Abstract
Background
Premature cardiovascular disease limits the duration and quality of life on long‐term hemodialysis. The objective of this study was to define the frequency of fatal and nonfatal cardiovascular events attributable to atherosclerotic and nonatherosclerotic mechanisms, risk factors for these events, and the effects of cinacalcet, using adjudicated data collected during the EValuation of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) Trial.
Methods and Results
EVOLVE was a randomized, double‐blind, placebo‐controlled clinical trial that randomized 3883 hemodialysis patients with moderate to severe secondary hyperparathyroidism to cinacalcet or matched placebo for up to 64 months. For this post hoc analysis, the outcome measure was fatal and nonfatal cardiovascular events reflecting atherosclerotic and nonatherosclerotic cardiovascular diseases. During the trial, 1518 patients experienced an adjudicated cardiovascular event, including 958 attributable to nonatherosclerotic disease. Of 1421 deaths during the trial, 768 (54%) were due to cardiovascular disease. Sudden death was the most frequent fatal cardiovascular event, accounting for 24.5% of overall mortality. Combining fatal and nonfatal cardiovascular events, randomization to cinacalcet reduced the rates of sudden death and heart failure. Patients randomized to cinacalcet experienced fewer nonatherosclerotic cardiovascular events (adjusted relative hazard 0.84, 95% CI 0.74 to 0.96), while the effect of cinacalcet on atherosclerotic events did not reach statistical significance.
Conclusions
Accepting the limitations of post hoc analysis, any benefits of cinacalcet on cardiovascular disease in the context of hemodialysis may result from attenuation of nonatherosclerotic processes.
Clinical Trials Registration
Unique identifier: NCT00345839. URL: ClinicalTrials.gov.
Keywords: atherosclerosis, cardiovascular diseases, heart failure, kidney, sudden death
Introduction
Patients with end‐stage kidney disease (ESKD) receiving hemodialysis are at high risk of death from cardiovascular diseases. In the United States, the mortality rate for patients receiving dialysis in 2010 was 193/1000 patient‐years, with 42% of deaths attributable to cardiovascular causes.1 Sudden death accounted for 27% of mortality and acute myocardial infarction for only 5%. A similar pattern of cardiovascular mortality has been observed in clinical trials that have recruited patients receiving hemodialysis.2–3 Traditional cardiovascular risk factors do not fully account for the increased burden of cardiovascular diseases in these patients, who are exposed to a wide range of physiological and metabolic stresses that may cause both myocardial and vascular injury.4 From a pathological perspective, the contribution of atherosclerosis to the increased burden of cardiovascular disease is unclear, and other processes such as myocardial fibrosis and arterial calcification are likely to be more important than in nonchronic kidney disease (CKD) populations.5 This is reflected by data from recent clinical trials exploring the benefits of lipid‐lowering interventions in the hemodialysis population. While statin‐based regimens reduce the risk of events attributable to atherosclerosis, the impact of lowering low‐density lipoprotein (LDL) cholesterol on cardiovascular events overall is small in diabetic and nondiabetic patients.3,6–7
Patients with progressive CKD develop disturbances in biochemical and endocrinological measures of mineral metabolism, abnormal bone histology, and extraskeletal calcification, collectively termed CKD mineral bone disorder.8 Elevated blood levels of calcium, phosphorus, and parathyroid hormone (PTH), which characterize this disorder, are associated with increased cardiovascular deaths in observational studies.9 The EValuation of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial was designed to establish whether cinacalcet (Sensipar/Mimpara), a calcimimetic that lowers PTH, calcium, and phosphorus, would reduce total mortality and nonfatal cardiovascular events in hemodialysis patients with moderate to severe secondary hyperparathyroidism.10 In the trial, 3883 patients from 22 countries with median baseline PTH concentration 693 pg/mL (10% to 90% range, 363 to 1694 pg/mL) were randomized to cinacalcet (dose titrated from 30 to 180 mg daily based on PTH values) or matched placebo for up to 64 months.11 The primary composite end point was time to death or first nonfatal myocardial infarction, hospitalization for unstable angina, heart failure, or peripheral vascular event. Cardiovascular mortality, stroke, clinical fracture, and parathyroidectomy were prespecified secondary end points. In an unadjusted analysis of the EVOLVE trial, cinacalcet did not significantly reduce the risk of death or nonfatal cardiovascular events (relative hazard 0.93, 95% CI 0.85 to 1.02). Adjusting for baseline characteristics yielded a 12% reduction in risk of death or nonfatal cardiovascular events (relative hazard 0.88, 95% CI 0.79 to 0.97) that was nominally significant (P=0.008). Rates of withdrawal from cinacalcet, often related to gastrointestinal symptoms, were high; this, along with crossover from placebo to commercially available cinacalcet, sharply reduced the trial's power to detect a statistically significant result.11 Another potential explanation for the lack of definitive efficacy was that the end point was a composite of both atherosclerotic and nonatherosclerotic events.
The aims of this post hoc analysis of the EVOLVE data set were to better characterize the epidemiology of atherosclerotic and nonatherosclerotic cardiovascular events, define associated risk factors, and assess the effects of cinacalcet on different categories of cardiovascular events. Categorization of cardiovascular events was based on the Study of Heart and Renal Protection (SHARP), in which the LDL cholesterol‐lowering combination therapy under investigation was considered more likely to reduce rates of atherosclerotic than nonatherosclerotic cardiovascular events, and the primary end point of the study was chosen accordingly.6 It was hypothesized that in EVOLVE, cinacalcet would exert a more potent effect in reducing events presumed to be of nonatherosclerotic etiology, including sudden death and heart failure, than on events presumed to be of atherosclerotic origin, such as myocardial infarction, hospitalization for unstable angina, ischemic stroke, and peripheral vascular disease.
Methods
Study Population and Design
In the EVOLVE trial, 3883 patients with secondary hyperparathyroidism receiving hemodialysis were randomized 1:1 to receive either cinacalcet or placebo in addition to conventional therapies for CKD–mineral bone disorder (ie, instructions for dietary phosphorus restriction, phosphate binders, and vitamin D sterols). The dose of the study drug was titrated once every 4 weeks during the first 20 weeks and every 8 weeks during the subsequent follow‐up period (from a starting dose of 30 mg to a maximum dose of 180 mg daily), depending on blood levels of PTH and calcium. Dosing of other medications was left to the discretion of the treating physicians. Details of the study design,10 characteristics of patients at baseline,12 and primary trial results11 have previously been published. The trial was led by an academic Executive Committee and sponsored by Amgen, Inc. Ethics committee approval was obtained at all participating sites; all patients gave informed consent.
Cardiovascular Events
An independent Clinical Events Committee adjudicated all primary and secondary end points.11 For purposes of our analyses, cardiovascular events were divided into those attributable to underlying atherosclerotic disease and those likely to be a consequence of nonatherosclerotic cardiac or vascular pathological processes. Atherosclerotic end points were defined as the first occurrence of myocardial infarction, nonhemorrhagic stroke, hospitalization for unstable angina, peripheral vascular event (including nontraumatic amputation), death associated with a cardiovascular procedure, or death due to aneurysm dissection or rupture. Nonatherosclerotic end points were defined as the first occurrence of heart failure, hemorrhagic stroke, sudden death, fatal pulmonary embolism, or death due to other or unknown cardiovascular cause. Patients could be dually classified with both an atherosclerotic and a nonatherosclerotic event.
Statistical Analysis
We used the Pearson χ2 test to compare the distribution of the component clinical events between cinacalcet and placebo groups for each subset of cardiovascular end points studied. The effect of cinacalcet was evaluated using the intention‐to‐treat principle. Gray's test was used to compare the cumulative incidence functions between treatment groups, accounting for competing events of death from other causes, loss to follow‐up, or consent withdrawal. The marginal Cox regression method of Wei et al13 was used for unadjusted and adjusted analyses of time to first atherosclerotic and nonatherosclerotic event, testing the difference between the relative hazard of the 2 event types. In a similar approach, we stratified Cox models based on the Prentice et al14 method for repeated events by event type to estimate and compare the relative hazard for repeated atherosclerotic and nonatherosclerotic events. Multivariable analyses adjusted for baseline characteristics, including age, sex, race (white, black, other), geographic region, history of diabetes mellitus, history of cardiovascular diseases, and other variables. Based on results from the primary analysis, a treatment assignment × age interaction term was also included, as the effect of cinacalcet on the primary composite end point, compared with placebo, varied by age.
In addition to the composite atherosclerotic and nonatherosclerotic end points, we examined individual components of each composite (eg, myocardial infarction, sudden death, and heart failure). All inference tests were performed without adjusting for multiple comparisons. As the effects of randomization to cinacalcet versus placebo on the primary composite end point in the primary analysis did not reach statistical significance on an unadjusted log rank test, all comparisons yielding P<0.05 were deemed nominally significant. All statistical analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
Results
Descriptive Findings
Cardiovascular disease was the most frequent cause of death in the EVOLVE population overall (Figure 1), with a distribution closely reflecting the distribution in registry data collected by the United States Renal Data System.1
Figure 1.
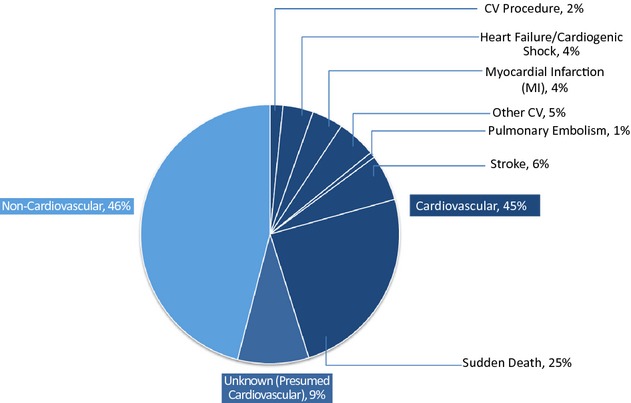
Adjudicated causes of death in the EVOLVE study population. CV indicates cardiovascular; EVOLVE, EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events.
Overall, 768 (54%) of 1421 deaths were adjudicated as being due to cardiovascular causes; of these, 127 (9%) were of unknown cause but were presumed to be cardiovascular. The most common mode of cardiovascular death was sudden death, accounting for 24.5% of all deaths and presumably reflecting cardiac arrhythmias. Of 348 sudden deaths, 45% were witnessed. Of the nonwitnessed deaths, 36% of patients had been last seen <24 hours earlier, and 19% were found dead >24 hours after last being seen. Only 56 (4%) deaths were attributable to acute myocardial infarction. Annualized event rates (95% CI) for cardiovascular mortality were 5.1% (95% CI 4.6% to 5.6%) in patients randomized to cinacalcet and 5.4% (95% CI 4.9% to 6.0%) in patients randomized to placebo.
Considering all adjudicated events (fatal and nonfatal) contributing to the end point of any cardiovascular event, an adjudicated cardiovascular event occurred in 746 patients randomized to cinacalcet and in 772 patients randomized to placebo; 377 and 391 fatal events, respectively, contributed to the cardiovascular mortality end point (Table 1).
Table 1.
Clinical End Points of Any Cardiovascular Event, Cardiovascular Death, and Composite Cardiovascular End Point by Clinical Event in Placebo (n=1935) and Cinacalcet Groups (n=1948)
| Event Type | Any Cardiovascular Event | Cardiovascular Death | ||
|---|---|---|---|---|
| Placebo | Cinacalcet | Placebo | Cinacalcet | |
| Myocardial infarction | 111 (14.4) | 129 (17.3) | 29 (7.4) | 27 (7.2) |
| Stroke | 79 (10.2) | 94 (12.6) | 34 (8.7) | 48 (12.7) |
| Heart failure | 203 (26.3) | 165 (22.1) | 34 (8.7) | 20 (5.3) |
| Hospitalization for unstable angina | 47 (6.1) | 31 (4.2) | — | — |
| Peripheral vascular event | 152 (19.7) | 150 (20.1) | — | — |
| Fatal pulmonary embolism | 2 (0.3) | 5 (0.7) | 4 (1.0) | 6 (1.6) |
| Sudden death | 115 (14.9) | 109 (14.6) | 182 (46.5) | 166 (44.0) |
| Death from cardiovascular procedure | 4 (0.5) | 6 (0.8) | 7 (1.8) | 15 (4.0) |
| Other fatal cardiovascular event | 15 (1.9) | 21 (2.8) | 33 (8.4) | 36 (9.5) |
| Unknown cardiovascular death cause | 44 (5.7) | 36 (4.8) | 68 (17.4) | 59 (15.6) |
| Total, n | 772 | 746 | 391 | 377 |
Values are n (%). There were no statistically significant differences in the distribution of the component clinical events between the group randomized to placebo and the group randomized to cinacalcet; χ2 P=0.159 and 0.155 for any cardiovascular event end point and cardiovascular death, respectively.
For fatal and nonfatal cardiovascular events of any type, distribution did not differ among patients randomized to cinacalcet versus placebo (Table 1). Likewise, distribution of atherosclerotic and nonatherosclerotic end point events, whether considering first event or all events, was similar (Table 2). Peripheral vascular events accounted for the largest fraction of first end point events attributed to atherosclerotic etiology (38.4% and 39.0% in patients randomized to cinacalcet and placebo, respectively). Heart failure events accounted for the largest fraction of first nonatherosclerotic end point events (44.3% and 47.5% in patients randomized to cinacalcet and placebo, respectively).
Table 2.
Clinical End Points of Atherosclerotic and Nonatherosclerotic Events in Placebo and Cinacalcet Groups
| Event Type | Atherosclerotic Event | Nonatherosclerotic Event | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Cinacalcet | Placebo | Cinacalcet | |||||
| First Event | All Events | First Event | All Events | First Event | All Events | First Event | All Events | |
| Myocardial infarction | 140 (32.0) | 248 (28.5) | 150 (35.1) | 256 (30.0) | — | — | ||
| Nonhemorrhagic stroke | 66 (15.1) | 83 (9.5) | 63 (14.8) | 86 (10.1) | — | — | ||
| Hemorrhagic stroke | — | — | 22 (4.4) | 27 (4.1) | 34 (7.4) | 41 (6.5) | ||
| Heart failure | — | – | 236 (47.5) | 343 (52.4) | 204 (44.3) | 327 (52.1) | ||
| Hospitalization for unstable angina | 54 (12.3) | 76 (8.7) | 39 (9.1) | 64 (7.5) | — | — | ||
| Peripheral vascular event | 171 (39.0) | 454 (52.1) | 164 (38.4) | 424 (49.7) | — | — | ||
| Fatal pulmonary embolism | — | — | 3 (0.6) | 4 (0.6) | 6 (1.3) | 6 (1.0) | ||
| Sudden death | — | — | 157 (31.6) | 182 (27.8) | 143 (31.0) | 166 (26.5) | ||
| Death from cardiovascular procedure | 6 (1.4) | 7 (0.8) | 6 (1.4) | 15 (1.8) | — | — | ||
| Other fatal cardiovascular event | 1 (0.2) | 3 (0.3) | 5 (1.2) | 8 (0.9) | 22 (4.4) | 30 (4.6) | 26 (5.6) | 28 (4.5) |
| Unknown cardiovascular death cause | — | — | 57 (11.5) | 68 (10.4) | 48 (10.4) | 59 (9.4) | ||
| Total, n | 438 | 871 | 427 | 853 | 497 | 654 | 461 | 627 |
Values are n (%). There were no statistically significant differences in the distribution of the component clinical events between the group randomized to placebo and the group randomized to cinacalcet. χ2 P=0.34 and 0.28 for first atherosclerotic and nonatherosclerotic events, respectively; 0.204 and 0.475 for all atherosclerotic and nonatherosclerotic events, respectively (χ2 test).
Cardiovascular Death and Nonfatal End Points: Intention‐to‐Treat Analysis
Table 3 shows the multivariable model analyzing the relative hazard of fatal and nonfatal cardiovascular events, based on time to event. In this analysis, randomization to cinacalcet resulted in a 10% (95% CI 0% to 19%) reduction in the cardiovascular event rate. Table 4 shows a corresponding model analyzing the relative hazard of cardiovascular death; randomization to cinacalcet resulted in a nominally statistically significant 16% (95% CI 2% to 28%) reduction in the cardiovascular death rate.
Table 3.
Multivariable Cox Regression Model on Time to the First of Any Cardiovascular Event Using Intent‐to‐Treat Analysis
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Treatment (cinacalcet/placebo) | 0.89 (0.80 to 0.99) | 0.029 |
| Age | 1.03 (1.02 to 1.03) | <0.001 |
| Sex (ref, female) | 1.18 (1.05 to 1.32) | 0.006 |
| Geographical region (ref, United States) | 0.002 | |
| Russia | 0.78 (0.60 to 1.03) | |
| Latin America | 0.80 (0.65 to 0.97) | |
| Europe | 0.76 (0.66 to 0.87) | |
| Canada | 0.74 (0.57 to 0.96) | |
| Australia | 0.97 (0.76 to 1.25) | |
| History of coronary artery disease | 1.41 (1.22 to 1.63) | <0.001 |
| History of cardiac arrhythmia | 1.28 (1.11 to 1.49) | 0.001 |
| History of diabetes | 1.77 (1.57 to 2.01) | <0.001 |
| History of heart failure | 1.14 (1.00 to 1.28) | 0.045 |
| History of peripheral vascular disease | 1.40 (1.23 to 1.60) | <0.001 |
| History of revascularization | 1.19 (1.03 to 1.38) | 0.022 |
| History of stroke | 1.24 (1.06 to 1.44) | 0.007 |
| History of transient ischemic attack | 1.16 (0.93 to 1.45) | 0.181 |
| Other cardiac disease history (valvular heart disease, angina) | 1.26 (1.11 to 1.42) | <0.001 |
| Tobacco use (ref, never) | <0.001 | |
| Current | 1.56 (1.34 to 1.81) | |
| Former | 1.13 (1.00 to 1.28) | |
| Type of vascular access (ref, natural fistula) | 0.165 | |
| Permanent catheter | 1.12 (0.96 to 1.32) | |
| Other | 1.17 (0.86 to 1.60) | |
| Graft | 1.15 (1.00 to 1.33) | |
| Baseline vitamin D use | 0.89 (0.79 to 0.99) | 0.032 |
| Baseline aspirin use | 1.02 (0.91 to 1.15) | 0.691 |
| Baseline amiodarone use | 0.89 (0.66 to 1.19) | 0.427 |
| Baseline proton pump inhibitor use | 1.10 (0.98 to 1.23) | 0.095 |
| Baseline warfarin use | 1.14 (0.94 to 1.38) | 0.200 |
| Systolic blood pressure per 10 mm Hg increase | 1.04 (1.02 to 1.07) | 0.000 |
| Baseline serum HDL per 10 mg/dL increase | 1.04 (1.00 to 1.08) | 0.033 |
| Baseline albumin, g/dL | 0.65 (0.56 to 0.76) | <0.001 |
| Dialysis duration, y | 1.01 (1.00 to 1.02) | 0.128 |
Variables were selected by backward elimination. The baseline variables corrected serum calcium, hemoglobin, serum phosphorus, and calcium phosphorus product were not included in the regression model due to lack of statistically significant independent association with the end point at α=0.25 in a separate model for each. Baseline use of aspirin, amiodarone, proton pump inhibitor, or warfarin was added to the final model. P=0.257 for interaction of age with treatment. Atrial fibrillation (chronic or paroxysmal) accounted for 67% of reported cardiac arrhythmias. HDL indicates high‐density lipoprotein cholesterol.
Table 4.
Multivariable Cox Regression on Time to Cardiovascular Death, Intention‐to‐Treat Analysis
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Treatment (cinacalcet/placebo) | 0.84 (0.72 to 0.97) | 0.019 |
| Age | 1.05 (1.04 to 1.05) | <0.001 |
| History of coronary artery disease | 1.33 (1.11 to 1.59) | 0.002 |
| History of cardiac arrhythmia | 1.23 (1.00 to 1.52) | 0.051 |
| History of diabetes | 1.86 (1.53 to 2.25) | <0.001 |
| History of heart failure | 1.22 (1.02 to 1.45) | 0.029 |
| History of peripheral vascular disease | 1.33 (1.11 to 1.60) | 0.002 |
| History of retinopathy | 1.15 (0.96 to 1.39) | 0.124 |
| History of stroke | 1.25 (1.01 to 1.55) | 0.044 |
| History of transient ischemic attack | 1.26 (0.93 to 1.71) | 0.135 |
| Tobacco use (ref, never) | 0.003 | |
| Current | 1.45 (1.17 to 1.81) | |
| Former | 1.06 (0.89 to 1.26) | |
| Type of vascular access (ref, natural fistula) | 0.071 | |
| Permanent catheter | 1.18 (0.94 to 1.49) | |
| Graft | 1.30 (1.05 to 1.59) | |
| Other | 1.23 (0.79 to 1.93) | |
| Baseline statin use | 0.85 (0.71 to 1.00) | 0.053 |
| Baseline aspirin use | 1.02 (0.86 to 1.20) | 0.858 |
| Baseline amiodarone use | 0.92 (0.62 to 1.39) | 0.706 |
| Baseline proton pump inhibitor use | 1.14 (0.97 to 1.35) | 0.118 |
| Baseline warfarin use | 0.95 (0.71 to 1.27) | 0.731 |
| Baseline serum phosphorus, mg/dL | 1.14 (1.08 to 1.21) | <0.001 |
| Baseline serum albumin, g/dL | 0.60 (0.48 to 0.74) | <0.001 |
| Baseline serum total cholesterol, mg/dL | 0.99 (0.99 to 1.00) | 0.014 |
| Baseline PTH per 100 pg/mL increase | 1.02 (1.01 to 1.03) | 0.005 |
| Baseline serum LDL per 10 mg/dL increase | 1.06 (1.00 to 1.12) | 0.057 |
Variables were selected by backward elimination. The baseline variables vitamin D binder use, hemoglobin level, sex, bone‐specific alkaline phosphatase, and high‐density lipoprotein cholesterol were not included initially due to lack of statistically significant independent association with the end point at α=0.25 in a separate model for each. Baseline use of aspirin, amiodarone, proton pump inhibitor, or warfarin was added to the final model. P=0.047 for interaction of treatment assignment with age. LDL indicates low‐density lipoprotein cholesterol; PTH, parathyroid hormone.
Atherosclerotic and Nonatherosclerotic Events
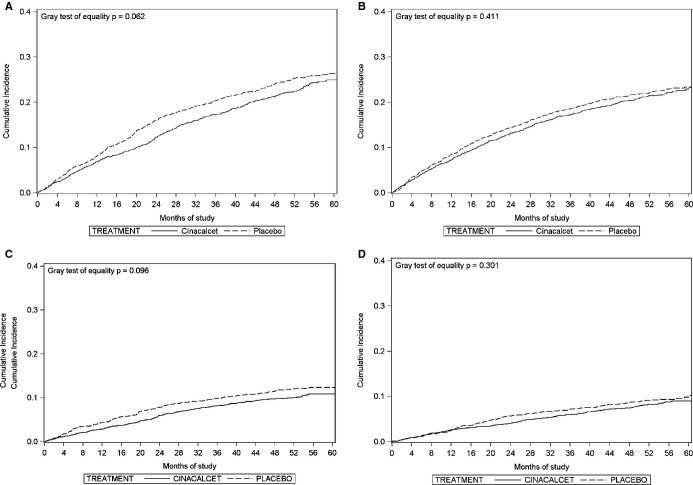
Figure 2A and 2B show unadjusted cumulative incidence function plots for nonatherosclerotic and atherosclerotic events accounting for mortality from other causes, loss to follow‐up and consent withdrawal as competing events. The cumulative incidence of nonatherosclerotic events (Figure 2A) was marginally lower in the cinacalcet than in the placebo group (P=0.062).
Figure 2.
Cumulative incidence function plots, intention‐to‐treat analysis, of (A) nonatherosclerotic events, (B) atherosclerotic events, (C) heart failure, and (D) sudden death.
Several correlates of time to first nonatherosclerotic events were noteworthy (Table 5), including geographic region (all regions had nominally significantly lower rates of nonatherosclerotic events relative to the United States), current smoking, and higher diastolic blood pressure at baseline. As hypothesized, there was a 16% (95% CI 4% to 26%) lower hazard of nonatherosclerotic events in patients randomized to cinacalcet. In contrast, while the hazard of atherosclerotic events was slightly lower in patients randomized to cinacalcet, the difference did not reach statistical significance (12%, 95% CI −1% to 24%) (Table 6). However, there was no significant treatment × categorized event (atherosclerotic versus nonatherosclerotic) type interaction, either for the time to first event or time to repeated events for atherosclerotic and nonatherosclerotic events (P=0.628 and 0.629 for first and repeated events, respectively). In contrast, in multivariable Cox regression models (intention‐to‐treat analysis), the relative hazards of heart failure (relative hazard 0.79, 95% CI 0.66 to 0.96), and sudden death (relative hazard 0.79, 95% CI 0.64 to 0.98) were reduced in patients randomized to cinacalcet. The number needed to treat for 1 year to prevent 1 sudden death was 145.
Table 5.
Multivariable Cox Regression Model on Time to First Nonatherosclerotic Event, Intention‐to‐Treat Analysis
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Treatment (cinacalcet/placebo) | 0.84 (0.73 to 0.96) | 0.009 |
| Age | 1.03 (1.03 to 1.04) | <0.001 |
| Geographical region (ref, United States) | <0.001 | |
| Russia | 0.57 (0.40 to 0.82) | |
| Latin America | 0.76 (0.59 to 0.97) | |
| Europe | 0.49 (0.39 to 0.61) | |
| Canada | 0.67 (0.48 to 0.93) | |
| Australia | 0.69 (0.49 to 0.99) | |
| Race (ref, white) | 0.090 | |
| Black | 0.86 (0.71 to 1.05) | |
| Other | 0.81 (0.66 to 0.99) | |
| History of coronary artery disease | 1.28 (1.08 to 1.51) | 0.004 |
| History of cardiac arrhythmia | 1.18 (0.97 to 1.43) | 0.100 |
| History of diabetes | 1.54 (1.32 to 1.80) | <0.001 |
| History of dyslipidemia | 0.87 (0.75 to 1.02) | 0.079 |
| History of heart failure | 1.35 (1.16 to 1.57) | 0.000 |
| Other cardiac disease history (valvular heart disease, angina) | 1.35 (1.15 to 1.58) | 0.000 |
| History of peripheral vascular disease | 1.21 (1.03 to 1.44) | 0.025 |
| Tobacco use (ref, never) | <0.001 | |
| Current | 1.66 (1.38 to 2.00) | |
| Former | 1.14 (0.98 to 1.33) | |
| Type of vascular access (ref, natural fistula) | 0.090 | |
| Permanent catheter | 0.99 (0.80 to 1.22) | |
| Other | 1.13 (0.73 to 1.74) | |
| Graft | 1.25 (1.04 to 1.49) | |
| Baseline aspirin use | 0.98 (0.85 to 1.14) | 0.809 |
| Baseline amiodarone use | 1.10 (0.75 to 1.62) | 0.617 |
| Baseline proton pump inhibitor use | 1.08 (0.94 to 1.25) | 0.285 |
| Baseline warfarin use | 0.97 (0.75 to 1.25) | 0.794 |
| Baseline serum cholesterol per 10 mg/dL increase | 0.98 (0.97 to 1.00) | 0.058 |
| Baseline serum HDL per 10 mg/dL increase | 1.04 (0.99 to 1.08) | 0.112 |
| Baseline serum albumin, g/dL | 0.68 (0.56 to 0.82) | <0.001 |
| Diastolic blood pressure per 10 mm Hg increase | 1.09 (1.04 to 1.15) | 0.001 |
Variables were selected by backward elimination. The baseline variables sex, hemoglobin, corrected serum calcium, serum phosphorus, and calcium phosphorus product were not included in the regression model due to lack of statistically significant independent association with the end point at α=0.25 in a separate model for each. Baseline use of aspirin, amiodarone, proton pump inhibitor, or warfarin was added to the final model. P=0.359 for interaction of treatment with age. HDL indicates high‐density lipoprotein.
Table 6.
Multivariable Cox Regression Model on Time to First Atherosclerotic Event Using Intention‐to‐Treat Analysis
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Treatment (cinacalcet/placebo) | 0.88 (0.76 to 1.01) | 0.066 |
| Age | 1.03 (1.02 to 1.03) | <0.001 |
| Sex (ref, female) | 1.17 (1.01 to 1.36) | 0.039 |
| History of transient ischemic attack | 1.43 (1.10 to 1.87) | 0.008 |
| History of coronary artery disease | 1.58 (1.30 to 1.91) | <0.001 |
| History of cardiac arrhythmia | 1.36 (1.12 to 1.67) | 0.002 |
| History of diabetes | 1.76 (1.47 to 2.10) | <0.001 |
| History of bone fracture | 1.11 (0.94 to 1.31) | 0.224 |
| History of peripheral vascular disease | 1.69 (1.43 to 2.00) | <0.001 |
| History of retinopathy | 1.27 (1.07 to 1.51) | 0.007 |
| History of revascularization | 1.37 (1.12 to 1.67) | 0.002 |
| Tobacco use (ref, never) | <0.001 | |
| Current | 1.50 (1.23 to 1.82) | |
| Former | 1.14 (0.97 to 1.34) | |
| Baseline aspirin use | 1.05 (0.90 to 1.23) | 0.543 |
| Baseline amiodarone use | 0.79 (0.53 to 1.19) | 0.260 |
| Baseline proton pump inhibitor use | 1.08 (0.93 to 1.25) | 0.333 |
| Baseline warfarin use | 1.03 (0.78 to 1.34) | 0.854 |
| Bone‐specific alkaline phosphatase, ng/L | 0.99 (0.98 to 1.01) | 0.222 |
| N‐telopeptide (log‐transformed) | 1.09 (0.98 to 1.21) | 0.131 |
| Baseline serum albumin, g/dL | 0.66 (0.54 to 0.82) | <0.001 |
| Systolic blood pressure per 10 mm Hg increase | 1.06 (1.02 to 1.09) | 0.001 |
Variables were selected by backward elimination. The baseline variables calcium‐containing phosphate binder use, corrected serum calcium, cholesterol, serum phosphorus, calcium phosphorus product, high‐density lipoprotein and low‐density lipoprotein cholesterol were not included in the regression model due to lack of statistically significant independent association with the end point at α=0.25 in a separate model for each. Baseline use of aspirin, amiodarone, proton pump inhibitor, or warfarin was added to the final model. P=0.11 for interaction of treatment with age. P value for difference between treatment assignment effect on atherosclerotic and nonatherosclerotic end points=0.622.
Discussion
In this post hoc analysis of the EVOLVE trial data set, adjudicated fatal and nonfatal cardiovascular events were examined in detail according to whether the likely underlying pathological process was presumed to be atherosclerotic or nonatherosclerotic. These results are inconclusive but suggest a greater relative benefit of cinacalcet on nonatherosclerotic cardiovascular events, including sudden death and heart failure, than on atherosclerotic events. We can speculate that the potential cardiovascular benefit of cinacalcet in the context of hemodialysis may be mediated via nonatherosclerotic disease mechanisms, for example, slowing of arterial calcification or reducing myocardial calcium accumulation. Our data suggest that treatment with cinacalcet may reduce the risk of cardiovascular death. As shown in Table 4, in a Cox regression model on time to cardiovascular death (by intention‐to‐treat analysis), there was a 16% reduction in the hazard of cardiovascular death for cinacalcet versus placebo (P=0.023).
The availability of the EVOLVE data set provided the opportunity to study cardiovascular epidemiology in a well‐defined study population of patients receiving hemodialysis, randomized in an international controlled trial with adjudicated end points. The results of this analysis deliver a clear message regarding cardiovascular epidemiology in patients with moderate to severe secondary hyperthyroidism receiving hemodialysis, and resolve any lingering doubts regarding the pre‐eminence of sudden death, which is unequivocally the single most frequent cause of death in this patient population. We were able to compare the EVOLVE data with United States Renal Data System registry data with cause of death obtained from the Death Notification (Centers for Medicaid & Medicare form CMS‐2746). The distribution of fatal cardiovascular events was similar in the EVOLVE population and the United States Renal Data System registry cohort, although the EVOLVE population included patients who were younger, had longer dialysis duration, and in general had more severe secondary hyperparathyroidism. More importantly, the adjudicated EVOLVE data independently validated the enormous contribution of sudden cardiac death to mortality in patients with ESKD worldwide.
This pattern of cardiovascular mortality observed in EVOLVE contrasts with that seen in nonhemodialysis populations such as those represented in the cholesterol trialists collaboration meta‐analysis of high‐risk patients recruited into randomized controlled trials of statin therapy.15 Among 129 536 participants in 21 such trials, most of whom did not have CKD, 26% of 15 969 recorded deaths were attributable to coronary artery disease, and only 8.7% were sudden in nature (Colin Baigent, BM, MSc, personal communication, unpublished data, 2013). The discrepancy among the lipid‐lowering trials and EVOLVE may reflect the fact that patients recruited to statin trials have atherosclerosis and are at high risk of cardiovascular events that complicate this disease. Cardiovascular events in patients receiving hemodialysis may be largely driven by nonatherosclerotic cardiovascular disease, perhaps resulting from long‐term exposure to hypertension, fluid overload, and disorders of mineral and endocrine metabolism associated with CKD.5
The SHARP trial examined the impact of LDL cholesterol‐lowering therapy in a population with advanced CKD, including over 2500 patients receiving hemodialysis. As in EVOLVE, clinical end points were adjudicated during the SHARP study, with the primary end point a composite of fatal and nonfatal cardiovascular events considered by the Steering Committee to be attributable to atherosclerotic cardiovascular disease. There was a 17% reduction in such events among patients allocated to the active intervention (simvastatin 20 mg plus ezetimibe 10 mg daily) compared with placebo, but no impact on other cardiovascular disease events, either fatal or nonfatal. Among the 2509 SHARP patients receiving hemodialysis at the time of randomization to study treatment, the benefits of the LDL cholesterol‐lowering regimen appeared to be attenuated, although this might be attributable to reduced adherence with study medication, reflected in a lesser absolute reduction in blood LDL‐cholesterol concentrations, limited statistical power in the ESKD subgroup, or a smaller or absent treatment effect. A recent meta‐analysis of lipid‐lowering trials in dialysis‐requiring and nondialysis‐requiring CKD patients, including data from SHARP, concluded that the effects of statin therapy are attenuated in patients with more advanced CKD stages.16 It can be inferred from these results that mechanisms other than atherosclerosis become more important drivers of cardiovascular events in advanced CKD and ESKD and that alternative treatment regimens should be explored in these patients. In this analysis of the cardiovascular events in the EVOLVE study, the 16% reduction in nonatherosclerotic events among patients randomized to cinacalcet is congruent with the statistically significant 17% reduction in atherosclerotic events attributable to the LDL cholesterol‐lowering regimen in the SHARP study.
Observational data have highlighted important associations among abnormalities of bone and mineral metabolism and adverse outcomes. Several studies in patients with ESKD have linked very low and very high PTH concentrations with mortality, sudden death, and cardiovascular events17–18; experimental studies have identified plausible biological mechanisms linking CKD‐associated mineral and endocrine abnormalities to both arterial19 and left ventricular disease.20 Further evidence for the role of secondary hyperparathyroidism in the pathogenesis of CKD‐related cardiovascular disease can be derived from clinical and experimental studies. Both primary and secondary hyperparathyroidism are associated with left ventricular hypertrophy,21–22 and in the context of hemodialysis, parathyroidectomy improves left ventricular function.23
Cardiovascular benefits of cinacalcet could also result from a reduction in calcification of the cardiovascular system. A Randomized Study to Evaluate the Effects of Cinacalcet plus Low‐Dose Vitamin D on Vascular Calcification in Subjects with Chronic Kidney Disease Receiving Hemodialysis (ADVANCE) was designed to test this hypothesis in a clinical setting. Inclusion and exclusion criteria and the dose titration schedule were similar to EVOLVE; 360 hemodialysis patients were randomized to cinacalcet or placebo with baseline and follow‐up computed tomography scans to assess coronary artery and aortic calcification. The ADVANCE trial results did not reach conventional levels of statistical significance for the primary outcome (change in Agatston coronary artery calcification score), but the change in volume score (a secondary outcome) was nominally significant and the results are consistent with a modest effect of cinacalcet on slowing progression of cardiovascular calcification.24 The physiological consequences of arterial calcification in the context of CKD may include arterial stiffening,25 which may in turn increase left ventricular afterload and hypertrophy and failure. Thus, a reduction in arterial calcification and stiffening in response to cinacalcet therapy might plausibly reduce cardiovascular events associated with left ventricular disease, such as heart failure and sudden death. Another potential benefit of cinacalcet might be a reduction in serum levels of fibroblast growth factor‐23, a phosphatonin that has been implicated in the development of left ventricular hypertrophy in the context of CKD.20 A modest reduction in fibroblast growth factor‐23 has been suggested in a recent open‐label single‐arm trial of cinacalcet involving 55 participants receiving hemodialysis,26 along with the expected reduction in blood PTH.
As shown in EVOLVE, sudden death is an important factor limiting the lifespan of hemodialysis patients. Risk factors for sudden death included higher baseline serum phosphorus and lower serum albumin concentrations. Further studies are required to define risk factors for sudden cardiac death and its relationship to the dialysis cycle and to better target methods to detect at‐risk patients and therapies (such as defibrillators) that might affect this end point.27–28
In determining the effects of cinacalcet on atherosclerotic and nonatherosclerotic events, strengths of these analyses include the randomized design; a large sample size; diversity in age, sex, race/ethnicity, and clinical features of ESKD; the breadth of comorbidity and laboratory data available at study entry; and the availability of carefully adjudicated end points. There are several important limitations. While several determinants of atherosclerotic and nonatherosclerotic events unrelated to treatment were identified, we did not include time‐varying covariates (eg, blood pressure, laboratory study results), so misclassification might have occurred, biasing the relations among these variables and cardiovascular events toward the null. Importantly, our estimate of the cinacalcet treatment effect is strongly influenced by adherence and crossover, and the confounding effects of parathyroidectomy. These factors in aggregate are likely to sharply attenuate the observed treatment effect. Perhaps most importantly, while the distinction of atherosclerotic and nonatherosclerotic is contextually of interest, the 2 obviously overlap. For example, some peripheral vascular events, including nontraumatic amputation, result primarily from arteriosclerosis of smaller caliber vessels, not atherosclerosis of larger caliber vessels. Although peripheral arterial disease is conventionally considered to be predominantly attributable to atherosclerotic mechanisms (eg, as illustrated by the SHARP trial design), we would suggest that in patients with ESKD, much of peripheral vascular disease could be related to severe obliterative arteriosclerosis with medial vascular calcification, as seen with calcific uremic arteriolopathy (calciphylaxis). Conversely, while many cases of heart failure result from impaired ventricular relaxation related to hypertension and vascular stiffness, some may reflect an anginal equivalent, without diagnosed unstable angina or myocardial infarction (or even chest pain). With respect to myocardial infarction, we did not distinguish between type 1 (eg, plaque rupture) and type 2 (eg, demand ischemia).
In summary, we highlight the contribution of presumed nonatherosclerotic mechanisms to cardiovascular disease in the hemodialysis population and the importance of sudden death as a preventable cause of premature mortality. Although the EVOLVE trial did not show an unequivocal benefit of cinacalcet on the primary composite end point, the analyses presented herein suggest the possibility of a modest (but clinically meaningful) benefit in cardiovascular end points, particularly cardiovascular death, sudden death, and heart failure. Recognizing the exceptionally high risk of death and cardiovascular disease in ESKD, the complex role of secondary hyperparathyroidism in dystrophic calcification, and the capacity of cinacalcet to effectively control parathyroid hormone levels, results from EVOLVE can (and should) inform clinical practice.
Supplementary Material
Appendix List of participating EVOLVE sites and affiliated investigators, with enrollment by country (# enrolled).
Sources of Funding
This study was supported by Amgen, Inc, Thousand Oaks, CA.
Disclosures
As EVOLVE executive committee members, Drs Wheeler, London, Parfrey, Block, Correa‐Rotter, Drüeke, Floege, Moe, Chertow, and Herzog have received consulting fees from Amgen. Drs Dehmel and Goodman and Ms Kubo and Ms Trotman are employed by and own stock options in Amgen. In addition, Dr Wheeler has received travel support, lecture fees, or grant support from Amgen, Merck, Genzyme, Abbott, Fresenius Medical Care, Otsuka, and Shire. Dr London has received consulting or lecture fees from Amgen, Genzyme, Shire, and Sandoz. Dr Parfrey has received lecture fees from Amgen. Dr Block has received consulting fees, travel support, lecture fees, or grant support from Amgen, KAI Pharmaceuticals, and Genzyme, and payment for development of educational presentations from Genzyme; he is employed by DaVita as a facility medical director. Dr Correa‐Rotter has received grant support, consulting fees, or lecture fees from Amgen, Fresenius Medical Care, AbbVie, Reata, Pfizer, Genzyme, Roche, and Abbott. Dr Drüeke has received consultation fees, travel support, grant support, or lecture fees from Amgen, Hoffman‐LaRoche, Abbott, Fresenius Medical Care, Genzyme, KAI Pharmaceuticals, Baxter, Theracion, Chugai Pharmaceutical, Kirin Brewery (Pharmaceutical Branch), Shire, and Sanofi. Dr Floege has received consulting fees, travel support, or lecture fees from Amgen, Abbott, Fresenius Medical Care, Genzyme, Chugai Pharmaceuticals, and Boehringer Ingelheim, and royalties from Elsevier. Dr Mahaffey's financial disclosures prior to August 1, 2013, can be viewed at https://www.dcri.org/about-us/conflict-of-interest/Mahaffey-COI_2011-2013.pdf; disclosures after August 1, 2013, can be viewed at http://med.stanford.edu/profiles/kenneth_mahaffey. Dr Moe has received consulting fees, travel support, or grant support from Amgen, KAI Pharmaceuticals, Shire, Genzyme, Novartis, and Vifor Pharma; is a board member at Litholink; has provided expert testimony regarding phosphate binders; has a patent on use of calcimimetics for treatment of polycystic kidney disease; has received royalties from Up to Date; and owns stock options in Eli Lilly. Dr Abdalla has received grant and travel support from Amgen. Dr Chertow has received grant support and travel support from Amgen; is a board member of Satellite Healthcare, Inc; has provided consultation to Reata Pharmaceuticals; and owns stock options in Allocure, Ardelyx, Home Dialysis Plus, PuraCath, and Thrasos. Dr Herzog has received travel support, lecture fees, and grant support from Amgen; consulting fees, grant support, or lecture fees from Abbott/AbbVie, Affymax, ClearView Healthcare Partners, GlaxoSmithKline, Fibrogen, Keryx, Medtronic, Gilead, Johnson & Johnson, Zoll, and Greenfield Health System; royalties from UpToDate; and he owns stock in Boston Scientific, Cambridge Heart, Johnson & Johnson, and Merck.
Acknowledgments
The authors thank Delaney Berrini, BS, and Nan Booth, MSW, MPH, ELS, of the Minneapolis Medical Research Foundation for manuscript preparation and editing, respectively.
References
- 1.U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States. 2012Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases [Google Scholar]
- 2.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO study. Kidney Int. 2004; 65:2380-2389. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005; 353:238-248. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, Sarnak MJ. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007; 50:217-224. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler DC, Haynes R, Landray MJ, Baigent C. In: Taal MW, Chertow GM, Marsden P, Skorecki K, Yu ASL, Brenner BM. (eds.). Cardiovascular aspects of kidney disease. Brenner and Rector's The Kidney. 20129th edPhiladelphia, PA: Elsevier Saunders; 2059-2080. [Google Scholar]
- 6.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt‐Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen‐Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011; 377:2181-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen‐Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009; 360:1395-1407. [DOI] [PubMed] [Google Scholar]
- 8.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006; 69:1945-1953. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004; 15:2208-2218. [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Pupim LB, Block GA, Correa‐Rotter R, Drueke TB, Floege J, Goodman WG, London GM, Mahaffey KW, Moe SM, Wheeler DC, Albizem M, Olson K, Klassen P, Parfrey P. Evaluation of cinacalcet therapy to lower cardiovascular events (EVOLVE): rationale and design overview. Clin J Am Soc Nephrol. 2007; 2:898-905. [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Block GA, Correa‐Rotter R, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012; 367:2482-2494. [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Correa‐Rotter R, Block GA, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Wheeler DC, Parfrey PS. Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant. 2012; 27:2872-2879. [DOI] [PubMed] [Google Scholar]
- 13.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989; 84:1065-1073. [Google Scholar]
- 14.Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika. 1981; 68:373-379. [Google Scholar]
- 15.Cholesterol Treatment Trialists' (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010; 376:1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upadhyay A, Earley A, Lamont JL, Haynes S, Wanner C, Balk EM. Lipid‐lowering therapy in persons with chronic kidney disease: a systematic review and meta‐analysis. Ann Intern Med. 2012; 157:251-262. [DOI] [PubMed] [Google Scholar]
- 17.Natoli JL, Boer R, Nathanson BH, Miller RM, Chiroli S, Goodman WG, Belozeroff V. Is there an association between elevated or low serum levels of phosphorus, parathyroid hormone, and calcium and mortality in patients with end stage renal disease? A meta‐analysis. BMC Nephrol. 2013; 14:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008; 52:519-530. [DOI] [PubMed] [Google Scholar]
- 19.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011; 109:697-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon‐Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John SM, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro O, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011; 121:4393-4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duprez D, Bauwens F, De Buyzere M, De Backer T, Kaufman JM, Van Hoecke J, Vermeulen A, Clement DL. Relationship between parathyroid hormone and left ventricular mass in moderate essential hypertension. J Hypertens Suppl. 1991; 9:S116-S117. [PubMed] [Google Scholar]
- 22.Harnett JD, Parfrey PS, Griffiths SM, Gault MH, Barre P, Guttmann RD. Left ventricular hypertrophy in end‐stage renal disease. Nephron. 1988; 48:107-115. [DOI] [PubMed] [Google Scholar]
- 23.Drueke T, Fauchet M, Fleury J, Lesourd P, Toure Y, Le Pailleur C, de Vernejoul P, Crosnier J. Effect of parathyroidectomy on left‐ventricular function in hemodialysis patients. Lancet. 1980; 1:112-114. [DOI] [PubMed] [Google Scholar]
- 24.Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, Moustafa M, Goodman WG, Lopez N, Downey G, Dehmel B, Floege J. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low‐dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011; 26:1327-1339. [DOI] [PubMed] [Google Scholar]
- 25.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant. 2008; 23:586-593. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi M, Komaba H, Nakanishi S, Fujimori A, Fukagawa M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol Dial Transplant. 2012; 27:784-790. [DOI] [PubMed] [Google Scholar]
- 27.Charytan DM, Patrick AR, Liu J, Setoguchi S, Herzog CA, Brookhart MA, Winkelmayer WC. Trends in the use and outcomes of implantable cardioverter‐defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011; 58:409-417. [DOI] [PubMed] [Google Scholar]
- 28.Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005; 68:818-825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix List of participating EVOLVE sites and affiliated investigators, with enrollment by country (# enrolled).