, Michel C, Melin JA. Hemodynamic and mechanical determinants of myocardial O2 consumption in normal human heart: effects of dobutamine. Am J Physiol. 1993; 265:H1884-H1892. [DOI] [PubMed] [Google Scholar]
- 34.Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA, Visser FC. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation. 2007; 115:918-927. [DOI] [PubMed] [Google Scholar]
- 35.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009; 54:402-409. [DOI] [PubMed] [Google Scholar]
- 36.Selby DE, Palmer BM, LeWinter MM, Meyer M. Tachycardia‐induced diastolic dysfunction and resting tone in myocardium from patients with a normal ejection fraction. J Am Coll Cardiol. 2011; 58:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschope C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008; 117:2051-2060. [DOI] [PubMed] [Google Scholar]
- 38.van Empel VP, Kaye DM, Borlaug BA. Effects of healthy aging on the cardiopulmonary hemodynamic response to exercise. Am J Cardiol. 2014; 114:131-135. [DOI] [PubMed] [Google Scholar]


