Abstract
Background
Metabolic syndrome (MetS) enhances salt sensitivity of blood pressure and is an important risk factor for cardiovascular disease. The effects of dietary salt restriction on cardiac pathology associated with metabolic syndrome remain unclear.
Methods and Results
We investigated whether dietary salt restriction might ameliorate cardiac injury in DahlS.Z‐Leprfa/Leprfa (DS/obese) rats, which are derived from a cross between Dahl salt‐sensitive and Zucker rats and represent a model of metabolic syndrome. DS/obese rats were fed a normal‐salt (0.36% NaCl in chow) or low‐salt (0.0466% NaCl in chow) diet from 9 weeks of age and were compared with similarly treated homozygous lean littermates (DahlS.Z‐Lepr+/Lepr+, or DS/lean rats). DS/obese rats fed the normal‐salt diet progressively developed hypertension and showed left ventricular hypertrophy, fibrosis, and diastolic dysfunction at 15 weeks. Dietary salt restriction attenuated all of these changes in DS/obese rats. The levels of cardiac oxidative stress and inflammation and the expression of cardiac renin–angiotensin–aldosterone system genes were increased in DS/obese rats fed the normal‐salt diet, and dietary salt restriction downregulated these parameters in both DS/obese and DS/lean rats. In addition, dietary salt restriction attenuated the increase in visceral adipose tissue inflammation and the decrease in insulin signaling apparent in DS/obese rats without reducing body weight or visceral adipocyte size. Dietary salt restriction did not alter fasting serum glucose levels but it markedly decreased the fasting serum insulin concentration in DS/obese rats.
Conclusions
Dietary salt restriction not only prevents hypertension and cardiac injury but also ameliorates insulin resistance, without reducing obesity, in this model of metabolic syndrome.
Keywords: diet, hypertension, obesity, remodeling, sodium
Introduction
Obesity is the central and causal component of metabolic syndrome (MetS), which is a growing medical problem in industrialized countries as a result of changes in lifestyle.1 Obesity is also associated with an increased incidence of hypertension and a consequent increase in cardiovascular disease risk.2 Adipose tissue is thought to play an important role in the development of hypertension and complications related to insulin resistance as a result of dysregulated secretion of adipocytokines from adipocytes in visceral fat of obese humans.3
Hypertension is a key feature of MetS, and up to one third of hypertensive individuals are thought to have MetS.4 Excessive consumption of dietary salt is an important contributor to hypertension in humans.5 A recent Chinese study showed that MetS enhances the blood pressure response to salt intake.6 Indeed, dietary salt restriction reduced blood pressure to a greater extent in obese individuals than in nonobese ones.6–7 In addition to its effects on blood pressure, high sodium intake elicits insulin resistance8 and is thought to have detrimental cardiovascular effects independent of blood pressure.9
The INTERSALT study showed that a reduction in sodium intake resulted in a lowering of the prevalence of hypertension.10 The Japanese Society of Hypertension Guidelines for the Management of Hypertension recommend a reduction in dietary salt intake to <6 g per day for the treatment of hypertension.11 A reduced intake of dietary sodium is especially effective in lowering blood pressure in individuals with several risk factors for MetS.6 In contrast, several surveys demonstrated an inverse association of cardiovascular mortality with salt intake.12–14 The relation between cardiovascular mortality and salt intake is still controversial, and the effects of dietary salt restriction on cardiac injury in individuals with MetS remain unclear.
We recently established a new animal model of MetS, the DahlS.Z‐Leprfa/Leprfa (DS/obese) rat, by crossing Dahl salt‐sensitive (DS) rats with Zucker rats harboring a missense mutation in the leptin receptor gene (Lepr). When fed a normal diet, DS/obese rats develop a phenotype similar to MetS in humans, including hypertension and cardiac hypertrophy as well as renal and liver damage.15 These observations suggested that salt sensitivity of blood pressure and target organ damage are enhanced in MetS. We have now investigated the effects of dietary salt restriction on cardiac and adipose tissue pathophysiology in male DS/obese rats.
Methods
Animals and Experimental Protocols
Animal experiments were approved by the Animal Experiment Committee of Nagoya University Graduate School of Medicine (Daiko district, approval Nos. 021‐029, 022‐009, 023‐028, 024‐012, 025‐010, and 026‐039). Eight‐week‐old male inbred DS/obese and DahlS.Z‐Lepr+/Lepr+ (DS/lean) rats were obtained from Japan SLC Inc (Hamamatsu, Japan) and were handled in accordance with the guidelines of Nagoya University Graduate School of Medicine as well as with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85‐23, revised 1996). After weaning, the rats were fed a 0.36% NaCl (normal‐salt) diet. DS/obese rats were fed a normal‐salt (NS) diet (0.36% NaCl in chow) or low‐salt (LS) diet (0.0466% NaCl in chow) from 9 weeks of age and were compared with similarly treated homozygous lean littermates, DS/lean rats (n=10, 10, 8, and 8 rats for DS/lean+LS, DS/lean+NS, DS/obese+LS, and DS/obese+NS groups, respectively). Both the normal‐salt and low‐salt diets and tap water were provided ad libitum throughout the experimental period. Body weight as well as food and water intake were measured weekly. At 15 weeks of age, the animals were anesthetized by intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg) and were subjected to echocardiographic and hemodynamic analyses. The heart and both visceral (retroperitoneal) and subcutaneous (inguinal) fat were subsequently excised, and left ventricular (LV) tissue was separated for analysis.
Echocardiographic and Hemodynamic Analyses
Systolic blood pressure (SBP) was measured weekly in conscious animals by tail‐cuff plethysmography (BP‐98A; Softron, Tokyo, Japan). At 15 weeks of age, rats were subjected to transthoracic echocardiography, as described previously.16 In brief, M‐mode echocardiography was performed with a 12.5‐MHz transducer (Xario SSA‐660A; Toshiba Medical Systems, Tochigi, Japan). LV end‐diastolic (LVDd) and end‐systolic (LVDs) dimensions as well as the thickness of the interventricular septum (IVST) and LV posterior wall (LVPWT) were measured. LV fractional shortening (LVFS), relative wall thickness (RWT), and LV mass were calculated as follows: LVFS (%)=[(LVDd−LVDs)/LVDd]×100; RWT=(IVST+LVPWT)/LVDd; and LV mass (g)=({[(IVST+LVDd+LVPWT)3−(LVDd)3]×1.04}×0.8)+0.14.17 For assessment of LV diastolic function, we calculated the ratio of early to late ventricular velocities, and the isovolumic relaxation time from the pulsed Doppler echocardiographic data. After echocardiography, cardiac catheterization was performed as described previously.16 Tracings of LV pressure and the ECG were digitized to determine LV end‐diastolic pressure. The time constant of isovolumic relaxation (tau) was calculated by the derivative method of Raff and Glantz as described previously.18
Measurement of Metabolic Parameters
Serum levels of glucose, triglyceride, total cholesterol, and free fatty acid were measured by routine enzymatic assays. The concentration of insulin in serum was measured using a mouse/rat enzyme‐linked immunosorbent assay kit (Morinaga Bioscience Institute, Yokohama, Japan). Insulin resistance was assessed from fasting insulin and glucose levels, using the previously validated homeostasis model assessment of insulin resistance (HOMA‐IR); HOMA‐IR=fasting glucose (mmol/L)×fasting insulin (μU/mL)/22.5.19 The serum concentration of adiponectin was measured with the use of a mouse/rat enzyme‐linked immunosorbent assay kit (Otsuka Pharmaceutical Co, Ltd, Tokyo, Japan). The plasma concentration of tumor necrosis factor (TNF)‐α and interleukin (IL)‐6 were measured with the use of mouse/rat enzyme‐linked immunosorbent assay kits (R&D Systems, Inc, Minneapolis, MN).
Histological Analysis
LV tissue was fixed in ice‐cold 4% paraformaldehyde for 48 to 72 hours, embedded in paraffin, and processed for histology as described.20 Transverse sections (thickness, 3 μm) of the left ventricle were stained either with hematoxylin‐eosin for routine histological examination or with Azan‐Mallory solution for evaluation of the extent of fibrosis. Image analysis was performed with NIH Scion Image software (Scion Corp, Frederick, MD) in a blinded manner to the experimental status of the animals.
Immunohistochemical Analysis
For evaluation of macrophage infiltration into the LV myocardium or visceral fat, tissue sections were subjected to immunostaining with antibodies to the monocyte–macrophage marker CD68. Frozen sections (thickness, 5 μm) of LV tissue were fixed with acetone, and the visceral fat pad was fixed in ice‐cold 4% paraformaldehyde for 48 to 72 hours, embedded in paraffin, and sectioned at a thickness of 5 μm.20 Endogenous peroxidase activity in all sections was blocked by their exposure to methanol containing 0.3% hydrogen peroxide. Sections were incubated at 4°C first overnight with mouse monoclonal antibodies to CD68 (1:100 dilution, clone ED1; Chemicon, Temecula, CA) and then for 30 minutes with Histofine Simple Stain Rat MAX PO (Nichirei Biosciences, Tokyo, Japan). Immune complexes were visualized with diaminobenzidine and hydrogen peroxide, and the sections were counterstained with hematoxylin. The number of immunoreactive myocardial interstitial macrophages was counted in 5 separate high‐power fields of each section and is expressed as CD68‐positive cells per square millimeter. The adipocyte cross‐sectional area was measured for 50 or more cells per animal, and macrophage infiltration in adipose tissue was quantified as the ratio of the number of nuclei of CD68‐positive cells to the total number of nuclei in 5 different low‐power fields of each section. Image analysis was performed with NIH Scion Image software (ImageJ) in a blinded manner to the experimental status of the animals.
Assay of Superoxide Production
Nicotinamide adenine dinucleotide phosphate (NADPH)‐dependent superoxide production by homogenates prepared from freshly frozen LV tissue was measured with an assay based on lucigenin‐enhanced chemiluminescence as described previously.21 The chemiluminescence signal was sampled every minute for 10 minutes with a microplate reader (WALLAC 1420 ARVO MX/Light; Perkin‐Elmer, Waltham, MA), and the respective background counts were subtracted from experimental values. Sections stained with dihydroethidium (Sigma) were examined with a fluorescence microscope equipped with a 585‐nm long‐pass filter. As a negative control, sections were incubated with superoxide dismutase (300 U/mL) before staining with dihydroethidium; such treatment prevented the generation of fluorescence signals (data not shown). The average of dihydroethidium fluorescence intensity values was calculated with NIH ImageJ software.
Quantitative Reverse Transcription Polymerase Chain Reaction (RT‐PCR) Analysis
Total RNA was extracted from LV tissue and treated with DNase with the use of a spin‐vacuum isolation kit (Promega, Madison, WI). Total RNA was extracted from adipose tissue homogenized with QIAzol reagent with the use of an RNeasy Lipid Tissue Mini Kit for adipose tissue (Qiagen, Hilden, Germany). Complementary DNA was synthesized from 2 μg (LV tissue) or 1 μg (adipose tissue) of the total RNA by RT with the use of random primers (Invitrogen, Carlsbad, CA) and MuLV reverse transcriptase (Applied Biosystems, Foster City, CA). After RT, real‐time PCR analysis was performed with the use of a Prism 7000 Sequence Detector (Perkin‐Elmer, Wellesley, MA)22 and with primers and TaqMan probes specific for rat cDNAs encoding atrial natriuretic peptide,23 brain natriuretic peptide,23 β‐myosin heavy chain,23 collagen type I or type III,24 fibronectin,25 the p22phox,26 gp91phox,26 p47phox,27 p67phox,27 and Rac127 subunits of NADPH oxidase, monocyte chemoattractant protein (MCP)‐1,28 osteopontin,28 cyclooxygenase (COX)‐2,16 angiotensin‐converting enzyme,23 the angiotensin II type 1A receptor,23 the mineralocorticoid receptor (MR),28 serum‐ and glucocorticoid‐regulated kinase 1,27 TNF‐α,27 or IL‐6.27 Reagents for detection of human 18S rRNA (Applied Biosystems) were used to quantify rat 18S rRNA as an internal standard.
Immunoblot Analysis
Total protein was isolated from LV and visceral adipose tissue and quantitated with the use of the Bradford reagent (Bio‐Rad, Hercules, CA). Equal amounts of protein were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and the separated proteins were transferred to a polyvinylidene difluoride membrane as described previously.16 The membrane was incubated first with a 1:200 dilution of rabbit polyclonal antibodies to the MR (Santa Cruz Biotechnology, Santa Cruz, CA) and a 1:1000 dilution of rabbit polyclonal antibodies to the Akt, Akt phosphorylated on Ser473, p70 S6 kinase, and p70 S6 kinase phosphorylated on Thr389 (Cell Signaling Technology, Danvers, MA) and then with a 1:10 000 dilution of horseradish peroxidase–conjugated goat antibodies to rabbit immunoglobulin G (KPL Laboratories, Gaithersburg, MD). Antibodies to GAPDH (Santa Cruz Biotechnology) were used to confirm equal loading of samples. Detection and quantification of immune complexes were performed as described previously.16
Statistical Analysis
Data are presented as means±SEM. Differences among groups of rats at 15 weeks of age were assessed by 1‐way factorial ANOVA followed by Fisher's multiple‐comparison test. The time courses of body weight, SBP, or food or water intake were compared among groups by 2‐way repeated‐measures ANOVA (time course [8 to 15 weeks of age]×salt loading [LS or NS]). Furthermore, we analyzed the data using 2‐way factorial ANOVA to evaluate the interactive influence of strains and salt loading on various parameters in the 4 experimental groups. A P value of <0.05 was considered statistically significant.
Results
Physiological Analysis and Metabolic Parameters
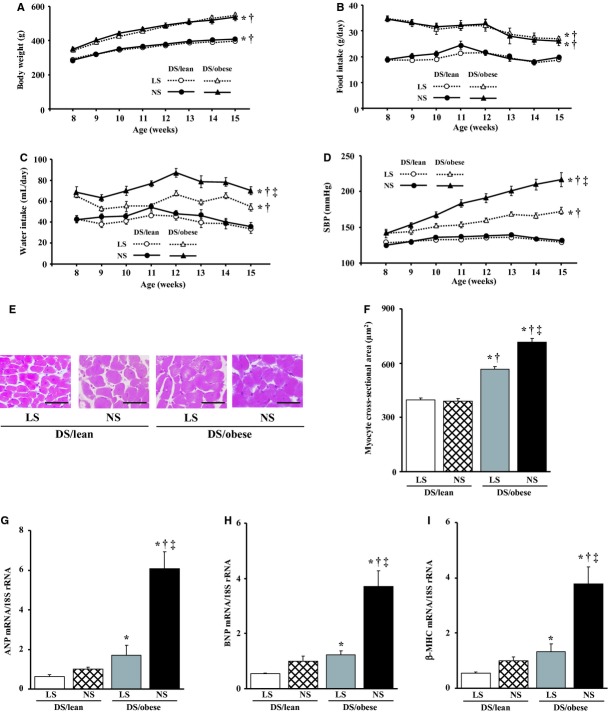
Body weight, visceral and subcutaneous fat mass, and the cross‐sectional area of visceral adipocytes were markedly greater in DS/obese rats than in DS/lean rats (Figure 1A, Table 1). These parameters did not differ significantly, however, between animals of the same genotype fed a NS‐ or LS diet, indicating that dietary salt restriction had no effect on body or fat mass. Food and water intake were significantly greater in DS/obese rats than in DS/lean rats throughout the experimental period (Figure 1B and 1C; Table 1). Given that food intake did not differ between animals of the same genotype fed a NS‐ or LS diet, dietary salt intake in each type of rat fed a LS diet was approximately one eighth of that in those fed a NS diet. Dietary salt restriction significantly reduced water intake in DS/obese rats.
Figure 1.
Time course of body weight (A), food intake (B), water intake (C), and SBP (D) and effects of salt restriction on cardiomyocyte hypertrophy (E through I). E, Hematoxylin‐eosin staining of transverse sections of the LV myocardium. Scale bars, 50 μm. F, Cross‐sectional area of cardiac myocytes determined from sections similar to those in (A). G through I, Quantitative RT‐PCR analysis of ANP, BNP, and β‐MHC mRNAs, respectively. The amount of each mRNA was normalized by that of 18S rRNA and then expressed relative to the corresponding mean value for DS/lean rats fed a normal‐salt diet. Data in (A through D) and (F through I) are means±SEM (n=10, 10, 8, and 8 rats for DS/lean+LS, DS/lean+NS, DS/obese+LS, and DS/obese+NS groups, respectively). *P<0.05 vs DS/lean+LS; †P<0.05 vs DS/lean+NS; ‡P<0.05 vs DS/obese+LS. ANP indicates atrial natriuretic peptide; BNP, brain natriuretic peptide; DS, Dahl salt; LS, low‐salt; LV, left ventricular; NS, normal‐salt; RT‐PCR, reverse transcription polymerase chain reaction; SBP, systolic blood pressure; β‐MHC, β‐myosin heavy chain.
Table 1.
Physiological and Metabolic Parameters in Rats of the 4 Experimental Groups at 15 Weeks of Age
| Parameter | DS/Lean | DS/Obese | ||
|---|---|---|---|---|
| LS | NS | LS | NS | |
| Tibial length, mm | 39.0±0.2 | 39.1±0.2 | 35.9±0.3*† | 35.6±0.3*† |
| Heart rate, beats/min | 370±10 | 382±8 | 350±10† | 368±19 |
| Heart weight/tibial length, mg/mm | 29.8±0.6 | 30.5±0.8 | 35.1±1.2*† | 40.3±0.9*†‡ |
| LV weight/tibial length, mg/mm | 21.5±0.3 | 22.1±0.5 | 26.1±0.9*† | 31.4±0.6*†‡ |
| Visceral fat weight/tibial length, mg/mm | 92.7±6.2 | 98.3±6.8 | 426.4±12.6*† | 431.6±26.6*† |
| Subcutaneous fat weight/tibial length, mg/mm | 62.8±5.5 | 63.3±6.5 | 642.4±23.7*† | 612.3±63.9*† |
| Visceral adipocyte cross‐sectional area, μm2 | 3380±131 | 3467±190 | 10 544±136*† | 10 698±300*† |
| Glucose, mg/dL | 130.1±4.3 | 131.8±4.5 | 148.0±18.9 | 147.0±14.3 |
| Insulin, ng/mL | 0.55±0.17 | 0.38±0.08 | 4.43±1.14*† | 7.17±1.35*†‡ |
| HOMA‐IR | 4.37±1.26 | 3.05±0.64 | 36.08±8.01*† | 72.39±9.11*†‡ |
| Triglyceride, mg/dL | 68.4±5.8 | 65.5±6.6 | 3469.3±869.2*† | 1304.9±250.4*†‡ |
| Total cholesterol, mg/dL | 73.6±2.99 | 84.8±4.47 | 469.25±85.66*† | 297.38±22.73*†‡ |
| Free fatty acid, mEq/L | 0.89±0.09 | 0.95±0.13 | 1.40±0.11*† | 1.54±0.19*† |
| Adiponectin, ng/mL | 3965±128 | 3729±229 | 6257±229*† | 6030±332*† |
| TNF‐α, pg/mL | 4.21±1.40 | 5.21±0.90 | 6.33±1.00*† | 17.71±3.13*†‡ |
| IL‐6, pg/mL | 37.18±8.30 | 35.88±5.00 | 53.88±9.60*† | 122.01±14.54*†‡ |
Analytes were measured in serum unless indicated otherwise. Data are means±SEM (n=10, 10, 8, and 8 rats for DS/lean+LS, DS/lean+NS, DS/obese+LS, and DS/obese+NS groups, respectively). DS indicates Dahl salt; HOMA‐IR, homeostasis model assessment of insulin resistance; IL, interleukin; LS, low‐salt; LV, left ventricular; NS, normal‐salt; TNF, tumor necrosis factor.
*P<0.05 vs DS/lean+LS; †P<0.05 vs DS/lean+NS; ‡P<0.05 vs DS/obese+LS.
The metabolic parameters are summarized in Table 1. Dietary salt restriction did not alter fasting serum glucose levels but it markedly decreased the fasting serum insulin concentration and HOMA‐IR index in DS/obese rats. In addition, dietary salt restriction significantly increased the serum levels of triglyceride and total cholesterol but it did not change the serum concentration of free fatty acid. Serum adiponectin levels were greater in DS/obese rats than in DS/lean rats and did not differ significantly between animals of the same genotype fed a NS‐ or LS diet. Plasma levels of TNF‐α and IL‐6 were increased in DS/obese rats compared with DS/lean rats, and these effects were significantly attenuated by dietary salt restriction.
Hemodynamics, LV Geometry, and Cardiac Function
DS/obese rats fed a NS diet progressively developed hypertension during the experimental period, and this change was significantly attenuated by dietary salt restriction (Figure 1D, Table 1). In contrast, DS/lean rats fed a NS‐ or LS diet maintained a normal SBP. At 15 weeks of age, the ratio of LV weight to tibial length, an index of LV hypertrophy, was increased in DS/obese rats compared with DS/lean rats, and this increase was significantly attenuated by dietary salt restriction (Table 1).
Echocardiography revealed that the thickness of the IVST and LVPWT, LVFS, RWT, and LV mass were significantly greater in DS/obese rats than in DS/lean rats, whereas the LVDd was similar in both rat strains (Table 2). Dietary salt restriction did not affect LVDd or LVFS, but it significantly attenuated the increases in IVST, LVPWT, RWT, and LV mass in DS/obese rats. Isovolumic relaxation time and tau, both of which are indices of LV relaxation, as well as the ratio of left ventricular end‐diastolic pressure to LVDd, an index of diastolic stiffness, were all increased in DS/obese rats compared with DS/lean rats. The early to late ventricular velocities were decreased in DS/obese rats compared with DS/lean rats. Dietary salt restriction attenuated all of these changes in parameters of LV relaxation and diastolic stiffness in DS/obese rats.
Table 2.
Cardiac Morphological and Functional Parameters of Rats in the 4 Experimental Groups at 15 Weeks of Age
| Parameter | DS/Lean | DS/Obese | ||
|---|---|---|---|---|
| LS | NS | LS | NS | |
| IVST, mm | 1.47±0.04 | 1.51±0.02 | 1.83±0.03*† | 2.08±0.03*†‡ |
| LVPWT, mm | 1.44±0.04 | 1.49±0.02 | 1.78±0.04*† | 2.06±0.03*†‡ |
| LVDd, mm | 8.09±0.19 | 8.36±0.19 | 8.60±0.32 | 8.51±0.22 |
| LVFS, % | 37.7±1.2 | 36.0±0.9 | 44.4±2.1*† | 45.7±1.9*† |
| RWT | 0.36±0.02 | 0.36±0.01 | 0.43±0.02*† | 0.49±0.02*†‡ |
| LV mass, mg | 834±30 | 920±40 | 1290±40*† | 1528±37*†‡ |
| E/A | 2.01±0.05 | 2.01±0.08 | 1.65±0.09*† | 1.34±0.06*†‡ |
| IRT, ms | 17.1±0.6 | 17.8±0.4 | 26.3±1.0*† | 31.5±0.9*†‡ |
| Tau, ms | 21.0±1.1 | 23.0±1.7 | 30.0±0.5*† | 39.8±2.5*†‡ |
| LVEDP, mm Hg | 2.62±0.36 | 2.50±0.20 | 5.07±0.48*† | 8.27±0.80*†‡ |
| LVEDP/LVDd, mm Hg/mm | 0.29±0.02 | 0.28±0.01 | 0.51±0.03*† | 0.85±0.01*†‡ |
Data are means±SEM (n=10, 10, 8, and 8 rats for DS/lean+LS, DS/lean+NS, DS/obese+LS, and DS/obese+NS groups, respectively). DS indicates Dahl salt; E/A, early to late ventricular; IRT, isovolumic relaxation time; IVST, interventricular septum; LS, low‐salt; LV, left ventricular; LVDd, LV end‐diastolic; LVEDP, LV end‐diastolic pressure; LVFS, LV fractional shortening; LVPWT, LV posterior wall thickness; NS, normal‐salt; RWT, relative wall thickness.
*P<0.05 vs DS/lean+LS; †P<0.05 vs DS/lean+NS; ‡P<0.05 vs DS/obese+LS.
Cardiomyocyte Hypertrophy as Well as Cardiac Fibrosis
The cross‐sectional area of cardiac myocytes was greater in DS/obese rats than in DS/lean rats, and this cardiomyocyte hypertrophy in DS/obese rats was significantly attenuated by dietary salt restriction (Figure 1E and 1F). Hemodynamic overload resulted in marked upregulation of the expression of atrial natriuretic peptide, brain natriuretic peptide, and β‐myosin heavy chain genes in the left ventricle of DS/obese rats, and this upregulation was greatly attenuated by dietary salt restriction (Figure 1G through 1I).
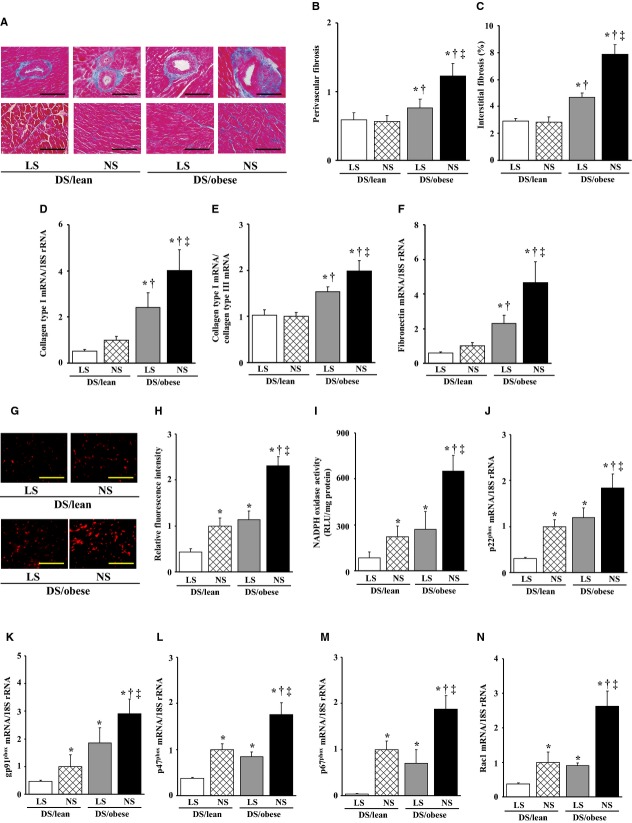
Azan‐Mallory staining revealed that fibrosis in perivascular and interstitial regions of the LV myocardium was increased in DS/obese rats compared with DS/lean rats and that this increase in myocardial fibrosis was significantly suppressed by dietary salt restriction (Figure 2A through 2C). The abundance of collagen type I and fibronectin mRNAs in the left ventricle as well as the ratio of the amount of collagen type I mRNA to that of collagen type III mRNA, which correlates with myocardial stiffness, were also increased in DS/obese rats in a manner sensitive to dietary salt restriction (Figure 2D through 2F).
Figure 2.
Effects of salt restriction on cardiac fibrosis and oxidative stress. A, Representative microscopic images of collagen deposition (blue) in perivascular (upper panels) or interstitial (lower panels) regions of the LV myocardium as revealed by Azan‐Mallory staining. Scale bars, 50 μm (upper panels) or 100 μm (lower panels). B and C, Relative extents of perivascular and interstitial fibrosis, respectively, in the LV myocardium as determined from sections similar to those in (A). D through F, Quantitative RT‐PCR analysis of collagen type I (D) and fibronectin (F) mRNAs as well as the ratio of the amount of collagen type I mRNA to that of collagen type III mRNA (E). G, Representative microscopic images of superoxide production in the LV myocardium as revealed by staining with dihydroethidium. Scale bars, 100 μm. H, Dihydroethidium fluorescence intensity as determined from sections similar to those in (G) and expressed relative to the value for DS/lean rats fed a normal‐salt diet. I, NADPH‐dependent superoxide production in LV homogenates. Data are expressed as relative light units (RLU) per milligram of protein. J through N, Quantitative RT‐PCR analysis of p22phox, gp91phox, p47phox, p67phox, and Rac1 mRNAs, respectively. Data in (B through F) and (H through N) are means±SEM (n values and symbols for statistical significance are as in Figure 1). DS indicates Dahl salt; LS, low‐salt; LV, left ventricular; NADPH, nicotinamide adenine dinucleotide phosphate; NS, normal‐salt; RT‐PCR, reverse transcription polymerase chain reaction.
Cardiac Oxidative Stress
Superoxide production in myocardial tissue sections revealed by staining with dihydroethidium as well as the activity of NADPH oxidase in LV homogenates were both increased in DS/obese rats compared with DS/lean rats (Figure 2G through 2I). Dietary salt restriction significantly attenuated superoxide production and NADPH oxidase activity in both DS/obese and DS/lean rats. In particular, dietary salt restriction in DS/obese rats reduced these parameters to the levels apparent in DS/lean rats fed a NS diet. The expression of genes for the p22phox and gp91phox membrane components and for the p47phox, p67phox, and Rac1 cytosolic components of NADPH oxidase in the left ventricle was also upregulated in DS/obese rats compared with DS/lean rats (Figure 2J through 2N). Dietary salt restriction reduced the expression of these NADPH oxidase subunit genes in both DS/obese and DS/lean rats.
Cardiac Inflammation
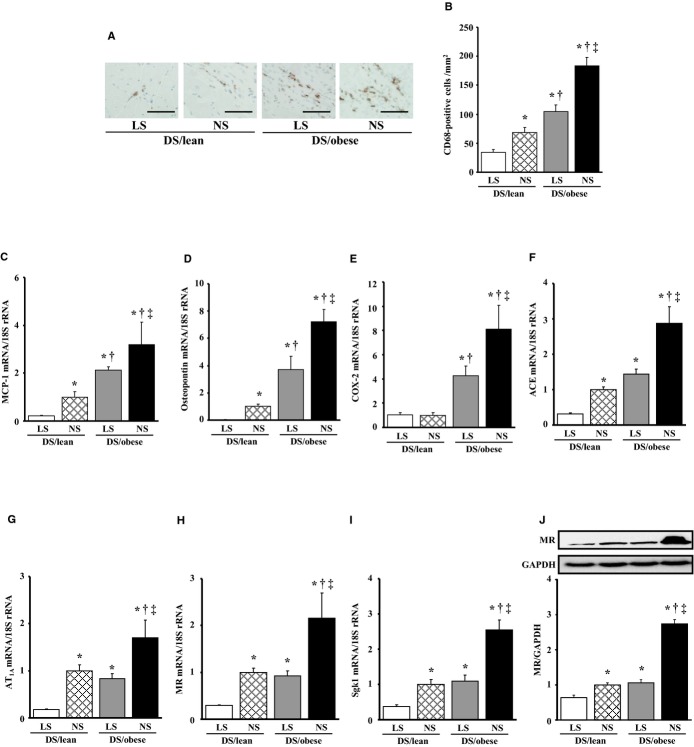
Immunostaining of the LV myocardium for the monocyte–macrophage marker CD68 revealed that the number of CD68‐positive cells was increased in DS/obese rats compared with DS/lean rats, and that dietary salt restriction reduced the extent of macrophage infiltration in both DS/lean and DS/obese rats (Figure 3A and 3B). The expression of MCP‐1, osteopontin, and COX‐2 genes in the left ventricle was also increased in DS/obese rats in a manner sensitive to dietary salt restriction (Figure 3C through 3E). The expression of MCP‐1 and osteopontin genes was also downregulated by dietary salt restriction in DS/lean rats.
Figure 3.
Effects of salt restriction on cardiac inflammation and RAAS genes. A, Representative microscopic images of immunohistochemical staining (brown) for the monocyte–macrophage marker CD68. Scale bars, 50 μm. B, Density of CD68‐positive cells in the LV myocardium as determined from sections similar to those in (A). C through I, Quantitative RT‐PCR analysis of MCP‐1, osteopontin, COX‐2, angiotensin‐converting enzyme, AT1A, MR, and Sgk1 mRNAs, respectively. J, Immunoblot analysis of the abundance of MR protein in the left ventricle. A representative immunoblot and the ratio of the amount of the MR to that of GAPDH (expressed relative to the corresponding value for the DS/lean+NS group) are shown. Data in (F through J) are means±SEM (n values and symbols for statistical significance are as in Figure 1). ACE indicates angiotensin‐converting enzyme; AT1A, angiotensin II type 1A receptor; COX, cyclooxygenase; DS, Dahl salt; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; LS, low‐salt; LV, left ventricular; MCP, monocyte chemoattractant protein; MR, mineralocorticoid receptor; NS, normal‐salt; RAAS, renin–angiotensin–aldosterone system; RT‐PCR, reverse transcription polymerase chain reaction; Sgk, serum‐ and glucocorticoid‐regulated kinase.
Cardiac Renin–Angiotensin–Aldosterone System (RAAS)
Cardiac expression of angiotensin‐converting enzyme, angiotensin II type 1A receptor, MR, and serum‐ and glucocorticoid‐regulated kinase 1 genes was upregulated in DS/obese rats compared with DS/lean rats, and dietary salt restriction downregulated the expression of these genes in both DS/lean and DS/obese rats (Figure 3F through 3I). The abundance of the MR protein in the left ventricle showed a pattern similar to that of the MR mRNA in the 4 experimental groups (Figure 3J). In particular, dietary salt restriction in DS/obese rats downregulated the expression of these genes of the RAAS and that of MR protein to the levels apparent in DS/lean rats fed a NS diet.
Inflammation in Adipose Tissue
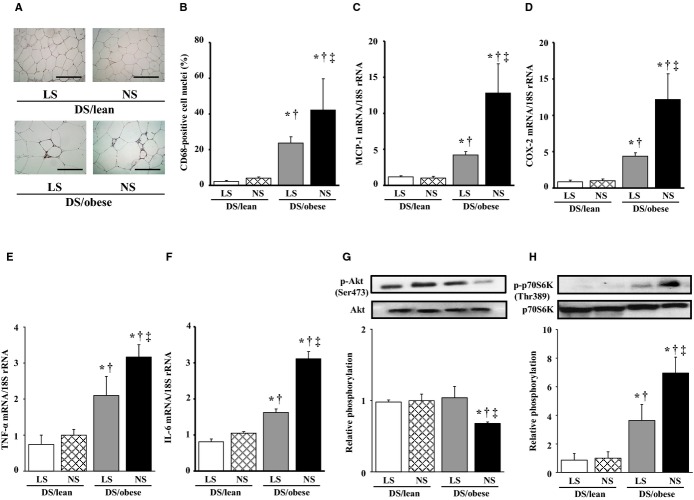
Immunostaining of visceral fat with antibodies to CD68 revealed the presence of more macrophages in DS/obese rats than in DS/lean rats (Figure 4A and 4B). DS/obese rats fed a NS diet also exhibited more areas of aggregated CD68‐positive cells surrounding adipocytes, forming a typical crownlike pattern, than did the other groups of animals. The increase in the extent of macrophage infiltration in adipose tissue of DS/obese rats was attenuated by dietary salt restriction. The expression of MCP‐1, COX‐2, TNF‐α, and IL‐6 genes in adipose tissue was also increased in DS/obese rats in a manner sensitive to dietary salt restriction (Figure 4C through 4F).
Figure 4.
Effects of salt restriction on adipose tissue inflammation and insulin signaling. A, Representative microscopic images of immunohistochemical staining for CD68. Scale bars, 100 μm. B, The number of nuclei of CD68‐positive cells as a percentage of total nuclei was determined from sections similar to those in (A). C through F, Quantitative RT‐PCR analysis of MCP‐1, COX‐2, and TNF‐α, and IL‐6 mRNAs, respectively. G and H, Representative immunoblots and the ratio of phosphorylated (p‐) to total forms of Akt and p70 S6 kinase. Data in (B through H) are means±SEM (n values and symbols for statistical significance are as in Figure 1). COX indicates cyclooxygenase; DS, Dahl salt; IL, interleukin; LS, low‐salt; MCP, monocyte chemoattractant protein; NS, normal‐salt; RT‐PCR, reverse transcription polymerase chain reaction; TNF, tumor necrosis factor.
Insulin Signaling in Adipose Tissue
The ratio of the amount of the phospho‐Ser473 form of Akt to that of total Akt was significantly decreased in DS/obese rats compared with DS/lean rats, and this effect was restored to the level apparent in DS/lean rats by dietary salt restriction (Figure 4G). The phosphorylation of p70 S6 kinase on Thr389 was significantly increased in DS/obese rats in a manner sensitive to dietary salt restriction (Figure 4H).
Analysis by 2‐Way Factorial ANOVA
There were no interactions between strains and salt loading in body weight, tibial length, food intake, or water intake (Table 3). The interactions were significant in SBP and LV weight, whereas it was not significant in heart rate. There were no interactions in adipose tissue weights or visceral adipocyte cross‐sectional area. Although the interactions were not significant in glucose, they were significant in insulin and HOMA‐IR. Regarding lipid metabolism, significant interactions were detected in triglyceride and total cholesterol, but they were not in free fatty acid. In regard to circulating adipocytokines, although there was no interaction in adiponectin, the interactions were significant in TNF‐α and IL‐6. With regard to echocardiographic and hemodynamic analyses, in IVST, LVPWT, RWT, LV mass, early to late ventricular velocities, isovolumic relaxation time, tau, left ventricular end‐diastolic pressure, and left ventricular end‐diastolic pressure/LVDd, the interactions were all significant. Significant interactions were observed in myocyte cross‐sectional area, perivascular and interstitial fibrosis, and the expression of fetal‐type cardiac genes and profibrotic genes. There were no significant interactions in cardiac oxidative stress, the expression of NADPH oxidase components, inflammation (except for COX‐2), or the expression of RAAS genes (except for MR protein). Finally, the interactions between strains and salt loading were all significant in adipose tissue analyses. Thus, the data analyzed by 2‐way factorial ANOVA were compatible with the original results analyzed by 1‐way factorial ANOVA.
Table 3.
Results of 2‐Way Factorial ANOVA in 4 Groups of Rats (DS/Lean+LS, DS/Lean+NS, DS/Obese+LS, and DS/Obese+NS)
| Variable | P Value for the Interaction | Variable | P Value for the Interaction |
|---|---|---|---|
| Body weight, g | 0.4098 | ANP | <0.0001 |
| Tibial length, mm | 0.3453 | BNP | 0.0002 |
| Food intake, g/day | 0.5898 | β‐MHC | 0.0004 |
| Water intake, mL/day | 0.0905 | Perivascular fibrosis | 0.0474 |
| SBP, mm Hg | 0.004 | Interstitial fibrosis, % | 0.0006 |
| Heart rate, beats/min | 0.7671 | Collagen type I | 0.0398 |
| Heart weight/tibial length, mg/mm | 0.0122 | Collagen type I/collagen type III | 0.0285 |
| LV weight/tibial length, mg/mm | 0.0005 | Fibronectin | 0.0217 |
| Visceral fat weight/tibial length, mg/mm | 0.9890 | DHE staining | 0.0762 |
| Subcutaneous fat weight/tibial length, mg/mm | 0.6207 | NADPH oxidase activity, RLU/mg protein | 0.060 |
| Visceral adipocyte cross‐sectional area, μm2 | 0.8717 | p22phox | 0.8784 |
| Glucose, mg/dL | 0.8876 | gp91phox | 0.4404 |
| Insulin, ng/mL | 0.0140 | p47phox | 0.3057 |
| HOMA‐IR | 0.0006 | p67phox | 0.5990 |
| Triglyceride, mg/dL | 0.0112 | Rac1 | 0.0708 |
| Total cholesterol, mg/dL | 0.0268 | CD68 positive cells (/mm2) | 0.0597 |
| Free fatty acid, mEq/L | 0.7467 | MCP‐1 | 0.5691 |
| Adiponectin, ng/mL | 0.9871 | Osteopontin | 0.0550 |
| TNF‐α, pg/mL | 0.0028 | COX‐2 | 0.0241 |
| IL‐6, pg/mL | 0.0038 | ACE | 0.0905 |
| IVST, mm | 0.0033 | AT1A | 0.8853 |
| LVPWT, mm | 0.0015 | MR | 0.2082 |
| LVDd, mm | 0.4473 | Sgk1 | 0.1095 |
| LVFS, mm | 0.3097 | MR protein | <0.0001 |
| RWT | 0.0333 | CD68 positive cells in adipose tissue, % | 0.0002 |
| LV mass, mg | 0.0284 | MCP‐1 in adipose tissue | 0.0105 |
| E/A | 0.0356 | COX‐2 in adipose tissue | 0.0205 |
| IRT, ms | 0.0023 | TNF‐α in adipose tissue | 0.0215 |
| Tau, ms | 0.0222 | IL‐6 in adipose tissue | 0.0455 |
| LVEDP, mm Hg | 0.0043 | Akt phosphorylation | 0.0359 |
| LVEDP/LVDd, mm Hg/mm | 0.0088 | p70S6K phosphorylation | 0.0080 |
| Myocyte cross‐sectional area, μm2 | <0.0001 |
ACE indicates angiotensin‐converting enzyme; ANP, atrial natriuretic peptide; AT1A, angiotensin II type 1A receptor; BNP, brain natriuretic peptide; COX, cyclooxygenase; DS, Dahl salt; E/A, early to late ventricular; HOMA‐IR, homeostasis model assessment of insulin resistance; IL, interleukin; IRT, isovolumic relaxation time; IVST, interventricular septum; LS, low‐salt; LV, left ventricular; LVDd, LV end‐diastolic; LVEDP, LV end‐diastolic pressure; LVFS, LV fractional shortening; LVPWT, LV posterior wall thickness; MCP, monocyte chemoattractant protein; β‐MHC, β‐myosin heavy chain; MR, mineralocorticoid receptor; NADPH, nicotinamide adenine dinucleotide phosphate; NS, normal‐salt; RLU, relative light unit; RWT, relative wall thickness; SBP, systolic blood pressure; Sgk, serum‐ and glucocorticoid‐regulated kinase; TNF, tumor necrosis factor.
Discussion
We have shown that dietary salt restriction attenuated hypertension as well as LV hypertrophy, fibrosis, and diastolic dysfunction and that these effects were accompanied by inhibition of cardiac oxidative stress, inflammation, and RAAS gene expression in DS/obese rats. The antioxidative and anti‐inflammatory effects of dietary salt restriction, without a lowering of blood pressure, were also apparent, in DS/lean rats. In addition, dietary salt restriction inhibited inflammation in visceral adipose tissue without reducing body weight or visceral fat mass in DS/obese rats. Attenuation of insulin resistance induced by dietary salt restriction may have contributed to beneficial effects on cardiac pathophysiology, in a manner independent of its antihypertensive effect, in this model of MetS. However, dietary salt restriction was not beneficial in ameliorating dyslipidemia in DS/obese rats.
Our recent report suggested that the presence of the fa allele of Lepr on the DahlS background is associated with increased salt sensitivity of blood pressure.15 In the present study, dietary salt restriction substantially attenuated the increase in blood pressure in DS/obese rats without affecting SBP in DS/lean rats. Insulin resistance, a key component of MetS, is also closely related to salt sensitivity of blood pressure.8 Reduced systemic insulin resistance resulting from dietary salt restriction may thus have contributed to the attenuation of hypertension by this manipulation in DS/obese rats in the present study. However, the mechanism by which salt intake affects cardiovascular function remains uncertain. Our results suggest that dietary salt restriction may alter LV structure and function as well as blood pressure in hypertensive obese individuals but not in normotensive nonobese controls. In the present study, the beneficial effects of dietary salt restriction on LV remodeling and diastolic dysfunction could not be separated from the antihypertensive effect. Nevertheless, the pleiotropic benefits of dietary salt restriction may help explain the relations between salt intake and cardiovascular and all‐cause mortality.29
The cardiac inflammatory changes may have contributed to myocardial fibrosis in DS/obese rats.30 Dietary salt restriction attenuated macrophage infiltration into the myocardium as well as the upregulation of MCP‐1, osteopontin, and COX‐2 gene expression in the heart of DS/obese rats, indicating that dietary salt restriction alleviated cardiac inflammation in these animals. Moreover, the increase in circulating levels of TNF‐α and IL‐6 in DS/obese rats was inhibited by dietary salt restriction. Since the elevations in circulating proinflammatory cytokines also result in cardiac fibrosis and dysfunction,31–32 the decrease in these cytokines by dietary salt restriction may have contributed to improvement of cardiac injury in DS/obese rats. Given that dietary salt restriction significantly attenuated cardiac inflammation and slightly but not significantly downregulated the expression of profibrotic genes without lowering blood pressure in DS/lean rats, salt loading appears to contribute to the development of cardiac inflammation and consequent upregulation of profibrotic genes in a manner independent of blood pressure. In this regard, however, dietary salt restriction did not affect cardiac fibrosis in DS/lean rats under our experimental conditions.
The decrease in cardiac oxidative stress induced by dietary salt restriction in both DS/obese and DS/lean rats was accompanied by a reduction in blood pressure only in DS/obese rats. Salt loading may be an important trigger for the accumulation of reactive oxygen species in MetS, which in turn may play a key role in salt‐induced progression of cardiac pathophysiology associated with this condition.33 Our observations that the cardiac RAAS was inhibited by dietary salt restriction in both DS/obese and DS/lean rats are consistent with previous results showing that high salt intake increases aldosterone production and upregulates angiotensin II type 1A receptor mRNA in the cardiovascular system of rats.34 Enhanced MR signaling in the myocardium results in increased cardiac oxidative stress and inflammation, leading to the development of cardiac remodeling and dysfunction.28,33 Although dietary salt restriction did not affect cardiac phenotype in DS/lean rats, our results suggest that the inhibition of cardiac oxidative stress and the cardiac RAAS induced by dietary salt restriction in DS/obese rats are not likely attributable solely to the reduction in blood pressure.
Visceral obesity gives rise to a state of chronic, low‐grade inflammation that contributes to cardiovascular disease.35 The low‐grade inflammation in adipose tissue associated with obesity is characterized by abnormal levels of circulating proinflammatory factors and an aberrant production of adipocytokines.35 Indeed, we detected adipocyte hypertrophy, macrophage infiltration, and upregulation of the expression of proinflammatory genes in visceral adipose tissue of DS/obese rats, consistent with previous studies showing macrophage infiltration into adipose tissue of obese animals36 as well as obesity‐induced inflammation and insulin resistance.37 Dietary salt restriction did not affect adipocyte size but attenuated macrophage infiltration and the increased expression of proinflammatory genes in visceral adipose tissue of DS/obese rats. It did not affect any of these parameters in DS/lean rats. These data suggest that salt can modulate adipose tissue inflammation, but not adipose tissue mass, in MetS, and that macrophage accumulation in adipose tissue is a feature of MetS rather than obesity.
Previous studies have reported that severe dietary salt restriction elicits insulin resistance38 and has caused adverse effects on glucose39 and lipid metabolism.40 Our data on lipid metabolism are consistent with a previous report showing that in obese mice, dyslipidemia induced by dietary salt restriction is attributable to an impairment in the removal rate of triglyceride‐rich lipoproteins.40 Moreover, our results are in good agreement with another report showing that free fatty acid levels did not vary on a low‐salt diet.41 Contrary to the previous report,40 we observed that in DS/obese rats, dietary salt restriction ameliorated systemic insulin resistance, as shown by the reductions in the fasting insulin concentration and HOMA‐IR index, in spite of increased triglyceride and total cholesterol concentrations. Because dietary salt restriction did not affect circulating adiponectin levels, adiponectin may not have played a major role in improvement of insulin resistance in this model of MetS. The phosphorylation of Akt clearly modulates the glucose transport and lipogenesis in the adipose tissue.8,38 The p70 S6 kinase has been reported to inhibit insulin receptor substrate (IRS)‐1 function through induction of serine phosphorylation of IRS‐1, which is believed to be the major mechanism of insulin resistance.42 Moreover, TNF‐α induces insulin resistance by increasing Ser/Thr phosphorylation of IRS‐1.43 Taken together, the present results suggest that p70 S6 kinase and consequent inhibition of the IRS‐1/Akt pathway in adipose tissue contributed to insulin resistance in DS/obese rats, and that attenuation of p70 S6 kinase phosphorylation by dietary salt restriction improved the IRS‐1/Akt kinase‐mediated insulin signaling. Since insulin resistance is induced by inflammatory adipocytokines and excess salt via Rac1 activation,44 suppression of Rac1 activation by dietary salt restriction might have contributed, at least in part, to improved insulin resistance. Thus, dietary salt restriction may have inhibited systemic and adipose tissue inflammation as well as improved insulin signaling in adipose tissue in DS/obese rats, resulting in a decrease in insulin levels.
In conclusion, dietary salt restriction ameliorated hypertension and cardiac pathophysiology in DS/obese rats. In addition, it did not alter adipose tissue mass but attenuated adipose tissue inflammation and improved insulin signaling in DS/obese rats. Dietary salt restriction in patients with MetS may be an effective strategy not only for preventing hypertension and cardiac injury but also for providing attenuation of insulin resistance, without reducing obesity.
Sources of Funding
This work was supported by unrestricted research grants from Nippon Boehringer Ingelheim Co, Ltd (Tokyo, Japan), Ajinomoto Pharmaceuticals Co, Ltd (Tokyo, Japan), Kyowa Hakko Kirin Co Ltd (Tokyo, Japan), Astellas Pharma Inc (Tokyo, Japan), Mochida Pharmaceutical Co, Ltd (Tokyo, Japan), Mitsubishi Tanabe Pharma Corporation (Osaka, Japan), Takeda Pharmaceutical Company Limited (Osaka, Japan), Daiichi‐Sankyo Company, Limited (Tokyo, Japan) and Dr Nagata (Nagoya University) as well as by Management Expenses Grants from the Japanese Government to Nagoya University.
Disclosures
None.
Acknowledgments
We thank Chieko Nakashima and Masafumi Ohtake for technical assistance.
References
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004; 109:433-438. [DOI] [PubMed] [Google Scholar]
- 2.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002; 288:2709-2716. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa Y. Therapy insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006; 3:35-42. [DOI] [PubMed] [Google Scholar]
- 4.Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catini E, Leonetti G, Magrini F, Zanchetti A. Metabolic syndrome and target organ damage in untreated essential hypertensives. J Hypertens. 2004; 22:1991-1998. [DOI] [PubMed] [Google Scholar]
- 5.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009; 23:363-384. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Chen J, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J. Metabolic syndrome and salt sensitivity of blood pressure in non‐diabetic people in China: a dietary intervention study. Lancet. 2009; 373:829-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, Martin M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989; 321:580-585. [DOI] [PubMed] [Google Scholar]
- 8.Ogihara T, Asano T, Ando K, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Abe M, Fukushima Y, Kikuchi M, Fujita T. High‐salt diet enhances insulin signaling and induces insulin resistance in Dahl salt‐sensitive rats. Hypertension. 2002; 40:83-89. [DOI] [PubMed] [Google Scholar]
- 9.Dyer AR, Elliott P, Shipley M, Stamler R, Stamler J. Body mass index and associations of sodium and potassium with blood pressure in INTERSALT. Hypertension. 1994; 23:729-736. [DOI] [PubMed] [Google Scholar]
- 10.INTERSALT Cooperative Research Group. INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988; 297:319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim‐Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014; 37:253-387. [DOI] [PubMed] [Google Scholar]
- 12.Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovsky J, Kawecka‐Jaszcz K, Nikitin Y, Staessen JA. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011; 305:1777-1785. [DOI] [PubMed] [Google Scholar]
- 13.Alderman MH, Cohen H, Madhavan S. Dietary sodium intake and mortality: the National Health and Nutrition Examination Survey (NHANES I). Lancet. 1998; 351:781-785. [DOI] [PubMed] [Google Scholar]
- 14.Cohen HW, Hailpern SM, Fang J, Alderman MH. Sodium intake and mortality in the NHANES II follow‐up study. Am J Med. 2006; 119:275.e277. [DOI] [PubMed] [Google Scholar]
- 15.Hattori T, Murase T, Ohtake M, Inoue T, Tsukamoto H, Takatsu M, Kato Y, Hashimoto K, Murohara T, Nagata K. Characterization of a new animal model of metabolic syndrome: the DahlS.Z‐Leprfa/Leprfa rat. Nutr Diabetes. 2011; 1:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murase T, Hattori T, Ohtake M, Nakashima C, Takatsu M, Murohara T, Nagata K. Effects of estrogen on cardiovascular injury in ovariectomized female DahlS.Z‐Leprfa/Leprfa rats as a new animal model of metabolic syndrome. Hypertension. 2012; 59:694-704. [DOI] [PubMed] [Google Scholar]
- 17.Reffelmann T, Kloner RA. Transthoracic echocardiography in rats. Evaluation of commonly used indices of left ventricular dimensions, contractile performance, and hypertrophy in a genetic model of hypertrophic heart failure (SHHF‐Mcc‐facp‐Rats) in comparison with Wistar rats during aging. Basic Res Cardiol. 2003; 98:275-284. [DOI] [PubMed] [Google Scholar]
- 18.Nagata K, Iwase M, Sobue T, Yokota M. Differential effects of dobutamine and a phosphodiesterase inhibitor on early diastolic filling in patients with congestive heart failure. J Am Coll Cardiol. 1995; 25:295-304. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412-419. [DOI] [PubMed] [Google Scholar]
- 20.Miyachi M, Yazawa H, Furukawa M, Tsuboi K, Ohtake M, Nishizawa T, Hashimoto K, Yokoi T, Kojima T, Murate T, Yokota M, Murohara T, Koike Y, Nagata K. Exercise training alters left ventricular geometry and attenuates heart failure in Dahl salt‐sensitive hypertensive rats. Hypertension. 2009; 53:701-707. [DOI] [PubMed] [Google Scholar]
- 21.Ichihara S, Noda A, Nagata K, Obata K, Xu J, Ichihara G, Oikawa S, Kawanishi S, Yamada Y, Yokota M. Pravastatin increases survival and suppresses an increase in myocardial matrix metalloproteinase activity in a rat model of heart failure. Cardiovasc Res. 2006; 69:726-735. [DOI] [PubMed] [Google Scholar]
- 22.Somura F, Izawa H, Iwase M, Takeichi Y, Ishiki R, Nishizawa T, Noda A, Nagata K, Yamada Y, Yokota M. Reduced myocardial sarcoplasmic reticulum Ca(2+)‐ATPase mRNA expression and biphasic force‐frequency relations in patients with hypertrophic cardiomyopathy. Circulation. 2001; 104:658-663. [DOI] [PubMed] [Google Scholar]
- 23.Nagata K, Somura F, Obata K, Odashima M, Izawa H, Ichihara S, Nagasaka T, Iwase M, Yamada Y, Nakashima N, Yokota M. AT1 receptor blockade reduces cardiac calcineurin activity in hypertensive rats. Hypertension. 2002; 40:168-174. [DOI] [PubMed] [Google Scholar]
- 24.Sakata Y, Yamamoto K, Mano T, Nishikawa N, Yoshida J, Hori M, Miwa T, Masuyama T. Activation of matrix metalloproteinases precedes left ventricular remodeling in hypertensive heart failure rats: its inhibition as a primary effect of angiotensin‐converting enzyme inhibitor. Circulation. 2004; 109:2143-2149. [DOI] [PubMed] [Google Scholar]
- 25.Hattori T, Murase T, Sugiura Y, Nagasawa K, Takahashi K, Ohtake M, Miyachi M, Murohara T, Nagata K. Effects of salt status and blockade of mineralocorticoid receptors on aldosterone‐induced cardiac injury. Hypertens Res. 2014; 37:125-133. [DOI] [PubMed] [Google Scholar]
- 26.Takatsu M, Nakashima C, Takahashi K, Murase T, Hattori T, Ito H, Murohara T, Nagata K. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension. 2013; 62:957-965. [DOI] [PubMed] [Google Scholar]
- 27.Murase T, Hattori T, Ohtake M, Abe M, Amakusa Y, Takatsu M, Murohara T, Nagata K. Cardiac remodeling and diastolic dysfunction in DahlS.Z‐Leprfa/Leprfa rats: a new animal model of metabolic syndrome. Hypertens Res. 2012; 35:186-193. [DOI] [PubMed] [Google Scholar]
- 28.Nagata K, Obata K, Xu J, Ichihara S, Noda A, Kimata H, Kato T, Izawa H, Murohara T, Yokota M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low‐aldosterone hypertensive rats. Hypertension. 2006; 47:656-664. [DOI] [PubMed] [Google Scholar]
- 29.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow‐up of the trials of hypertension prevention (TOHP). BMJ. 2007; 334:885-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber KT. From inflammation to fibrosis: a stiff stretch of highway. Hypertension. 2004; 43:716-719. [DOI] [PubMed] [Google Scholar]
- 31.Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor‐alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002; 53:822-830. [DOI] [PubMed] [Google Scholar]
- 32.Melendez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010; 56:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsui H, Ando K, Kawarazaki H, Nagae A, Fujita M, Shimosawa T, Nagase M, Fujita T. Salt excess causes left ventricular diastolic dysfunction in rats with metabolic disorder. Hypertension. 2008; 52:287-294. [DOI] [PubMed] [Google Scholar]
- 34.Schmid C, Castrop H, Reitbauer J, Della Bruna R, Kurtz A. Dietary salt intake modulates angiotensin II type 1 receptor gene expression. Hypertension. 1997; 29:923-929. [DOI] [PubMed] [Google Scholar]
- 35.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C‐reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999; 19:972-978. [DOI] [PubMed] [Google Scholar]
- 36.Baudrand R, Lian CG, Lian BQ, Ricchiuti V, Yao TM, Li J, Williams GH, Adler GK. Long‐term dietary sodium restriction increases adiponectin expression and ameliorates the proinflammatory adipokine profile in obesity. Nutr Nutr Metab Cardiovasc Dis. 2014; 24:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka‐Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein‐1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006; 281:26602-26614. [DOI] [PubMed] [Google Scholar]
- 38.Prada PO, Coelho MS, Zecchin HG, Dolnikoff MS, Gasparetti AL, Furukawa LN, Saad MJ, Heimann JC. Low salt intake modulates insulin signaling, JNK activity and IRS‐1ser307 phosphorylation in rat tissues. J Endocrinol. 2005; 185:429-437. [DOI] [PubMed] [Google Scholar]
- 39.Prada P, Okamoto MM, Furukawa LN, Machado UF, Heimann JC, Dolnikoff MS. High‐ or low‐salt diet from weaning to adulthood: effect on insulin sensitivity in Wistar rats. Hypertension. 2000; 35:424-429. [DOI] [PubMed] [Google Scholar]
- 40.Catanozi S, Rocha JC, Nakandakare ER, Passarelli M, Mesquita CH, Silva AA, Dolnikoff MS, Harada LM, Quintao EC, Heimann JC. The rise of the plasma lipid concentration elicited by dietary sodium chloride restriction in Wistar rats is due to an impairment of the plasma triacylglycerol removal rate. Atherosclerosis. 2001; 158:81-86. [DOI] [PubMed] [Google Scholar]
- 41.Townsend RR, Kapoor S, McFadden CB. Salt intake and insulin sensitivity in healthy human volunteers. Clin Sci (Lond). 2007; 113:141-148. [DOI] [PubMed] [Google Scholar]
- 42.Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M. A rapamycin‐sensitive pathway down‐regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate‐1. Mol Endocrinol. 2000; 14:783-794. [DOI] [PubMed] [Google Scholar]
- 43.Ueno M, Carvalheira JB, Tambascia RC, Bezerra RM, Amaral ME, Carneiro EM, Folli F, Franchini KG, Saad MJ. Regulation of insulin signalling by hyperinsulinaemia: role of IRS‐1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia. 2005; 48:506-518. [DOI] [PubMed] [Google Scholar]
- 44.Fujita T. Mineralocorticoid receptors, salt‐sensitive hypertension, and metabolic syndrome. Hypertension. 2010; 55:813-818. [DOI] [PubMed] [Google Scholar]