Abstract
Background
It is unclear whether the incidence of non‐valvular atrial fibrillation (NVAF) has been increasing over time. We aimed to provide contemporary estimates of the incidence and case‐fatality of NVAF in a well‐defined population.
Methods and Results
We used the computerized databases of the Régie de l'assurance maladie du Québec (RAMQ), responsible for administering the universal health care services for all its residents, to identify a population‐based cohort of 243 800 patients with an incident diagnosis of NVAF during 2000–2009. The incidence rate of NVAF, age‐ and sex‐standardized to the 2004 population of Québec, was 32.4 (95% confidence interval, 32.3 to 32.5) per 10 000 per year. There was no evidence of an increasing incidence of NVAF during the 10‐year study period. The incidence rate was higher in men compared with women (age‐adjusted incidence rate ratio 1.51; 95% CI 1.50 to 1.52). The 30‐day case‐fatality was 9.2% (95% CI 9.0 to 9.3), higher for men (10.0%; 95% CI 9.8 to 10.1) than women (8.5; 95% CI 8.3 to 8.6), and increasing with age, ranging from around 1% for cases aged <40 years to around 16% for cases aged ≥80.
Conclusion
Current incidence estimates illustrate the significant burden of NVAF. Mortality remains particularly high around the time of diagnosis, and case‐fatality increases with age, being systematically higher in men than women.
Keywords: arrhythmia, case‐fatality, epidemiology, fibrillation, mortality, sex difference
Introduction
Atrial fibrillation (AF) is the most common cardiac dysrhythmia,1–2 with an estimated prevalence from <0.5% in young adults to >8% after the age of 80.3 Several population‐based studies in developed countries have suggested an increasing incidence and prevalence of AF.1,4–5 Owing to the sustained prolongation of life expectancy and aging of the population, the proportion of patients with AF is expected to more than double in the next decades.1 These projections have been reported in several North American and European studies.1,4,6–8
AF is associated with significant morbidity and mortality.9–10 In particular, AF is associated with a 3‐ to 5‐fold increased risk of ischemic stroke, and the attributable risk of stroke associated with AF increases from 1.5% in subjects aged 50 to 59 years of age to 23.5% among those aged 80 to 89 years.3 The prevention of arterial thromboembolic events is therefore crucial in the management of AF and relies on the use of oral anticoagulants such as vitamin K antagonists. Recently, novel oral anticoagulants have entered the market and they are currently recommended for non‐valvular AF (NVAF) only,11–13 the most common form of AF in developed countries. In this context of increasing societal and economic burden of AF, and changing preventive therapeutic options for thromboembolic risk in NVAF, recent population‐based measures are necessary for effective prevention strategies and planning of health care resources. However, very few epidemiologic studies focused specifically on NVAF, included hospital‐diagnosed patients only, or were limited to a particular age or non‐treated subgroup. Therefore, we aimed to provide contemporary estimates of the incidence and mortality rates of NVAF in a well‐defined population‐based cohort of patients with incident NVAF in the province of Québec, Canada.
Methods
Source of Data
This study was conducted using computerized heath care databases of the Régie de l'assurance maladie du Québec (RAMQ), the Maintenance et exploitation des données pour l'étude de la clientèle hospitalière (MED‐ÉCHO) and the Institut de la statistique du Québec (ISQ). The RAMQ is responsible for administering the universal health care services for all its residents currently living in the province of Québec. Health care coverage is mandatory for Québec residents. Visitors, non‐Canadian students, and individuals residing outside of Québec for >183 days in the year are not eligible for coverage. In 2004, the calendar year used to estimate standardized rates in our study, 7 396 325 Québec residents were eligible for health coverage, 49% were men, 23% were 19 years old or younger, 58% were 20 to 59, and 19% were 60 or older.
The RAMQ has three computerized databases. The demographic database contains the age, sex, and postal code of all individuals registered. The medical services database contains information on the medical services program, including nature of the service rendered, specialty of treating and referring physician, date and location, as well as the diagnostic code of the service (International Classification of Diseases, 9th Revision, Clinical Modification [ICD‐9‐CM] or enhanced version of ICD‐10 for Canada ICD‐10‐CA). This program is universal for all Québec residents and is a fee‐for‐service. The prescription database, which was not used for this study, contains information on outpatient prescription medications. Since 1996, this program covers all Québec residents aged 65 years or older, welfare recipients, and individuals who do not have private medical insurance.
MED‐ÉCHO has maintained the hospital inpatient database since 1980. The database contains data pertaining to all Québec hospitalisations including date and type of admission and discharge, type of establishment, primary and secondary diagnoses, in addition to procedure codes (with corresponding procedure dates). Prior to 2006, diagnoses were classified according to the ICD‐9‐CM and procedures were coded according to the Canadian Classification of Diagnostic, Therapeutic, and Surgical Procedures (CCDTC). Since 2006, diagnoses and procedures are coded according to ICD‐10‐CA and the Canadian Classification of Health Interventions (CCI), respectively. Finally, the cause of death database is administered by the Institut de la statistique du Québec (ISQ) and contains vital statistics such as the date of death, the medical code corresponding to the underlying cause of death, as well as the establishment where the death took place (if any).
Each of these databases contains the individual's numéro d'assurance‐maladie (Health Insurance Number). This unique number, acquired at birth or at the time of residency, remains unchanged throughout the life of the individual and is used for record linkage within the RAMQ databases and the MED‐ECHO. The general accuracy of linkage between the prescription database and the medical services database was found to be 98.2%. The 1.8% rate of unfeasible linkages arises primarily from name changes, and the quality of the data has been documented.14–15
The study protocol was approved by the Research Ethics Committee of the Jewish General Hospital, Montreal, Canada.
Cohort Definition
From the source population of all individuals in the RAMQ database, we first identified all patients with an inpatient or outpatient diagnosis for atrial fibrillation (ICD‐9: 427.3, 427.31, 427.32; ICD‐10: I48, I48.0, I48.1) between January 1, 2000 and December 31, 2009.
Cohort entry (time zero) for all patients was defined at the date of the first diagnosis of NVAF during the study period. If the diagnosis occurred during a hospitalization, cohort entry was set as the date of hospital discharge. To confirm the incident nature of the NVAF diagnosis, all subjects with any mention of AF in the 2 years prior to the cohort entry diagnosis were excluded. Patients with a history of valvular aortic or mitral heart disease, previous valvular repair or hyperthyroidism (either a treatment or a diagnosis) in the 2 years prior to cohort entry were also excluded.
All cohort members were followed until RAMQ deregistration, death, or the end of the study period (December 31, 2009), whichever occurred first. The occurrence of death was determined from RAMQ and ISQ. MED‐ÉCHO was used to identify in‐hospital deaths.
Data Analysis
The study cohort was characterized by tabulating cohort members by age, sex, comorbidities measured during the 2 years prior to cohort entry as well as the CHADS2 risk score (congestive heart failure, hypertension, age over 75 years, diabetes mellitus, prior stroke, or transient ischemic attack).16 Crude incidence rates of first NVAF episodes were calculated from the number of new cases of NVAF divided by the total person‐years at risk in the population of Québec eligible for RAMQ between 2000 and 2009. Incidence rates were age and sex standardized to the population of Québec eligible for RAMQ in 2004 (www.ramq.gouv.qc.ca). Crude and age‐adjusted incidence rate ratios (IRR), comparing rates among women and men, were estimated using Poisson regression. Thirty‐day case‐fatality was calculated using all‐cause deaths as the numerator and the number of patients with incident NVAF as the denominator, stratified by age and sex. Linear trends in the incidence of NVAF were assessed using Poisson regression with calendar year as a continuous variable.
Confidence intervals (CI) were calculated using a significance level of 5%. All statistical procedures were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
The source population included 309 556 subjects with at least one atrial fibrillation diagnosis code during the period 2000–2009. From these, we identified 243 800 patients satisfying cohort inclusion and exclusion criteria (Figure 1). Table 1 describes the baseline characteristics of the cohort. The mean age at NVAF diagnosis was 71.5 years, 63% of the patients were 70 years or older at diagnosis, 52% were males, and the mean follow‐up was 3.46 years. With respect to CHADS2 score at baseline, 20.2% had a score of 0, 27.5% had a score of 1, 28.1% had a score of 2, 15.4% had a score of 3, and 8.8% had a score of 4 or higher. The most prevalent comorbidities at cohort entry were hypertension (56%), hyperlipidemia (35%), diabetes (25%), and congestive heart failure (21%). Comparing characteristics of men and women, 24% of males were 80 years or older at the time of diagnosis compared with 41% of the women. The prevalence of cardiovascular comorbidities was higher in men than in women except for hypertension. The occurrence of a previous stroke or transient ischemic attack was similar in both groups. The age‐specific incidence rates of NVAF are shown in Table 2 and the corresponding age‐ and sex‐specific rates in Figure 2. The population‐based incidence of NVAF was 3.28 (95% CI, 3.27 to 3.29) per 1000 per year, increasing with age for men and women. The age‐ and sex‐adjusted (to the 2004 Québec population eligible to RAMQ) incidence rate was 3.24 (95% CI, 3.23 to 3.25) per 1000 per year. The incidence rate of NVAF was higher in men compared with women in virtually all age groups with a crude and age‐adjusted IRR of 1.13 (95% CI, 1.12 to 1.14) and 1.51 (1.50 to 1.52), respectively. There was no evidence of an increasing incidence of NVAF during the 10‐year study period (P=0.13).
Figure 1.
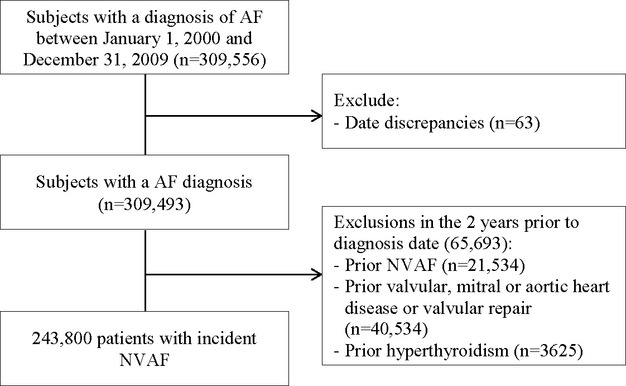
Details of incident non‐valvular atrial fibrillation cohort definition. AF indicates atrial fibrillation; NAVF, non‐valvular atrial fibrillation.
Table 1.
Baseline Characteristics of the Incident NVAF Cohort
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Cohort size | 243 800 | 126 833 | 52.02 | 116 967 | 47.98 | |
| Follow‐up (years), mean (SD) | 3.46 (2.86) | 3.48 (2.87) | 3.45 (2.85) | |||
| Age (y) | ||||||
| 0 to 19 | 1230 | 0.5 | 660 | 0.52 | 570 | 0.49 |
| 20 to 29 | 2487 | 1.02 | 1323 | 1.04 | 1164 | 1 |
| 30 to 39 | 4788 | 1.96 | 2840 | 2.24 | 1948 | 1.67 |
| 40 to 49 | 11 172 | 4.58 | 6914 | 5.45 | 4258 | 3.64 |
| 50 to 59 | 24 776 | 10.16 | 15 967 | 12.59 | 8809 | 7.53 |
| 60 to 69 | 45 985 | 18.86 | 28 669 | 22.60 | 17 316 | 14.8 |
| 70 to 79 | 73 973 | 30.34 | 39 586 | 31.21 | 34 387 | 29.4 |
| ≥80 | 79 389 | 32.56 | 30 874 | 24.34 | 48 515 | 41.48 |
| Comorbidities at diagnosis | ||||||
| Hypertension | 137 871 | 56.55 | 65 841 | 51.91 | 72 030 | 61.58 |
| Myocardial infarction | 49 690 | 20.38 | 30 345 | 23.93 | 19 345 | 16.54 |
| Angina pectoris | 45 301 | 18.58 | 26 191 | 20.65 | 19 110 | 16.34 |
| Congestive heart failure | 51 886 | 21.28 | 27 557 | 21.73 | 24 329 | 20.8 |
| Diabetes | 59 937 | 24.58 | 33 169 | 26.15 | 26 768 | 22.89 |
| Hyperlipidemia | 86 517 | 35.49 | 48 564 | 38.29 | 37 953 | 32.45 |
| Vascular disease | 28 321 | 11.62 | 17 505 | 13.80 | 10 816 | 9.25 |
| Prior stroke or TIA | 20 149 | 8.26 | 10 211 | 8.05 | 9938 | 8.5 |
| Prior hemorrhage | 2617 | 1.07 | 1409 | 1.11 | 1208 | 1.03 |
| Chronic renal failure | 26 670 | 10.94 | 15 495 | 12.22 | 11 175 | 9.55 |
| Cancer | 46 249 | 18.97 | 27 267 | 21.50 | 18 982 | 16.23 |
| CHADS2 score | ||||||
| 0 | 49 214 | 20.19 | 29 654 | 23.38 | 19 560 | 16.72 |
| 1 | 67 100 | 27.52 | 37 019 | 29.19 | 30 081 | 25.72 |
| 2 | 68 445 | 28.07 | 32 363 | 25.52 | 36 082 | 30.85 |
| 3 | 37 590 | 15.42 | 17 799 | 14.03 | 19 791 | 16.92 |
| 4 | 15 454 | 6.34 | 7114 | 5.61 | 8340 | 7.13 |
| 5 | 4780 | 1.96 | 2315 | 1.83 | 2465 | 2.11 |
| 6 | 1217 | 0.5 | 569 | 0.45 | 648 | 0.55 |
NAVF indicates non‐valvular atrial fibrillation; TIA, transient ischemic attack.
Table 2.
Incidence Rates of Non‐Valvular Atrial Fibrillation Per 1000 Person‐Years, by Age
| Age (y) | NVAF Events | Person‐Years | Incidence Rate Per 1000 Per Year | Incidence Rate Ratio (95% CI) |
|---|---|---|---|---|
| Overall | 243 800 | 74 297 764 | 3.28 (3.27 to 3.29) | |
| 0 to 19 | 1230 | 17 300 850 | 0.07 (0.07 to 0.08) | 1.00 (Reference) |
| 20 to 29 | 2487 | 9 391 349 | 0.26 (0.25 to 0.28) | 3.72 (3.48 to 3.99) |
| 30 to 39 | 4788 | 10 360 523 | 0.46 (0.45 to 0.48) | 6.50 (6.11 to 6.92) |
| 40 to 49 | 11 172 | 12 421 796 | 0.90 (0.88 to 0.92) | 12.65 (11.93 to 13.42) |
| 50 to 59 | 24 776 | 10 585 383 | 2.34 (2.31 to 2.37) | 32.92 (31.09 to 34.86) |
| 60 to 69 | 45 985 | 7 035 336 | 6.54 (6.48 to 6.60) | 91.93 (86.88 to 97.29) |
| 70 to 79 | 73 973 | 4 700 614 | 15.74 (15.62 to 15.85) | 221.35 (209.22 to 234.18) |
| ≥80 | 79 389 | 2 501 913 | 31.73 (31.51 to 31.95) | 446.32 (421.88 to 472.18) |
NAVF indicates non‐valvular atrial fibrillation.
Figure 2.
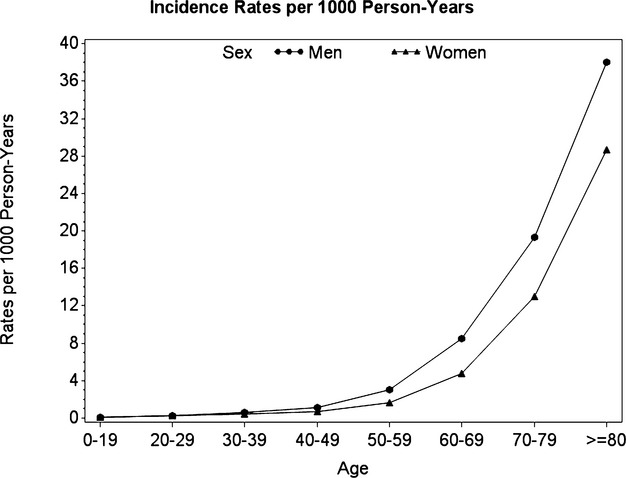
Incidence rates of non‐valvular atrial fibrillation per 1000 person‐years stratified by age and sex.
Among the cohort, 16 815 patients died at the time of the NVAF diagnosis (or during the entry NVAF hospitalisation) and overall, 22 325 died within 1 month of diagnosis. The most common causes of death in the 30 days following NVAF diagnostic were cardiac (28.5%), cancer (24%), pulmonary (9.5%), infections (7.3%), and stroke (6.4%). The corresponding 30‐day case‐fatality risk was 9.2% (95% CI 9.0 to 9.3), with 83% of these deaths occurring in the first week. Compared with women, men had a higher 30‐day case‐fatality (age‐standardized case‐fatality 10.0% (95% CI 9.8 to 10.1) and 8.46 (95% CI 8.30 to 8.61), respectively, P<0.0001). As expected, the 30‐day case‐fatality increased steadily with CHADS2 score, ranging from 3.04% (95% CI 2.89 to 3.19) for a score of 1 to 26.79% (95% CI 24.30 to 29.28) for a score of 6, and was much higher for patients diagnosed in hospital, whether it was a primary or a secondary diagnosis (Table 3). Also, case‐fatality varied greatly according to associated comorbidities at the time of diagnosis, from 22.24% (95% CI 21.69 to 22.80) in patients with recent acute coronary syndrome, to 5.85% (95% CI 5.69 to 6.02) in patients with hypertension without cardiac or chronic kidney disease (Table 4). Figure 3 describes the 30‐day case‐fatality stratified by age and sex, showing that the difference in case‐fatality was more pronounced in the elderly.
Table 3.
Crude Case‐Fatality in the 30 Days Following Atrial Fibrillation According to CHADS2 Score
| CHADS2 | People At Risk | Deaths | Case‐Fatality (%) | 95% CI | |
|---|---|---|---|---|---|
| Overall | 0 | 49 214 | 1496 | 3.04 | 2.89 to 3.19 |
| 1 | 67 100 | 4413 | 6.58 | 6.39 to 6.76 | |
| 2 | 68 445 | 6755 | 9.87 | 9.65 to 10.09 | |
| 3 | 37 590 | 5671 | 15.09 | 14.72 to 15.45 | |
| 4 | 15 454 | 2756 | 17.83 | 17.23 to 18.44 | |
| 5 | 4780 | 908 | 19.00 | 17.88 to 20.11 | |
| 6 | 1217 | 326 | 26.79 | 24.30 to 29.28 | |
| Outpatient NVAF diagnosis | 0 | 37 533 | 304 | 0.81 | 0.72 to 0.90 |
| 1 | 42 276 | 968 | 2.29 | 2.15 to 2.43 | |
| 2 | 33 084 | 878 | 2.65 | 2.48 to 2.83 | |
| 3 | 11 152 | 420 | 3.77 | 3.41 to 4.12 | |
| 4 | 3567 | 140 | 3.92 | 3.29 to 4.56 | |
| 5 | 928 | 34 | 3.66 | 2.46 to 4.87 | |
| 6 | 165 | 14 | 8.48 | 4.23 to 12.74 | |
| Hospital NVAF diagnosis | 0 | 11 681 | 1192 | 10.20 | 9.66 to 10.75 |
| 1 | 24 824 | 3445 | 13.88 | 13.45 to 14.31 | |
| 2 | 35 361 | 5877 | 16.62 | 16.23 to 17.01 | |
| 3 | 26 438 | 5251 | 19.86 | 19.38 to 20.34 | |
| 4 | 11 887 | 2616 | 22.01 | 21.26 to 22.75 | |
| 5 | 3852 | 874 | 22.69 | 21.37 to 24.01 | |
| 6 | 1052 | 312 | 29.66 | 26.90 to 32.42 | |
| Primary diagnosis | 0 | 2454 | 25 | 1.02 | 0.62 to 1.42 |
| 1 | 3888 | 84 | 2.16 | 1.70 to 2.62 | |
| 2 | 4489 | 150 | 3.34 | 2.82 to 3.87 | |
| 3 | 2753 | 146 | 5.30 | 4.47 to 6.14 | |
| 4 | 969 | 62 | 6.40 | 4.86 to 7.94 | |
| 5 | 255 | 17 | 6.67 | 3.60 to 9.73 | |
| 6 | 61 | 10 | 16.39 | 7.10 to 25.68 | |
| Secondary diagnosis | 0 | 9227 | 1167 | 12.65 | 11.97 to 13.33 |
| 1 | 20 936 | 3361 | 16.05 | 15.56 to 16.55 | |
| 2 | 30 872 | 5727 | 18.55 | 18.12 to 18.98 | |
| 3 | 23 685 | 5105 | 21.55 | 21.03 to 22.08 | |
| 4 | 10 918 | 2554 | 23.39 | 22.60 to 24.19 | |
| 5 | 3597 | 857 | 23.83 | 22.43 to 25.22 | |
| 6 | 991 | 302 | 30.47 | 27.61 to 33.34 |
CI indicates confidence intervals.
Table 4.
Case‐Fatality in the 30 Days Following Atrial Fibrillation According to Associated Comorbidities
| People at Risk | Deaths | Case‐Fatality (%) | 95% CI | |
|---|---|---|---|---|
| Acute coronary syndrome | 21 458 | 4773 | 22.24 | 21.69 to 22.80 |
| Heart failure | 55 144 | 9523 | 17.27 | 16.95 to 17.58 |
| Chronic kidney disease | 27 673 | 5940 | 21.46 | 20.98 to 21.95 |
| Recent cardiac (and related structure) surgery | 13 222 | 1384 | 10.47 | 9.95 to 10.99 |
| Cardiomyopathy | 7921 | 854 | 10.78 | 10.10 to 11.46 |
| Hypertension* | 80 417 | 4708 | 5.85 | 5.69 to 6.02 |
| Other* | 77 707 | 3852 | 4.96 | 4.80 to 5.11 |
CI indicates confidence intervals.
This category included only patients without any of the diagnostics listed in the table except hypertension.
This category included only patients without any of the diagnostics listed in the table.
Figure 3.
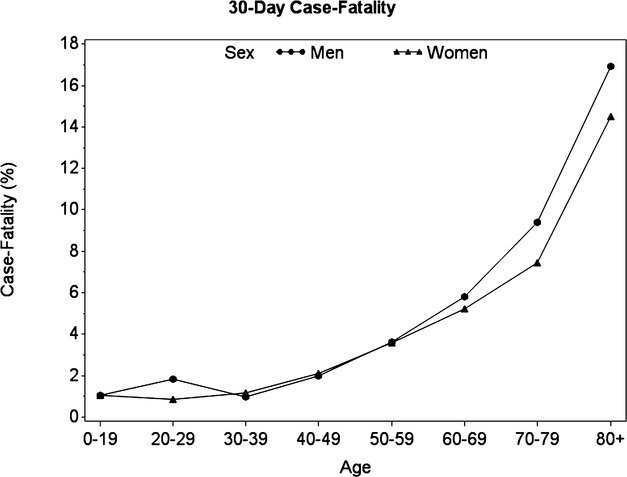
Case‐fatality in the 30 days following atrial fibrillation stratified by age and sex.
Discussion
Using a large population‐based cohort of patients with a first diagnosis of NVAF, we provided contemporary estimates of the burden of disease in Québec in terms of incidence and mortality following NVAF diagnosis during the last decade. The mean age at diagnosis was 71.5 years and, as expected, a large number of patients had associated comorbidities. Nearly 10% of the patients died within a month following NVAF diagnosis, and up to 27% of the patients with a CHADS2 score of 6. Compared with women, men had a higher 30‐day case‐fatality in all age groups and the difference increased in older age groups.
Population‐based contemporary estimates of the incidence of NVAF are missing. Large studies estimated the incidence of AF overall and did not specifically assess NVAF. Moreover, comparison between studies is also hampered by the various settings for case identification (hospital or population‐based), as well as the different algorithms for AF definitions, and exclusion criteria. Regarding the incidence of AF overall, previous large population‐based studies conducted in the 80s and the early 90s reported estimates slightly lower or comparable to ours,2,17–19 except the cardiovascular health study.20 More recent estimates of AF incidence from population‐based cohorts are in line with our results. All these previous cohorts included all AF whereas we focused specifically on NVAF; However, NVAF is the most common form of AF, which allows meaningful comparison between our respective estimates. A German study, which used health insurance data to estimate incidence rates of AF in 2008 by age and sex, reported results closely matching ours with a very low incidence rate of 0.06 per 1000 person‐years under the age of 20 and up to 24.4 for the 80 to 84 years age group.21 Two population‐based US studies, including all adults with a first AF diagnosis, reported very similar age‐ and sex‐adjusted rates of 3.5 per 1000 person‐years6 and 3.68 per 1000 person‐years in 2000.4 Only two studies, focusing on patients 65 years or older, reported higher age‐standardized incidence in this age group than ours (27.4 for 1000 person‐years and 28.4 for 1000 person‐years in 2007).22–23 All studies to date have confirmed the higher incidence of AF among men compared with women, as reported in our study focusing on NVAF, with an incidence rate ratio ranging from 1.5 to 1.8.4,17–18
Our case‐fatality estimates are higher than those reported in a Scottish cohort of 22 968 patients hospitalized for incident AF between 1986 and 1995.24 A likely explanation lies in their cohort definition, which was restricted to patients hospitalized with a principal diagnosis of AF, whereas we selected all patients with a first diagnosis of AF, either as primary or secondary diagnosis. The handful number of studies that described all causes mortality shortly after AF diagnosis consistently pointed out the highest mortality rate immediately following diagnosis.23,25–26 The mortality rate in the first month has been estimated around 10 per 100 person‐years in one study of Medicare beneficiaries 65 years and older, decreasing thereafter.23 In a Québec cohort of AF sampled from the RAMQ database, the decline in the survival curve was steepest in the first 2 months after diagnosis with a 6% probability of death.26 Two previous hospital‐based cohorts of incident AF, with >10 years of follow‐up, also showed that the mortality rate was highest in the year following diagnosis.25,27 We extend previous work by precisely quantifying all‐cause mortality in the first month following diagnosis in patients with NVAF only, and showing that case‐fatality varied greatly according to associated comorbidities at the time of NVAF diagnosis. We also provided case‐fatality estimates in all age‐strata including the younger age groups, and showed that case‐fatality after NVAF was higher in men than in women in all age groups, in agreement with the findings from the original Framingham heart study cohort of 5209 patients reporting Kaplan‐Meier death rates.9
Our study is based on one of the largest and complete population‐based cohort of patients diagnosed with NVAF in an outpatient and inpatient setting, allowing precise estimates of the incidence and mortality following NVAF diagnosis. Moreover, the use of an administrative database covering the entire Québec population excludes the possibility of selection bias. We applied broad exclusion criteria to define subjects with NVAF among all patients with AF diagnosis, as previously done.28–29 However, using less restrictive criteria such as excluding only subjects with mitral stenosis, valvular replacement, or prosthetic heart valve as done by others,1 hence privileging sensitivity over specificity of NVAF diagnosis, only resulted in minor changes to our results. Some limitations relate to the characteristics of the RAMQ database such as the inability to identify the different types of NVAF, paroxysmal, persistent, and permanent. In addition, it is possible that some patients were not true incident cases and had been diagnosed for some time before cohort entry. Physician billings for outpatient visit only contain one diagnostic code, limiting the medical information available for each medical visit. However, we also used the MED‐ÉCHO database, which allows up to 30 diagnostic codes for each hospital episode and all patients had a minimum of 2 years of information available before cohort entry to ensure sufficient baseline information to exclude prevalent cases. Finally, the prevalence of comorbidities at the time of diagnosis may have been underestimated for the same reason, especially for patients who did not have any hospital admission in the 2 years before cohort entry. However, the baseline comorbidities were also measured during the 2 years before cohort entry, which increases the likelihood of capturing most of the important chronic diseases. Also, some vascular risk factors such as smoking and body mass index are not routinely collected in the RAMQ database and their prevalence could not be estimated.
In conclusion, we provide precise and up‐to‐date estimates of the incidence of NVAF in a large well‐defined population‐based cohort, illustrating the significant burden related to NVAF. Mortality remains high in subjects with NVAF around the time of diagnosis, and case‐fatality increases with age, being constantly higher in men compared with women across all age categories.
Sources of Funding
Dr Renoux is a recipient of a Chercheur‐Boursier Award from the Fonds de la recherche en santé du Québec (FRSQ), and Dr Suissa is the recipient of the James McGill Chair. Funding for the study was from an infrastructure grant from the Canadian Foundation for Innovation (CFI) and an unrestricted grant from Bayer Pharma AG.
Disclosures
Dr. Samy Suissa has received research grants and participated in advisory board meetings and/or as a speaker at conferences for Bayer, Boehringer‐Ingelheim and Bristol‐Myers‐Squibb.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285:2370-2375. [DOI] [PubMed] [Google Scholar]
- 2.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006; 27:949-953. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22:983-988. [DOI] [PubMed] [Google Scholar]
- 4.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114:119-125. [DOI] [PubMed] [Google Scholar]
- 5.Wattigney WA, Mensah GA, Croft JB. Increased atrial fibrillation mortality: United States, 1980–1998. Am J Epidemiol. 2002; 155:819-826. [DOI] [PubMed] [Google Scholar]
- 6.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013; 112:1142-1147. [DOI] [PubMed] [Google Scholar]
- 7.Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013; 34:2746-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009; 104:1534-1539. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98:946-952. [DOI] [PubMed] [Google Scholar]
- 10.Lake FR, Cullen KJ, de Klerk NH, McCall MG, Rosman DL. Atrial fibrillation and mortality in an elderly population. Aust N Z J Med. 1989; 19:321-326. [DOI] [PubMed] [Google Scholar]
- 11.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin LCommittee R‐LS Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139-1151. [DOI] [PubMed] [Google Scholar]
- 12.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin LCommittees A Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981-992. [DOI] [PubMed] [Google Scholar]
- 13.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, Investigators RA. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883-891. [DOI] [PubMed] [Google Scholar]
- 14.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995; 48:999-1009. [DOI] [PubMed] [Google Scholar]
- 15.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004; 57:131-141. [DOI] [PubMed] [Google Scholar]
- 16.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864-2870. [DOI] [PubMed] [Google Scholar]
- 17.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994; 271:840-844. [PubMed] [Google Scholar]
- 19.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995; 98:476-484. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997; 96:2455-2461. [DOI] [PubMed] [Google Scholar]
- 21.Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Breithardt G. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013; 15:486-493. [DOI] [PubMed] [Google Scholar]
- 22.Ohlmeier C, Mikolajczyk R, Haverkamp W, Garbe E. Incidence, prevalence, and antithrombotic management of atrial fibrillation in elderly Germans. Europace. 2013; 15:1436-1444. [DOI] [PubMed] [Google Scholar]
- 23.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Cir Cardiovas Qual Outcomes. 2012; 5:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart S, MacIntyre K, Chalmers JW, Boyd J, Finlayson A, Redpath A, Pell JP, Capewell S, McMurray JJ. Trends in case‐fatality in 22968 patients admitted for the first time with atrial fibrillation in Scotland, 1986–1995. Int J Cardiol. 2002; 82:229-236. [DOI] [PubMed] [Google Scholar]
- 25.Andersson T, Magnuson A, Bryngelsson IL, Frobert O, Henriksson KM, Edvardsson N, Poci D. All‐cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long‐term case‐control study. Eur Heart J. 2013; 34:1061-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guertin JR, Dorais M, Khairy P, Sauriol L, Matteau A, Poulin F, Talajic M, Roy D, LeLorier J. Atrial fibrillation: a real‐life observational study in the Quebec population. Can J Cardiol. 2011; 27:794-799. [DOI] [PubMed] [Google Scholar]
- 27.Frost L, Engholm G, Moller H, Husted S. Decrease in mortality in patients with a hospital diagnosis of atrial fibrillation in Denmark during the period 1980–1993. Eur Heart J. 1999; 20:1592-1599. [DOI] [PubMed] [Google Scholar]
- 28.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp‐Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011; 342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk–treatment paradox in patients with newly diagnosed non‐valvular atrial fibrillation. Heart. 2011; 97:2046-2050. [DOI] [PubMed] [Google Scholar]


