Abstract
Background
Hypertensive cardiomyopathy is characterized by myocyte hypertrophy and interstitial fibrosis. The effects of renal denervation (RD) on the heart are poorly understood. New magnetic resonance imaging techniques (extracellular volume fraction) permit the quantitative assessment of myocardial fibrosis. Our aim was to study the effects of RD on myocardial fibrosis.
Methods and Results
Twenty‐three patients with resistant hypertension undergoing RD and 5 resistant hypertensive controls were prospectively included. Cardiac magnetic resonance imaging at 1.5 T was performed before RD and at 6‐month follow‐up. Indexed left ventricular mass, septal extracellular volume fraction, and indexed absolute extracellular volume (a quantitative measure of extracellular matrix) were quantified. All data are reported as mean±SD deviation (median). Decreases in systolic (161.96±19.09 [160] versus 144.78±16.48 [143] mm Hg, P<0.0001) and diastolic (85.61±12.88 [83] versus 80.39±11.93 [81] mm Hg, P=0.018) blood pressures and in indexed left ventricular mass (41.83±10.20 [41.59] versus 37.72±7.44 [38.49] g/m1.7, P=0.001) were observed at follow‐up only in RD patients. No significant differences in extracellular volume were found (26.24±3.92% [26.06%] versus 25.74±4.53% [25.63%], P=0.605). A significant decrease in absolute extracellular volume was observed after 6 months in RD patients exclusively (10.36±2.25 [10.79] versus 9.25±2.38 [9.79] mL/m1.7, P=0.031). This effect was observed independently of blood pressure reduction.
Conclusions
RD significantly decreases left ventricular mass, while extracellular volume remains stable. Our results suggest that the observed left ventricular mass decrease was due not exclusively to a reversion of myocyte hypertrophy but also to an additional reduction in collagen content, indicating interstitial myocardial fibrosis.
Keywords: cardiac, cardiac magnetic resonance, extracellular volume fraction, interstitial myocardial fibrosis, renal denervation
Introduction
Despite the wide availability of pharmacological antihypertensive treatment, it is estimated that 1 of 50 patients with newly diagnosed hypertension will develop resistant hypertension,1 a condition associated with an increased risk for cardiovascular events and chronic renal disease compared with that of nonresistant hypertensive patients.2
The therapeutic options for patients with resistant hypertension have recently improved with the introduction of renal denervation (RD), which was demonstrated in the Symplicity HTN‐1 and Symplicity HTN‐2 trials,3–4 as well as in multiple smaller trials and animal studies,5–7 to achieve a sustained blood pressure reduction. The results of the Symplicity HTN‐3 study8 have cast some doubt on the use of this procedure, and some methodological issues (eg, recent changes in medication and varying degree of expertise) limit its conclusions.9–10 In addition to its blood pressure–lowering effects, RD has been demonstrated to have positive effects on left ventricular (LV) morphology, geometry, and function.11–12 In a recent study using cardiac magnetic resonance (CMR), significant decreases in LV mass and improvements in LV ejection fraction and circumferential strain were observed in patients treated with RD, whereas no such changes were seen in controls at 6‐month follow‐up.12 Because hypertensive cardiomyopathy is characterized by myocyte hypertrophy and interstitial fibrosis,13 it is unclear whether the observed decrease in LV mass following RD is due to the reversal of myocyte hypertrophy, a reduction in interstitial fibrosis, or both. Although data from animal studies have shown that sympathectomy is associated with interstitial fibrosis reduction independently of blood pressure decrease,14 the mechanisms in humans are unknown.
CMR is currently the noninvasive modality of choice for fibrosis and scar assessment.15 CMR had been limited to the evaluation of localized forms of fibrosis, but the recent extracellular volume fraction (ECV) technique permits the assessment and quantification of its interstitial and diffuse forms.16–17 In particular, ECV allows the quantification of extracellular matrix expansion and has been proposed as a useful parameter with which to quantify diffuse fibrosis. ECV has been validated in several human studies versus biopsy, demonstrating a good correlation with histological collagen volume fraction.16,18–19 In addition, ECV is reproducible,19–20 independent of field strength (unlike native or postcontrast T1 measurements),21 and, most important, a predictor of mortality and events,22–23 with higher mortality at follow‐up in patients with higher ECV values.
Our study aimed to investigate the effects of RD on diffuse myocardial fibrosis noninvasively, by quantifying ECV fraction with the use of CMR.
Patients and Methods
Patients with resistant hypertension referred to our institution for RD between January 2012 and October 2013, and those for whom complete clinical and CMR data were available were enrolled. “Resistant hypertension” was defined as an office systolic blood pressure (SBP) above the target (≥140 mm Hg) or mean ambulatory 24‐hour SBP >135 mm Hg despite the use of ≥3 antihypertensive agents of different classes, including a diuretic at maximum or highest tolerated doses.24 A stable antihypertensive medication regimen (>3‐month treatment on stable dosing) was necessary before inclusion. Twenty‐three patients who met these criteria and underwent renal denervation were included, and they constituted our RD group. One patient with multiple allergies to antihypertensive preparations was also included. Five resistant hypertensive patients with the aforementioned criteria and with contraindications to RD (eg, significant renal artery stenosis, renal arteries with a diameter <4 mm or a length <20 mm, presence of multiple renal arteries25) or unwillingness to undergo this procedure) served as controls. Exclusion criteria included pseudo‐resistant hypertension (defined as mean ambulatory 24‐hour SBP <130 mm Hg), secondary causes of hypertension, and glomerular filtration rate <45 mL/min per 1.73 m2, as well as other general contraindications for the performance of CMR, such as noncompatible biometallic implants, severe claustrophobia, or known allergy to gadolinium contrast. All included patients underwent 2 CMR studies: 1 at baseline (≥1 week before RD in the patients who underwent this procedure) and 1 at 6 months. Clinical assessment, including review of medication compliance and blood pressure determination according to the Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),26 and blood hematocrit determination were also performed at both time points. In patients who underwent RD, the procedure was performed using the Symplicity Flex system (Medtronic) as described previously,27 with a mean number of ablation points per artery of 6.3±1.2 (median 6). A positive response to RD was defined as a reduction of ≥10 mm Hg in SBP at 6‐month follow‐up.3–4 The local institutional review board approved the study, and all patients gave written informed consent.
CMR Protocol
All CMR studies were performed with use of a 1.5‐T magnetic resonance scanner (Achieva; Philips Healthcare). A 32‐element cardiac synergy coil was used for signal detection. Patients were placed in the supine position, and images were acquired during breath‐holds of ≈10 to 15 seconds by using vector electrocardiogram gating. To localize the heart in the 3 standard planes (axial, coronal, and sagittal), a single‐shot steady‐state free precession sequence was used. Short‐axis cine images covering the entire LV myocardium and 2‐, 3‐, and 4‐chamber cine images were obtained. All cine images were recorded with a balanced gradient‐echo cine sequence (spatial resolution 1.8×1.8×8 mm3, 50 heart phases, repetition time (TR)/echo time (TE)=3.2/1.6 ms, flip angle 60°).
Late gadolinium enhancement images were acquired 10 to 15 minutes after bolus injection of 0.2 mmol/kg gadobenate meglumine (Dotarem; Guerbet) with an inversion‐recovery 3‐dimensional spoiled gradient echo sequence. Typical parameters were voxel size 1.7×1.7×5 mm3, TR/TE=3.3/1.6 ms, and flip angle of 15°. Inversion time was assessed individually with use of a Look‐Locker sequence using an individually adapted prepulse delay sequence. Short‐axis late gadolinium enhancement (LGE) views of the entire LV myocardium and 2‐, 3‐, and 4‐chamber LGE views were obtained.
For diffuse fibrosis assessment, we acquired a single breath‐hold modified Look‐Locker inversion‐recovery sequence28 in a basal and a mid‐ventricular short‐axis view, before and 10 minutes after contrast administration. An apical slice was not included to avoid partial volume effects. The sequence consisted of 3 inversion pulses with different prepulse delays (300, 210, and 130 ms), after which the images were acquired following a 3‐3‐5 scheme. Typical imaging parameters were a voxel size of 1.7×2.1×10 mm, TR/TE 2.4/1 ms, flip angle 35°, heart rate–adapted trigger delay, and the use of parallel imaging.
Image Analysis
The images obtained were analyzed using commercially available software (Qmass 7.5.20.0; Medis Medical Image Systems). Endocardial and epicardial borders were drawn in all short‐axis slices at end‐diastole and end‐systole to calculate the global myocardial mass and LV ejection fraction by using the disc summation method.15 The myocardial mass was indexed by body surface area and height (g/m1.7).29 To calculate the ECV fraction, a region of interest was drawn in the septum in each slice (basal and medial, precontrast and postcontrast T1 maps), with care taken to exclude the interface between myocardium and bordering structures (eg, blood) to avoid partial volume effects (Figure 1). Regions containing ischemic LGE were excluded. A second region of interest was drawn in the blood pool; this allowed the quantification of the T1 values of the septum and blood pool. ECV was calculated as previously described16 as:
where myo refers to the septal myocardial T1 value, blood to the blood pool T1 value, and pre and post to the measurement before and after contrast administration, respectively. For all T1 values, the averages of the basal and medial values were used.
Figure 1.
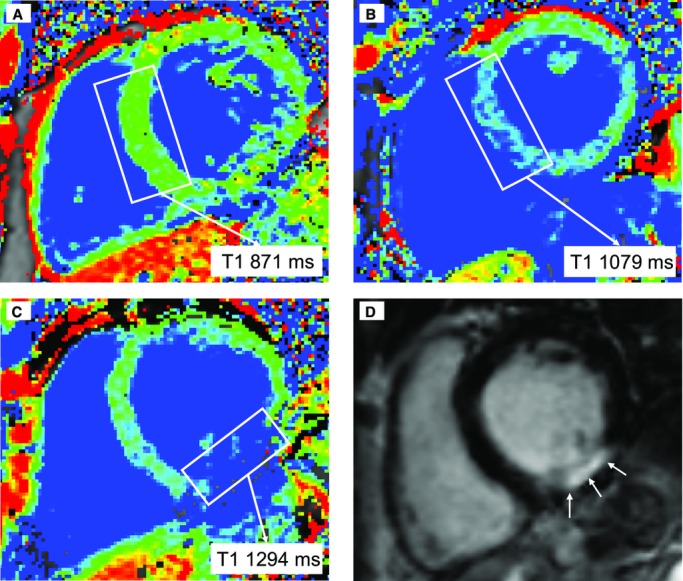
Examples of precontrast T1 quantification. Blue color reflects high T1 values, and areas depicted in green or red have lower T1 values. Whereas in A the T1 value in the septum is low (871 ms, green), in B an increased septal T1 can be found (1079 ms, blue). Finally, C and D correspond to a patient with an inferior myocardial infarction: in C, a high T1 value in the infarcted area can be seen, while D demonstrates a hyperenhanced area in the inferior wall, corresponding to the infarction.
Finally, we quantified the absolute volume of the extracellular myocardial space in each patient by using the following formula:
LV myocardial volume corresponds to the global LV volume expressed in milliliters and is calculated as:
where 1.05 is the myocardial density given in g/mL.30
Statistical Analysis
Statistical analysis was performed using SPSS for Windows (version 19; SPSS Inc). All continuous parameters are given as mean±SD (median). Categorical data are summarized as frequencies and percentages. The significance of mean differences between RD patients and controls was evaluated by using the Mann–Whitney test, whereas the differences between baseline and 6‐month follow‐up values for a particular patient group were tested with use of the Wilcoxon signed rank test for paired data. The Pearson χ2 test was used to compare categorical data. Intraclass correlation coefficient (ICC) was calculated to evaluate intraobserver and interobserver variabilities of myocardial T1 (precontrast and postcontrast), blood T1 (precontrast and postcontrast), and LV mass; an ICC >0.6 was considered “good” and >0.7 was considered “excellent.”31 ICC is given as “ICC (95% CI).” A value of P<0.05 was regarded as statistically significant.
Results
A total of 23 RD patients and 5 resistant hypertensive controls were included. A total of 3 segments had to be excluded: 2 in a patient due to the presence of artifacts and 1 in another patient due to LGE secondary to myocardial infarction. In those patients, the remaining segments were averaged to permit ECV quantification. No patients had to be excluded. Baseline characteristics of the included population are given in Tables 1 and 2. There were no significant differences between RD patients and controls regarding baseline clinical characteristics, renal function, or antihypertensive treatment, although the control group showed a trend toward a higher rate of coronary artery disease (12 [52.2%] in RD patients versus 5 [100%] in controls, P=0.063). Baseline blood pressure values, both SBP (161.96±19.09 [160] in RD versus 152.80±22.97 [150] mm Hg in controls, P=0.290) and diastolic blood pressure (DBP) (85.61±12.88 [83] in RD versus 80.60±12.28 [78] mm Hg in controls, P=0.318), did not differ between the groups. Although no statistically significant baseline characteristics were noted between RD patients and controls, it should be noted that this lack of statistical significance may be secondary to a lack of power due to small sample size.
Table 1.
Baseline Characteristics
| Renal Denervation (n=23) | Controls (n=5) | P Value | |
|---|---|---|---|
| Age, y | 67.09±8.51 (68) | 72.20±3.77 (73) | 0.193 |
| Female sex | 9 (39.1) | 3 (60) | 0.357 |
| BMI, kg/m2 | 28.12±3.58 (28.73) | 26.90±2.81 (25.69) | 0.560 |
| Heart rate, bpm | 71.04±12.77 (69) | 76.20±13.99 (78) | 0.521 |
| SBP, mm Hg | 161.96±19.09 (160) | 152.80±22.97 (150) | 0.290 |
| DBP, mm Hg | 85.61±12.88 (83) | 80.60±12.28 (78) | 0.318 |
| Creatinine, mg/dL | 0.88±0.13 (0.88) | 0.82±0.17 (0.77) | 0.318 |
| GFR, mL/min per 1.73 m2 | 80.70±15.33 (80.50) | 79.80±19.12 (73.30) | 0.727 |
| Hyperlipidemia | 17 (73.9) | 4 (80) | 0.633 |
| T2D | 11 (47.8) | 3 (60) | 0.500 |
| Current smoker | 2 (8.7) | 1 (20) | 0.459 |
| CAD | 12 (52.2) | 5 (100) | 0.063 |
| AF | 3 (13) | 1 (20) | 0.568 |
| Stroke | 3 (13) | 0 (0) | 0.541 |
Results expressed as mean±SD (median) or number (%). BMI indicates body mass index, SBP, systolic blood pressure, DBP, diastolic blood pressure, GFR, glomerular filtration rate; T2D, type 2 diabetes; CAD, coronary artery disease; AF, atrial fibrillation.
Table 2.
Antihypertensive Medication at Baseline
| Renal Denervation (n=23) | Controls (n=5) | P Value | |
|---|---|---|---|
| Antihypertensive medication | 4.78±1.48 (5) | 4.80±0.84 (5) | 0.954 |
| ACEI/ARB | 21 (91.3) | 5 (100) | 0.669 |
| Renin inhibitor | 1 (4.3) | 0 (0) | 0.821 |
| β‐Blocker | 20 (87) | 5 (100) | 0.541 |
| CCI | 19 (82.6) | 5 (100) | 0.432 |
| Diuretics | 22 (95.7) | 5 (100) | 0.821 |
| Sympatholytic | 11 (47.8) | 2 (40) | 0.572 |
Results expressed as mean±SD (median) or number (%). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCI, calcium channel inhibitor.
After 6‐month follow‐up, a significant decrease in SBP (161.96±19.09 [160] mm Hg at baseline versus 144.78±16.48 [143] mm Hg at follow‐up, P<0.0001) and DBP (85.61±12.88 [83] mm Hg at baseline versus 80.39±11.93 [81] mm Hg at follow‐up, P=0.018), as well as in heart rate (71.04±12.77 [69] beats per minute [bpm] at baseline versus 66.48±9.88 [67] bpm at follow‐up, P=0.016), was observed in the RD group, whereas no such changes were observed in control patients. A nonsignificant trend toward a reduction in the number of antihypertensive drugs was also observed in RD patients (4.78±1.48 [5] at baseline versus 4.35±1.67 [4] at follow‐up, P=0.083). There were no significant differences in body mass index or parameters of renal function between the groups (Table 3).
Table 3.
Clinical Parameters, Baseline Versus 6 months
| Renal Denervation (n=23) | Controls (n=5) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 mo | P Value | Baseline | 6 mo | P Value | |
| BMI, kg/m2 | 28.12±3.58 (28.73) | 27.72±3.80 (28.40) | 0.709 | 26.90±2.81 (25.69) | 26.89±3.92 (24.22) | 1.000 |
| Heart rate, bpm | 71.04±12.77 (69) | 66.48±9.88 (67) | 0.016 | 76.20±13.99 (78) | 74.20±18.03 (66) | 0.343 |
| SBP, mm Hg | 161.96±19.09 (160) | 144.78±16.48 (143) | <0.0001 | 152.80±22.97 (150) | 145.00±13.64 (140) | 0.273 |
| DBP, mm Hg | 85.61±12.88 (83) | 80.39±11.93 (81) | 0.018 | 80.60±12.28 (78) | 71.00±7.35 (75) | 0.144 |
| Creatinine, mg/dL | 0.88±0.13 (0.88) | 0.92±0.20 (0.89) | 0.080 | 0.82±0.17 (0.77) | 0.82±0.13 (0.77) | 1.000 |
| GFR, mL/min per 1.73 m2 | 80.70±15.33 (80.50) | 79.09±17.03 (78.95) | 0.503 | 79.80±19.12 (73.30) | 80.39±18.56 (73.30) | 0.715 |
| Hematocrit, % | 39.9±3.3 (39.7) | 39.9±3.8 (40.2) | 0.819 | 37.8±3.6 (39.7) | 38.9±4.7 (39.9) | 0.498 |
| Antihypertensive drugs (n) | 4.78±1.48 (5) | 4.35±1.67 (4) | 0.083 | 4.80±0.84 (5) | 4.60±1.14 (5) | 0.655 |
Results expressed as mean±SD (median) or number (%). BMI indicates body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GFR, glomerular filtration rate.
CMR Parameters
Excellent reproducibility was observed in all our measurements, both intraobserver and interobserver (Table 4). Our results regarding changes in CMR parameters are summarized in Table 5. At 6 months after RD, a significant decrease of 9% in indexed LV mass was observed in RD patients (41.83±10.20 [41.59] g/m1.7 at baseline versus 37.72±7.44 [38.49] g/m1.7 at follow‐up, P=0.001), whereas in control patients, a nonsignificant increase of 2% in indexed LV mass at follow‐up was found (37.92±6.29 [35.75] g/m1.7 at baseline versus 38.79±6.65 [38.84] g/m1.7 at follow‐up, P=0.686). No significant changes in myocardial T1 values (neither before nor after contrast) were observed in any group.
Table 4.
ICC Values
| Intraobserver ICC | Interobserver ICC | |
|---|---|---|
| LV mass | 0.965 (0.880 to 0.990) | 0.713 (0.196 to 0.920) |
| T1 myo native | 0.989 (0.959 to 0.997) | 0.966 (0.868 to 0.991) |
| T1 blood native | 0.935 (0.765 to 0.983) | 0.986 (0.944 to 0.997) |
| T1 myo postcontrast | 0.983 (0.936 to 0.996) | 0.947 (0.802 to 0.987) |
| T1 blood postcontrast | 0.987 (0.947 to 0.997) | 0.996 (0.984 to 0.999) |
ICC indicates intraclass correlation coefficient; LV, left ventricular; T1 blood, blood T1 measured in the medial slice; T1 myo, myocardial T1 measured in the medial slice.
Table 5.
Results for Renal Denervation Versus Controls
| Renal Denervation (n=23) | Controls (n=5) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 mo | P Value | Baseline | 6 mo | P Value | |
| LV mass, g/m1.7 | 42.02±9.67 (41.59) | 37.72±7.44 (38.49) | 0.001 | 37.92±6.29 (35.75) | 38.79±6.65 (38.84) | 0.686 |
| T1 myo native, ms | 955.02±69.63 (954.50) | 955.98±54.44 (967.50) | 0.988 | 1037.70±90.71 (1008.0) | 978.00±50.47 (971.50) | 0.138 |
| T1 myo postcontrast, ms | 433.91±48.80 (417.00) | 425.07±57.41 (408.50) | 0.581 | 496.00±111.34 (452.0) | 428.50±13.10 (431.0) | 0.138 |
| ECV septum, % | 26.24±3.92 (26.06) | 25.74±4.53 (25.63) | 0.605 | 28.06±2.64 (27.50) | 29.51±2.15 (29.44) | 0.225 |
| Absolute extracellular volume, mL/m1.7 | 10.36±2.25 (10.79) | 9.25±2.38 (9.79) | 0.031 | 10.08±1.50 (10.18) | 10.90±2.02 (11.51) | 0.345 |
LV indicates left ventricular; T1 myo, myocardial T1.
Regarding ECV fraction, no significant changes were observed in RD patients at 6‐month follow‐up (26.24±3.92% [26.06%] at baseline versus 25.74±4.53% [25.63%] at follow‐up, P=0.605), with a nonsignificant increasing trend in the control group (28.06±2.64% [27.50%] at baseline versus 29.51±2.15% [29.44%] at follow‐up, P=0.225) (Figure 2). Finally, when absolute extracellular volume was quantified, a significant decrease in absolute extracellular volume at follow‐up was observed in the RD group (10.36±2.25 [10.79] mL/m1.7 at baseline versus 9.25±2.38 [9.79] mL/m1.7 at follow‐up, P=0.031), with a nonsignificant increase in extracellular volumes in control patients (10.08±1.50 [10.18] mL/m1.7 at baseline versus 10.90±2.02 [11.51] mL/m1.7 at follow‐up, P=0.345) (Figure 3).
Figure 2.
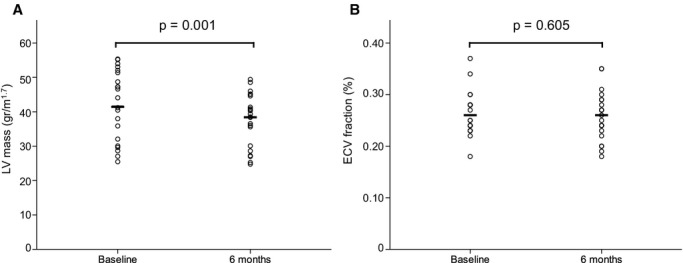
Indexed LV mass (A) and extracellular volume fraction (B) before and 6 months after renal denervation. Whereas a significant decrease in indexed LV mass (g/m1.7) can be observed at follow‐up, no significant changes are noted in ECV fraction (%). ECV, extracellular volume fraction LV indicates left ventricular.
Figure 3.
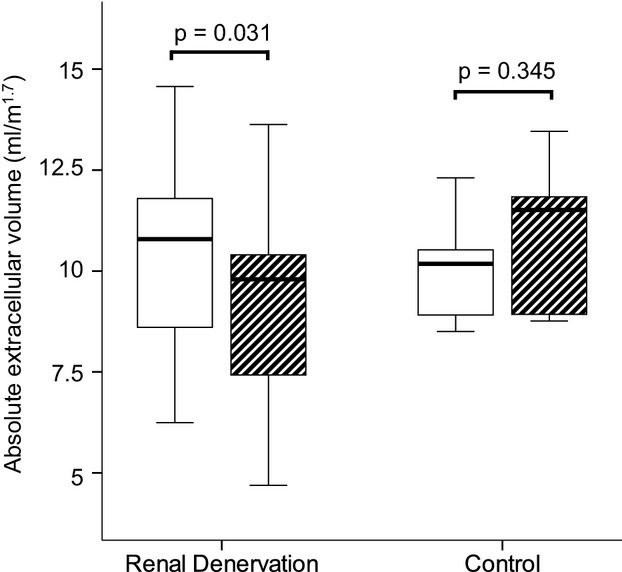
Change in indexed absolute extracellular volume (mL/m1.7) before and after 6‐month follow‐up in renal denervation patients and controls. Whereas in RD patients a significant decrease is documented, only a nonsignificant trend toward an increase can be seen in controls. RD indicates renal denervation.
Responders Versus Nonresponders
Fifteen (65.2%) patients in the RD group presented a decrease of at least 10 mm Hg in SBP and, thus, were considered responders to RD, whereas 8 (34.8%) were nonresponders. Our results for responders and nonresponders to RD are summarized in Table 6. A significant decrease in indexed LV mass was observed in both responders (41.83±10.20 [41.59] g/m1.7 at baseline versus 37.36±7.23 [38.37] g/m1.7 at follow‐up, P=0.011) and nonresponders to RD (42.37±9.23 [(44.32] g/m1.7 at baseline versus 38.40±8.28 [40.59] g/m1.7 at follow‐up, P=0.017). No significant differences between baseline and follow‐up were observed in either group regarding T1 values, ECV fraction, or absolute extracellular volume, although a trend toward a decrease in absolute extracellular volume after 6 months was observed in both groups (responders: 10.40±2.55 [11.57] mL/m1.7 at baseline versus 9.46±2.48 [10.03] mL/m1.7 at follow‐up, P=0.156; nonresponders: 10.29±1.71 [10.15] mL/m1.7 at baseline versus 8.86±2.30 [8.93] mL/m1.7 at follow‐up; P=0.123).
Table 6.
Results for Responders Versus Nonresponders
| Responders (n=15) | Nonresponders (n=8) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 mo | P Value | Baseline | 6 mo | P Value | |
| LV mass, g/m1.7 | 41.83±10.20 (41.59) | 37.36±7.23 (38.37) | 0.011 | 42.37±9.23 (44.32) | 38.40±8.28 (40.59) | 0.017 |
| T1 myo native, ms | 963.23±67.56 (959.0) | 961.47±55.83 (974.5) | 0.955 | 939.63±75.46 (952.25) | 945.69±53.81 (957.0) | 0.944 |
| T1 myo postcontrast, ms | 427.20±45.5 (416.5) | 410.23±33.89 (404.0) | 0.201 | 448.29±56.14 (445.5) | 452.88±81.90 (448.0) | 0.612 |
| ECV septum, % | 26.39±4.22 (26.06) | 26.48±4.86 (26.83) | 0.691 | 25.97±3.55 (25.11) | 24.34±3.72 (23.65) | 0.161 |
| Absolute extracellular volume, mL/m1.7 | 10.40±2.55 (11.57) | 9.46±2.48 (10.03) | 0.156 | 10.29±1.71 (10.15) | 8.86±2.30 (8.93) | 0.123 |
Response defined as decrease of systolic blood pressure ≥10 mm Hg after 6 months. LV indicates left ventricular; T1 myo, myocardial T1.
Discussion
The results of our study show that RD leads to a decrease in LV mass independently of blood pressure reduction. Additionally, we have demonstrated that while LV mass decreases, ECV remains stable, suggesting a concomitant reducing effect of RD on extracellular matrix expansion.
Our results confirm the findings from previous work demonstrating that RD is associated with a decrease in LV mass. Other studies, using different imaging techniques, have shown a consistent reduction in LV mass in patients undergoing RD.11–12 A recent study by Mahfoud et al12 demonstrated a decrease in indexed LV mass, as assessed by using CMR, in patients undergoing RD. Similarly, another study that used echocardiography found a slightly higher degree of LV mass reduction.11 Interestingly, the LV mass–decreasing effect seems to occur independently of blood pressure reduction (as demonstrated by the fact that we observed it in both responders and nonresponders). Both the previous magnetic resonance and echocardiography studies also reported LV mass reduction following RD, independently of RD blood pressure–lowering effects.11–12 Because LV hypertrophy is associated with an increased probability of events and mortality at follow‐up,32–33 these findings are important, suggesting that RD may also improve the prognosis of hypertensive patients. This possibility, however, has not been investigated in the present work and deserves further evaluation.
On the other hand, and taking into account that hypertensive cardiomyopathy is characterized by myocyte hypertrophy and interstitial fibrosis,13 the reduction in total LV mass following RD may be secondary to either myocyte hypertrophy reversal or fibrosis regression. We investigated this issue noninvasively by using ECV fraction, a parameter that permits the quantification of extracellular matrix expansion and correlates with fibrosis as defined by an increase in collagen concentration.16 While LV mass is reduced, ECV fraction remains stable, reflecting a simultaneous regression of both cell mass and extracellular matrix, and ECV fraction (reflecting extracellular space) would be expected to increase at follow‐up if the only mechanism for LV mass regression were the reversal of myocyte hypertrophy. We analyzed this further by calculating the absolute amount of extracellular space in each patient individually (absolute extracellular volume), which was significantly reduced after RD.
Interestingly, we observed a nonsignificant trend toward an increase in both indexed LV mass and ECV fraction in controls, which may reflect a progression of hypertensive cardiomyopathy in this subgroup of patients and which may have not reached statistical significance due to small sample size. This needs to be further analyzed in a larger population.
Although we did not perform histological analysis in our patients, our present findings are in agreement with data arising from prior studies, suggesting an involvement of the sympathetic nervous system in the development of myocardial fibrosis.14 In a study by Perlini et al,14 sympathectomy was performed in a rodent hypertension model.14 The procedure was associated with a reduction in histologically verified myocardial interstitial fibrosis. Interestingly, this effect was independent of blood pressure reduction because sympathectomy did not achieve a significant blood pressure reduction in this model. In the same experiment, doxazosine, but not propranolol, was also associated with fibrosis reversal, suggesting that the profibrotic effect is mediated via α‐adrenergic receptors.14 Recent studies have further demonstrated the role of sympathectomy in myocardial fibrosis reduction.34 Additionally, a recent report focusing exclusively on the effects of RD further support this data: in a study with obese hypertensive rats, RD was associated with reduced LV interstitial fibrosis formation.35
An important point to take into account when interpreting our results is the follow‐up period (6 months), which may be too short to observe a significant reduction in ECV fraction. It has been pointed out that myocite hypertrophy regression occurs faster than fibrosis reduction.36 It is then possible that a longer follow‐up is necessary to demonstrate a substantial, detectable reduction in ECV fraction. However, the results of the previous reports together with the fact that ECV fraction does not increase at follow‐up suggest that RD has a decreasing effect on interstitial myocardial fibrosis.
When dividing our population between responders and nonresponders to RD, we observed that ECV fraction remained stable although indexed LV mass significantly decreased in both groups, suggesting an effect independent of blood pressure reduction. Human studies have found a lack of correlation between extracellular matrix expansion reversal and blood pressure reduction, by demonstrating that not all antihypertensive medication has the same effect on myocardial fibrosis. Although angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, and mineralocorticoids have been demonstrated in histology‐validated studies to reduce interstitial fibrosis,37–40 less effect is observed with β‐blockers.41 In our study, the effect of RD on LV mass was observed in addition to that of antihypertensive medication, demonstrating its independent effect.
This potential beneficial effect of RD on extracellular matrix expansion could have prognostic implications. Prior data have shown that extracellular expansion is associated with a higher probability of events and mortality.22–23 What is more important is a recent report42 that demonstrated that extracellular matrix expansion measured with CMR ECV fraction is a powerful independent marker of prognosis beyond that of indexed LV mass.
Limitations
Our study has several limitations. On the one hand, our sample size is small and, thus, our results need to be confirmed in further work. The small sample size may explain the lack of statistical significance in some baseline characteristics (particularly the presence of coronary artery disease), as well as in changes at follow‐up. Furthermore, a higher presence of coronary artery disease in controls may have influenced the change in ECV fraction in this subgroup of patients; these issues should be taken into account when interpreting our results. In addition, we did not perform a histological study of our patients. However, other studies have previously validated ECV against histology, demonstrating a good correlation with histological collagen volume fraction.16,18
Further studies should investigate this topic, ideally at a multicentric level, with a larger number of patients and a longer follow‐up period. This would be useful not only to confirm our findings but also to possibly prove prognostic value.
Conclusions
RD is associated with a decrease in LV mass independent of blood pressure reduction. Our results suggest that RD may additionally reduce myocardial interstitial fibrosis in terms of absolute collagen content, because if the observed LV mass decrease was exclusively due to a reversion of myocyte hypertrophy, ECV fraction would be expected to increase. Whether this has a potential effect on prognosis and event reduction should be investigated in future studies.
Sources of Funding
Dr Doltra is supported by a Research Grant from the European Society of Cardiology.
Disclosures
None.
Acknowledgments
We thank Drs Alexander Berger, Ernst Wellnhofer, and Stephan Dreysse, as well as our magnetic resonance technicians Gudrun Großer, Janina Denzer, Christine Löffler, Johanna Schlee, and Corinna Else, for helping in the performance of high‐quality cardiac magnetic resonance examinations and Anne Gale for editorial assistance.
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008; 117:e510-e526. [DOI] [PubMed] [Google Scholar]
- 2.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O'Connor PJ, Selby JV, Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012; 125:1635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter‐based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet. 2009; 373:1275-1281. [DOI] [PubMed] [Google Scholar]
- 4.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010; 376:1903-1909. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira VL, Irigoyen MC, Moreira ED, Strunz C, Krieger EM. Renal denervation normalizes pressure and baroreceptor reflex in high renin hypertension in conscious rats. Hypertension. 1992; 192 suppl:II17-II21. [DOI] [PubMed] [Google Scholar]
- 6.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995; 25:878-882. [DOI] [PubMed] [Google Scholar]
- 7.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multi‐electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013; 34:2132-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha‐Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014; 370:1393-1401. [DOI] [PubMed] [Google Scholar]
- 9.Baker NC, Waksman R. Editorial: renal sympathetic denervation: a true lack of efficacy, or the victim of a “perfect storm”? Cardiovasc Revasc Med. 2014; 15:61-62. [DOI] [PubMed] [Google Scholar]
- 10.Luscher TF, Mahfoud F. Renal nerve ablation after SYMPLICITY HTN‐3: confused at the higher level? Eur Heart J. 2014; 35:1706-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Bohm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012; 59:901-909. [DOI] [PubMed] [Google Scholar]
- 12.Mahfoud F, Urban D, Teller D, Linz D, Stawowy P, Hassel JH, Fries P, Dreysse S, Wellnhofer E, Schneider G, Buecker A, Schneeweis C, Doltra A, Schlaich MP, Esler MD, Fleck E, Bohm M, Kelle S. Effect of renal denervation on left ventricular mass and function in patients with resistant hypertension: data from a multi‐centre cardiovascular magnetic resonance imaging trial. Eur Heart J. 2014; 35:2224-2231. [DOI] [PubMed] [Google Scholar]
- 13.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998; 16:1031-1041. [DOI] [PubMed] [Google Scholar]
- 14.Perlini S, Palladini G, Ferrero I, Tozzi R, Fallarini S, Facoetti A, Nano R, Clari F, Busca G, Fogari R, Ferrari AU. Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure‐overload hypertrophy. Hypertension. 2005; 46:1213-1218. [DOI] [PubMed] [Google Scholar]
- 15.Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch‐Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010; 55:2614-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010; 122:138-144. [DOI] [PubMed] [Google Scholar]
- 17.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub‐clinical myocardial pathology. Eur Heart J. 2012; 33:1268-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging. 2013; 6:955-962. [DOI] [PubMed] [Google Scholar]
- 19.Neilan TG, Coelho‐Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, Chen Y, Mandry D, Pierre‐Mongeon F, Blankstein R, Kwong RY, Jerosch‐Herold M. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013; 6:672-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chin CW, Semple S, Malley T, White AC, Mirsadraee S, Weale PJ, Prasad S, Newby DE, Dweck MR. Optimization and comparison of myocardial T1 techniques at 3T in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging. 2014; 15:556-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA. T1 mapping of the myocardium: intra‐individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012; 14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short‐term mortality. Circulation. 2012; 126:1206-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014; 35:657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck‐Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker‐Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013; 34:2159-2219. [DOI] [PubMed] [Google Scholar]
- 25.Mahfoud F, Luscher TF, Andersson B, Baumgartner I, Cifkova R, Dimario C, Doevendans P, Fagard R, Fajadet J, Komajda M, Lefevre T, Lotan C, Sievert H, Volpe M, Widimsky P, Wijns W, Williams B, Windecker S, Witkowski A, Zeller T, Bohm M. Expert consensus document from the European Society of Cardiology on catheter‐based renal denervation. Eur Heart J. 2013; 34:2149-2157. [DOI] [PubMed] [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560-2572. [DOI] [PubMed] [Google Scholar]
- 27.Tsioufis C, Mahfoud F, Mancia G, Redon J, Damascelli B, Zeller T, Schmieder RE. What the interventionalist should know about renal denervation in hypertensive patients: a position paper by the ESH WG on the interventional treatment of hypertension. EuroIntervention. 2014; 9:1027-1035. [DOI] [PubMed] [Google Scholar]
- 28.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look‐Locker inversion recovery (MOLLI) for high‐resolution T1 mapping of the heart. Magn Reson Med. 2004; 52:141-146. [DOI] [PubMed] [Google Scholar]
- 29.Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St John‐Sutton M, Rietzschel ER. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010; 56:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Geest RJ, Buller VG, Jansen E, Lamb HJ, Baur LH, van der Wall EE, de Roos A, Reiber JH. Comparison between manual and semiautomated analysis of left ventricular volume parameters from short‐axis MR images. J Comput Assist Tomogr. 1997; 21:756-765. [DOI] [PubMed] [Google Scholar]
- 31.Oppo K, Leen E, Angerson WJ, Cooke TG, McArdle CS. Doppler perfusion index: an interobserver and intraobserver reproducibility study. Radiology. 1998; 208:453-457. [DOI] [PubMed] [Google Scholar]
- 32.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996; 275:1557-1562. [PubMed] [Google Scholar]
- 33.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlof B. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004; 292:2343-2349. [DOI] [PubMed] [Google Scholar]
- 34.Levick SP, Murray DB, Janicki JS, Brower GL. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension. 2010; 55:270-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linz D, Schuetze J, Linz B, Hohl M, Mahfoud F, Ewen S, Boehm M. Renal denervation attenuates progression of kidney and heart injury in obese spontaneously hypertensive rats. Eur Heart J. 2014; 35:702 [Google Scholar]
- 36.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin‐angiotensin‐aldosterone system. Circulation. 1991; 83:1849-1865. [DOI] [PubMed] [Google Scholar]
- 37.Brilla CG, Funck RC, Rupp H. Lisinopril‐mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000; 102:1388-1393. [DOI] [PubMed] [Google Scholar]
- 38.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan‐dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002; 105:2512-2517. [DOI] [PubMed] [Google Scholar]
- 39.Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005; 112:2940-2945. [DOI] [PubMed] [Google Scholar]
- 40.Coelho‐Filho OR, Shah RV, Neilan TG, Mitchell R, Moreno H, Jr, Kwong R, Jerosch‐Herold M. Cardiac magnetic resonance assessment of interstitial myocardial fibrosis and cardiomyocyte hypertrophy in hypertensive mice treated with spironolactone. J Am Heart Assoc. 2014; 3:e00079010.1161/JAHA.114.000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciulla MM, Paliotti R, Esposito A, Diez J, Lopez B, Dahlof B, Nicholls MG, Smith RD, Gilles L, Magrini F, Zanchetti A. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation. 2004; 110:552-557. [DOI] [PubMed] [Google Scholar]
- 42.Wong TC, Piehler K, Kellman P, Schelbert E. Extracellular matrix expansion is more strongly associated with cardiovascular outcomes than left ventricular mass. J Am Coll Cardiol. 2014; 63:A986 [Google Scholar]


