Abstract
Background
Exercise is associated with age‐related penetrance and arrhythmic risk in carriers of arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C)‐associated desmosomal mutations; however, its role in patients without desmosomal mutations (gene‐elusive) is uncertain. This study investigates whether exercise is (1) associated with onset of gene‐elusive ARVD/C and (2) has a differential impact in desmosomal and gene‐elusive patients.
Methods and Results
Eighty‐two ARVD/C patients (39 desmosomal, all probands) were interviewed about regular physical activity from age 10. Participation in endurance athletics, duration (hours/year), and intensity (MET‐Hours/year) of exercise prior to clinical presentation were compared between patients with desmosomal and gene‐elusive ARVD/C. All gene‐elusive patients were endurance athletes. Gene‐elusive patients were more likely to be endurance athletes (P<0.001) and had done significantly more intense (MET‐Hrs/year) exercise prior to presentation (P<0.001), particularly among cases presenting < age 25 (P=0.027). Family history was less prevalent among gene‐elusive patients (9% versus 40% desmosomal, P<0.001), suggesting a greater environmental influence. Gene‐elusive patients without family history did considerably more intense exercise than other ARVD/C patients (P=0.004). Gene‐elusive patients who had done the most intense (top quartile MET‐Hrs/year) exercise prior to presentation had a younger age of presentation (P=0.025), greater likelihood of meeting ARVD/C structural Task Force Criteria (100% versus 43%, P=0.02), and shorter survival free from a ventricular arrhythmia in follow‐up (P=0.002).
Conclusions
Gene‐elusive, non‐familial ARVD/C is associated with very high intensity exercise suggesting exercise has a disproportionate role in the pathogenesis of these cases. As exercise negatively modifies cardiac structure and promotes arrhythmias, exercise restriction is warranted.
Keywords: arrhythmogenic right ventricular dysplasia/cardiomyopathy, desmosome cardiomyopathy, etiology, exercise, genetics—human
Introduction
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is a rare cardiomyopathy characterized by ventricular arrhythmias, an increased risk of sudden cardiac death, and predominant right ventricular (RV) dysfunction.1–2 Beginning with the observation that young athletes in the Veneto region of Italy had a 5‐fold risk of dying of ARVD/C compared with non‐athletes,3 evidence has emerged that endurance exercise is associated with ARVD/C. The discovery that up to 60% of ARVD/C patients have mutations in genes encoding the cardiac desmosome (PKP2, DSG2, DSP, DSC2, and JUP)4–11 suggested one reason for this association. Since cardiac desmosomes provide a mechanical connection between myocytes, dysfunction of desmosomal proteins is thought to render these junctions susceptible to pathogenic remodelling when exposed to mechanical load.12–13 We recently reported that endurance exercise increases risk of developing ARVD/C, ventricular arrhythmias, and heart failure among patients with desmosomal mutations.14
Whether the association of exercise with disease extends to ARVD/C patients without desmosomal mutations (gene‐elusive) is uncertain. While relatively rare non‐desmosomal ARVD/C‐associated mutations have been discovered,15–17 the genetic and environmental causes of gene‐elusive ARVD/C are poorly understood. Heidbüchel and colleagues have postulated that there is an exercise‐induced form of ARVD/C in which excessive, repetitive wall stress causes disruption of normal desmosomes eventually resulting in ARVD/C.18 Their hypothesis is supported by a relatively low prevalence of both familial disease and desmosomal mutations in their athletic cohort.19
Discerning whether exercise has a role in pathogenesis of ARVD/C among patients without desmosomal mutations (gene‐elusive), and whether duration or intensity of exercise associated with disease expression differs in patients with and without desmosomal mutations has significant implications both for affected families and for scientific discovery. Taking advantage of our large population of well‐phenotyped ARVD/C patients, we developed a study to answer these questions by (1) characterizing exercise history in ARVD/C index gene‐elusive cases, (2) comparing the exercise history of patients with and without desmosomal mutations, and (3) assessing whether exercise history influences clinical phenotype among gene‐elusive cases.
Methods
Study Population
The study population was recruited from the Johns Hopkins ARVD/C Registry (arvd.com). Participants are contacted and medical records are updated annually. ARVD/C patients who met revised 2010 ARVD/C Task Force Criteria (TFC)20 and (1) were the proband (first identified case) in their family, (2) had undergone sequencing of the ARVD/C‐associated genes PKP2, DSG2, DSC2, DSP, JUP, PLN, and TMEM43 through either our research9,21 or commercially available testing, and (3) could speak English, were invited to participate in a detailed interview to document exercise history. Parents were co‐informants for adolescents (one 16‐year‐old). Thirty‐seven desmosomal mutation carriers were included in a prior publication.14 The study was approved by the Johns Hopkins School of Medicine Institutional Review Board.
Clinical Characterization
Clinical, demographic, and family history data were drawn from the Johns Hopkins ARVD/C Registry. Clinical data included date, circumstances, symptoms, and arrhythmia associated with clinical presentation. Clinical presentation was defined as the first medical visit for a cardiac complaint related to ARVD/C. We considered an arrhythmic presentation as one associated with a documented spontaneous sustained ventricular arrhythmia, resuscitated cardiac arrest, or syncope adjudicated as likely arrhythmic due to associated circumstances and presentation by an experienced electrophysiologist (HC). Results of non‐invasive and invasive studies (12‐lead electrocardiogram [ECG], exercise testing, 24‐hour Holter monitoring, 2‐dimensional transthoracic echocardiography, cardiac magnetic resonance imaging, signal averaged ECG, RV angiogram) were reviewed and diagnosis of ARVD/C confirmed based on the presence of major and minor diagnostic criteria according to the TFC. Per ARVD/C Registry protocol, a detailed family history was obtained through patient interview by genetic counselors with a special interest in ARVD/C and review of cardiac medical records of family members. A positive family history was defined as meeting either a major or minor Family History criterion according to the TFC. The first sustained ventricular arrhythmia in follow‐up (VT/VF) was a composite measure of the occurrence of spontaneous sustained ventricular tachycardia, aborted sudden cardiac death, or appropriate implantable cardioverter‐defibrillator (ICD) intervention. In patients without an ICD, VT/VF outcome was adjudicated based on reviewing ECGs and medical records. In patients with an ICD, the device stored ECGs were reviewed for appropriateness of ICD therapy.
Exercise Interviews
Structured telephone or in‐person interviews were conducted by 3 genetic counselors and 2 physicians. We have previously described our interview methodology and data collection.14 Briefly, we prompted participants to list regular exercise since age 10. Participants were then asked to estimate how many months of the year, days of the month, and hours a day he/she did each activity and asked to rate intensity as “light,” “moderate,” or “vigorous” using language and definitions from the Multi‐Ethnic Study of Atherosclerosis Typical Week Physical Activity Survey.22
Exercise History Analysis
The focus of our analysis was exercise done prior to clinical presentation. We first classified participants as endurance athletes and non‐athletes. We defined endurance athletes as participants in sports with a high dynamic demand (>70% maximum O2), as defined by the 36th Bethesda Conference Classification of Sports (Task Force 8),23 done for at least 50 hours/year at vigorous intensity as we have done previously.14 Next, 2 characteristics of exercise were calculated: duration and intensity. Exercise duration was calculated for every activity separately and summed to achieve the total hours spent exercising prior to clinical presentation. This value was then annualized. Exercise intensity was calculated using MET‐Hours, a robust tool for measuring energy expenditure associated with physical activity.24–25 We assigned a Metabolic Equivalent (MET) for every exercise activity based on values assigned in the 2011 Compendium of Physical Activities.26 (One MET is the energy spent sitting at rest, 3 METs walking at a slow pace on even ground, 7 METs jogging, and 12 METs running vigorously.) We subsequently multiplied the MET value for each exercise activity by its duration, which was annualized to obtain MET‐Hours per year (MET‐Hrs/year).
Statistical Analysis
Categorical variables were reported as frequency (%) and compared between groups by the chi‐square or Fisher's exact test. Continuous variables were summarized as either mean±SD or median (interquartile range) and compared across groups using a t test, Mann‐Whitney U test, or Kruskal‐Wallis test as appropriate. Cumulative freedom from first VT/VF in follow‐up was determined by the Kaplan‐Meier method. Difference in survival between groups was evaluated with a log‐rank test. Regression analysis was used to perform age‐adjusted comparisons of exercise parameters between desmosomal and gene‐elusive patients. A P value of <0.05 was considered significant. SPSS (version 22; SPSS Inc, Chicago, IL) statistical software was used.
Results
Study Population
Of 132 eligible probands with ARVD/C, 82 (62%) responded to our invitation and were interviewed. There was no significant difference in response rate between patients with (39/67, 58%) and without (43/65, 66%) desmosomal mutations. Response rate also did not differ by sex or age. The final study population included 82 individuals (53 male) aged 16 to 76 (mean 43±14 years) with ARVD/C. Nearly half (39/82, 48%) carried a pathogenic desmosomal mutation (30 PKP2, 4 DSG2, 2 DSP, 1 DSC2, 2 with compound heterozygosity [1 DSG2/DSG2, 1 DSP/DSP]). Throughout the manuscript these patients are referred to as “desmosomal.” The 43 patients without a pathogenic mutation in the desmosomal genes are referred to as “gene‐elusive.” None carried a PLN or TMEM43 mutation. Two individuals were of Asian ancestry. The remainder were white.
Clinical Characteristics
Clinical characteristics of our study population are presented in Table 1. As per study design, all met TFC and were probands. Mean age at first clinical presentation was 35±14 years (range 12 to 64). Most (59/82, 72%) had an arrhythmic presentation: 47 (57%) with sustained ventricular tachycardia, 4 (5%) with resuscitated sudden cardiac arrest, and 8 (10%) with cardiac syncope. The presenting arrhythmia was associated with exercise in the majority (43/59, 73%). Following presentation, 79 (96%) elected ICD implantation, of which 55 (70%) had at least one appropriate ICD intervention (median 6.4 year follow‐up, [IQR 2.9, 12.7]). Five (6%) had undergone cardiac transplant; 2 predominantly for ventricular arrhythmias, 3 for heart failure.
Table 1.
Clinical Characteristics of the Study Population
| All (n=82) | Desmosomal Mutation (n=39) | No Desmosomal Mutation (n=43) | P Value* | |
|---|---|---|---|---|
| Male | 53 (65) | 27 (69) | 26 (61) | 0.41 |
| Proband | 82 (100) | 39 (100) | 43 (100) | 1.0 |
| Age at interview, y | 43±14 | 40±14 | 46±13 | 0.043 |
| Presentation | ||||
| Age at presentation, y | 35±14 | 32±14 | 39±14 | 0.007 |
| Symptomatic presentation | 79 (96) | 37 (95) | 42 (98) | 0.46 |
| Arrhythmic presentation | 59 (72) | 31 (79) | 28 (65) | 0.15 |
| Sustained VT | 47 | 22 | 25 | |
| Resuscitated SCA | 4 | 4 | 0 | |
| Syncope | 8 | 5 | 3 | |
| Exercise‐associated arrhythmia | 43/59 (73) | 22/31 (71) | 21/28 (75) | 0.73 |
| 2010 Task Force Criteria | ||||
| Structural alterations | 58 (71) major | 28 (72) major | 30 (70) major | 0.83 |
| 8 (10) minor | 3 (8) minor | 5 (12) minor | ||
| Repolarization abnormalities | 62 (76) major | 33 (85) major | 29 (67) major | 0.11 |
| 13 (16) minor | 5 (13) minor | 8 (19) minor | ||
| Depolarization abnormalities | 7 (9) major | 3 (8) major | 4 (9) major | 0.86 |
| 52 (63) minor | 24 (62) minor | 28 (65) minor | ||
| Arrhythmias | 35 (43) major | 15 (39) major | 20 (47) major | 0.42 |
| 43 (52) minor | 23 (59) minor | 20 (47) minor | ||
| Family history/Genetics | 41 (50) major | 39 (100) major | 2 (5) major | <0.001 |
| 9 (11) minor | 7 (18) minor | 2 (5) minor | ||
| ICD implantation | 79 (96) | 39 (100) | 40 (93) | 0.24 |
| Cardiac transplantation | 5 (6) | 4 (10) | 1 (2) | 0.19 |
Values are in n (%) or mean±SD. ICD indicates implantable cardioverter‐defibrillator; SCA, sudden cardiac arrest; VT, occurrence of spontaneous sustained ventricular tachycardia.
P value represents comparison between participants with (n=39) and without (n=43) desmosomal mutations.
As shown in Table 1, gene‐elusive patients were older at clinical presentation (age 39±14 years gene‐elusive versus 32±14 years desmosomal, P=0.007), and correspondingly older at study interview (46±13 years gene‐elusive versus 40±14 years desmosomal, P=0.04). Other demographic and clinical characteristics were similar. Desmosomal and gene‐elusive patients both predominantly had an arrhythmic presentation and the presenting arrhythmia was equally likely to be associated with exercise. There was also no difference in likelihood of meeting each domain of the TFC except family history/genetics as all patients with a desmosomal mutation meet this major criterion.
Exercise History
Most participants (70/82, 85%) had been endurance athletes prior to clinical presentation. Median age of starting a first endurance sport was 14 (range 10 to 45, IQR 10, 18). The most common endurance sports were long‐ and middle‐distance running (45/70, 64%), basketball (22/70, 31%), soccer (18/70, 26%), and cycling (13/70, 19%). Other qualifying sports included competitive swimming (9), tennis/squash/racquetball (8), lacrosse/field hockey/ice hockey (6), and rowing (2). Most (40/70, 57%) endurance athletes competed in multiple sports.
Interviewees participated in a median of 375 hours/year (IQR 216, 671) of regular exercise of all types prior to clinical presentation. These activities involved a median 4168 MET‐Hrs/year (IQR 2020, 7633). There was no difference by sex in participation in endurance athletics (P=0.28), annual exercise duration (P=0.90) or intensity [MET‐Hrs/year] (P=0.87).
Association of Exercise History With Genotype
We compared exercise history of gene‐elusive patients and patients with desmosomal mutations. As shown in Table 2, all gene‐elusive patients (43/43) had been endurance athletes, significantly more than the two‐thirds of desmosomal mutation carriers (27/39 [69%], P<0.001). Gene‐elusive and desmosomal patients began endurance exercise at a similarly young age (median 13 years gene‐elusive, 14 years desmosomal, P=0.93).
Table 2.
Exercise Preceding Clinical Presentation
| Total (n=82) | Desmosomal Mutation (n=39) | No Desmosomal Mutation (n=43) | P Value* | |
|---|---|---|---|---|
| Endurance athlete (% yes) | 70 (85) | 27 (69) | 43 (100) | <0.001 |
| Duration | ||||
| Total hours, median (IQR) | 7315 (4041, 15 588) | 6074 (2880, 9858) | 9850 (5400, 17 872) | 0.18 |
| Hours/year | 375 (216, 671) | 387 (179, 572) | 367 (233, 759) | 0.401 |
| Hours in year of presentation | 400 (160, 720) | 274 (96, 580) | 458 (184, 906) | 0.134 |
| Intensity | ||||
| MET‐Hrs/year, median (IQR) | 4168 (2020, 7633) | 2637 (1223, 4687) | 5640 (2692, 11 824) | 0.004 |
| MET‐Hrs in year of presentation | 3337 (1231, 8389) | 2784 (672, 5096) | 4505 (1570, 12 096) | 0.012 |
Values are in n (%) or median (interquartile range); MET‐Hrs, metabolic equivalent hours.
P value represents age‐adjusted comparison between patients with (n=39) and without (n=43) desmosomal mutations.
While gene‐elusive ARVD/C patients had done a similar duration of annual exercise as desmosomal mutation carriers, their exercise was significantly more intense, expending greater MET‐Hrs/year, despite their later age of onset (P=0.004). This is shown graphically in Figure 1A in which our population is stratified by age of presentation. Gene‐elusive patients had done more intense exercise in each age grouping. This difference was particularly notable among those presenting in the youngest tertile (≤age 25). Desmosomal patients were 3 times more likely to present prior to age 25 years than gene‐elusive patients (OR=3.38, 95% CI: 1.25 to 9.15, P=0.02). While gene‐elusive patients were less likely to present by age 25 (8/43 [19%] gene‐elusive versus 17/39 [44%] desmosomal, P=0.014) those who did so had done 5‐fold greater MET‐Hrs/year of exercise prior to presentation than young desmosomal patients (Figure 1B, P=0.027). Among those presenting after age 25, gene‐elusive patients had also done more intense annual exercise (P=0.001, Figure 1B), but the difference was only 2‐fold. A similar pattern was seen when only pre‐presentation exercise done prior to age 25 (referred to as “youth exercise”) was considered. Gene‐elusive patients had done more intense youth exercise, not only among those who presented by age 25 but also and among those presenting at an older age (6197 MET‐Hrs/year [IQR 2775, 11 251] gene‐elusive versus 2317 MET‐Hrs/year [IQR 1013, 6104] desmosomal, P=0.003).
Figure 1.

Exercise intensity (median MET‐Hours/year) stratified by (A) quartiles of age of clinical presentation and (B) age of clinical presentation before and after age 25. Gene‐elusive patients had done more intense exercise regardless of age of presentation. Among patients presenting by age 25 there is a 5‐fold difference in intensity. MET‐Hours indicates metabolic equivalent hours.
Association of Family History With Genotype
We next compared frequency of familial disease between desmosomal and gene‐elusive patients. Gene‐elusive patients were significantly less likely to meet family history TFC (not including mutation status) (4/43 [9%] gene‐elusive versus 15/38 [40%] desmosomal, P<0.001). Two gene‐elusive patients met major family history TFC (pathological diagnosis in a family member) and 2 met minor TFC. None had an affected second‐degree relative. Among desmosomal mutation carriers, 10 met major family history criteria (all TFC in a first degree relative) and 5 met only minor family history TFC. Two met criteria for disease in a second‐degree relative. (One desmosomal patient was adopted and therefore family history was unavailable.)
Among desmosomal patients, there was no difference in either intensity (P=0.56) or duration (P=0.39) of exercise between those with and without a family history of ARVD/C (Figure 2). In contrast, among gene‐elusive patients, those without a family history of ARVD/C expended more than double annual MET‐Hrs prior to presentation as those with a family history (median 6711 MET‐Hrs/year no family history versus 3040 MET‐Hrs/year with family history). As shown in Figure 2, patients with neither a desmosomal mutation nor family history did by far the most intense exercise (P=0.004).
Figure 2.
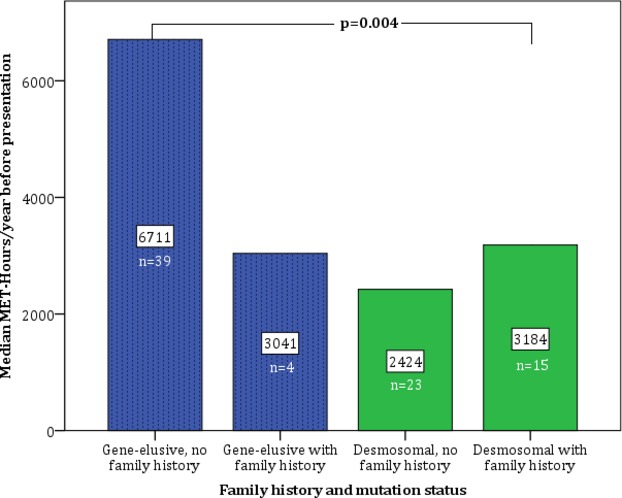
Exercise intensity among patients stratified by genotype and 2010 family history Task Force Criteria. Gene‐elusive, non‐familial patients participated in significantly higher‐intensity exercise than those with family history or desmosomal mutations (P=0.004, Kruskal‐Wallis one‐way analysis of variance). MET‐Hours indicates metabolic equivalent hours
Association of Exercise With Phenotype in Gene‐Elusive ARVD/C Patients
Finally, we investigated the association of duration and intensity of pre‐presentation exercise on clinical phenotype at presentation and arrhythmic outcomes during follow‐up among gene‐elusive patients. Those who did the greatest (top quartile) duration of annual exercise (>760 hours/year) presented approximately a decade earlier than those doing less (quartiles I–III) annual exercise (mean age 31±12 top quartile versus age 42±14 quartiles I–III; P=0.025). A similar trend was appreciated when intensity (MET‐Hrs/year) was considered (mean age 33±14 top quartile versus age 42±14 quartiles I–III; P=0.09). Since 9 out of 11 patients who had done top quartile duration also had done top quartile intensity, further comparisons were limited to MET‐Hrs/year.
Despite their relatively young age, patients who had done the most (top quartile) intense exercise prior to presentation had significant structural disease at presentation. Three‐quarters (34/43) of gene‐elusive patients had imaging available for review that allowed the structural TFC to be applied (32 cardiac magnetic resonance imaging, 1 echocardiogram, and 1 RV angiogram). Among these patients, 18/34 (53%) met major structural TFC, 4/34 (12%) met minor structural TFC, and 12/33 (35%) patients did not meet any TFC. Patients who had done top quartile MET‐Hrs/year exercise were significantly more likely to meet major structural TFC than those doing less intense exercise (100% top quartile MET‐Hrs/year versus 43% quartiles I–III; P=0.020).
Finally, we assessed the influence of exercise on arrhythmic course. Patients doing the most intense (top quartile MET‐Hrs/year) exercise prior to presentation were no more likely to have an arrhythmic presentation than those doing less intense exercise (6/11 top quartile [55%] versus 22/32 [69%] quartiles I–III, P=0.39). However, as shown in Figure 3, although all patients had an arrhythmic course, survival free from VT/VF was significantly lower among patients who had done the most intense exercise (P=0.002).
Figure 3.
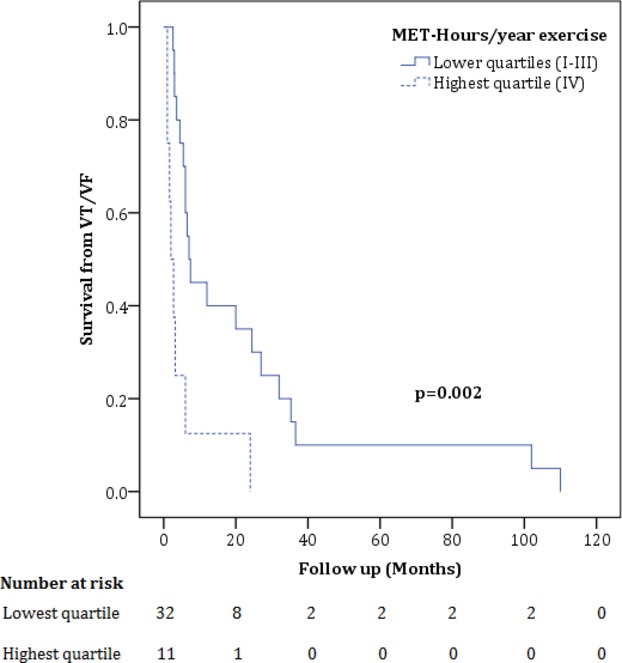
Cumulative survival free from ventricular arrhythmia (VT/VF) during follow‐up in gene‐elusive patients. Among gene‐elusive patients, survival from a VT/VF during follow‐up was significantly lower among patients who had done the most intense exercise (P=0.002, log‐rank test). MET‐Hours indicates metabolic equivalent‐hours; VT/VF, composite measure of spontaneous sustained ventricular tachycardia, aborted sudden cardiac death, or appropriate implantable cardioverter‐defibrillator (ICD) intervention.
Discussion
Main Findings
This study tests the hypothesis that exercise is an important environmental factor in the development of gene‐elusive ARVD/C, and that exercise has a differential impact on the development of ARVD/C in patients with and without desmosomal mutations. Based on 3 lines of evidence we suggest that frequent high‐intensity exercise may play an even greater role in the pathogenesis of gene‐elusive ARVD/C. First, we found ARVD/C patients without desmosomal mutations did significantly more intense exercise prior to presentation than desmosomal mutation carriers. This is particularly notable since it has been previously well established that desmosomal mutation carriers with ARVD/C are disproportionately athletes. Second, patients without desmosomal mutations were significantly less likely to have a family history of ARVD/C, suggesting a greater importance of an environmental influence on pathogenesis in this group. The fact that gene‐elusive, nonfamilial ARVD/C patients had done the most intense exercise by far suggests exercise as a key environmental factor. Finally, exercise history influences disease course in gene‐elusive ARVD/C patients. Top intensity exercise was associated with significantly earlier age of onset, worse structural disease at clinical presentation, and shorter freedom from ventricular arrhythmia in follow‐up.
Exercise and the Pathogenesis of ARVD/C
Shortly following the first description of ARVD/C 30 years ago,1,27 evidence emerged suggesting that exercise influences both the development of ARVD/C and its clinical course. A study of sudden cardiac death in the Veneto region of Italy found that young athletes had a 5‐fold risk of dying of ARVD/C.3 Consistent with this observation, implementation of a pre‐participation screening program for athletes resulted in a sharp decline in ARVD/C‐associated deaths.28 The subsequent discovery of ARVD/C‐associated mutations in desmosomal genes a decade ago4–11 suggested a mechanistic explanation for the association between ARVD/C and exercise as the cardiac desmosome provides a mechanical connection between myocytes. The association of exercise and disease pathogenesis in the setting of desmosome mutations was confirmed in a plakoglobin‐deficient mouse model29 and subsequently in human desmosomal mutation carriers.14 However, whether mutations in desmosomes accounted for most or all of the association of exercise with ARVD/C remained uncertain.
There is emerging evidence that the right ventricle (RV) may be particularly susceptible to exercise‐induced injury.18 The RV and left ventricle (LV) respond differently to exercise.30 Compared with rest, RV wall stress at peak exercise in athletes rises by 170%, compared with a 23% increase in LV wall stress. Intense endurance exercise causes acute dysfunction of the RV, but not the LV.31 It has been suggested that the RV response to frequent exercise can be maladaptive and result in fibrosis.32–33 Although RV changes are typically reversible, they can be persistent with frequent training.34
La Gerche and colleagues integrated these observations with a study of 47 athletes with definite or probable ARVD/C, 6 of whom were reported to have desmosomal mutations.19 To the best of our knowledge, this was the first study that quantified the athletic history of ARVD/C patients. Similar to our study results, they found patients with desmosomal mutations had done less exercise, and few athletes with what they dubbed “exercise‐induced RV cardiomyopathy” had a family history of ARVD/C. Based on these results, they developed a hypothesis that “extreme doses” of exercise may be sufficient to damage the RV and cause “exercise‐induced RV cardiomyopathy” even in the absence of a desmosomal mutation.
Study Findings
The results of our study confirm and extend the results of prior studies, which have examined the interaction between exercise and ARVD/C.14,19 We found that gene‐elusive ARVD/C patients were all endurance athletes, significantly more than the two‐thirds of patients with desmosomal mutations or the one‐third of unaffected desmosomal mutation carriers we previously reported.14 Our results strengthen the prior observation of La Gerche, Heidbüchel, and colleagues as we included 39 patients with pathogenic desmosomal mutations, a much more robust comparison group than the 6 patients (4 definite mutation carriers and 2 patients with variants of uncertain significance) in their prior report.19 The results of our study also revealed for the first time that the primary difference in exercise between ARVD/C patients with and without desmosomal mutations is in the intensity of exercise. We found a relatively low prevalence of familial disease among gene‐elusive cases. Furthermore, gene‐elusive cases with familial disease in our study had done exercise indistinguishable from that of desmosomal mutation carriers. This provides additional evidence that among patients with neither a mutation, nor a family history, a greater exercise “dose” may be required to cause phenotypic expression. Finally, our findings reveal for the first time that exercise influences phenotype among patients without desmosomal mutations. Greater exercise intensity was associated with both worse structural disease and younger age at clinical presentation. Furthermore, patients with the most intense pre‐presentation exercise had the most aggressive arrhythmic course.
While these results provide additional strong evidence for the importance of exercise in the pathogenesis of at least some cases of gene‐elusive ARVD/C, we feel it would be premature and inappropriate to conclude that this group of ARVD/C patients has an entirely acquired disease. There are several lines of evidence that lead us to conclude that exercise is not the only important environmental and genetic influence to impact the development of gene‐elusive ARVD/C. First, 10% of gene‐elusive cases in our study had clear familial disease. This is an underestimate of the true incidence of familial disease among patients without desmosomal mutations since by study design we excluded families in which the proband was deceased. Second, while our findings suggest a strong influence of exercise on pathogenesis, ARVD/C is a rare disease. Clearly only a small proportion of elite athletes are susceptible. This susceptibility is likely based largely on genetic background or on other environmental influences. Therefore, gene‐elusive ARVD/C may be caused in part by mutations in genes with lower penetrance or by combinations of rather low penetrant variants in both desmosomes and other genes (Figure 4).
Figure 4.

Relative influence of exercise and genetics in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy.
To identify these genes associated with gene‐elusive ARVD/C, this study points to investigating candidates in which function or expression could be influenced by regular exercise. One group of attractive candidates are genes encoding proteins of the adherens junction. Recent research indicates that in mammalian hearts there is a mixed‐type cardiac junction, the “area composita,” composed of both desmosomal and adherens junctional proteins.35 Indeed, mutations in the adherens junction gene CTNNA2 were recently identified in 2 ARVD/C patients.36 It is also possible that a subset of these patients have a unique or pathologic hemodynamic response to exercise, which in turn leads to higher right ventricular load and eventually right ventricular dilatation and dysfunction. Genes involved in regulating these responses may also merit study.
In addition to providing evidence that can be used for scientific discovery, the results of our study also have clinical implications. As exercise negatively influences cardiac structure and function in at least some ARVD/C patients without desmosomal mutations, it is likely important to restrict ongoing exercise. Further studies are needed to determine if our observation that gene‐elusive patients engaged in higher‐intensity exercise can be used to tailor recommendations for the degree of exercise restriction based on genotype of patients and at‐risk family members.
Study Limitations
Our retrospective interview‐based exercise history collection may limit interpretation of study findings. Recall of athletic participation may have been inaccurate in a way that varied across subpopulations (recall bias). Additionally, participants may have deliberately altered their responses in a way they considered desirable (social desirability). Our study population also may limit findings. First, while one‐third of ARVD/C cases present with sudden death, these individuals were not included in the study. The influence of exercise in this subpopulation may differ. Furthermore, because families with deceased probands were not included, the proportion of patients meeting family history criteria is likely lower in this study than in the ARVD/C population.37–38 Additionally, it is possible that some probands had a family history of disease that was not recognized. This may have been more likely in the gene‐elusive families for whom genetic testing for risk stratification could not be offered. Finally, those interviewed are participants in a research registry run by a tertiary care center creating a potential selection bias.
Conclusions
In conclusion, the results of this study strongly suggest that exercise plays an important role in the pathogenesis of gene‐elusive ARVD/C. Exercise has a differential impact on development of ARVD/C among gene‐elusive patients who appear to require higher intensity exercise for developing disease compared to desmosomal ARVD/C patients. Our findings have implications for both clinical care and scientific discovery. As exercise appears to negatively modify cardiac structure and promote arrhythmias in gene‐elusive ARVD/C patients, we will recommend exercise restriction. Our findings suggest avenues for discovery of new genes involved in pathways influenced significantly by exercise as well as further exploration into the mechanisms of exercise induced RV dysfunction.
Sources of Funding
The authors wish to acknowledge funding from the Alexandre Suerman Stipend (to AT), St. Jude Medical Inc, and Medtronic Inc. The Johns Hopkins ARVD/C Program (ARVD.com) is supported by the Leyla Erkan Family Fund for ARVD Research, the Dr. Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Dr. Francis P. Chiaramonte Private Foundation, the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments.
Disclosures
Dr Calkins receives research support from Medtronic Inc and St. Jude Medical Inc. Dr James receives salary support from these grants. The other authors report no conflict of interest.
Acknowledgments
The authors are grateful to the patients who made this work possible.
References
- 1.Marcus F, Fontaine G, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982; 65:384-398. [DOI] [PubMed] [Google Scholar]
- 2.Fontaine G. Arrhythmogenic right ventricular dysplasia. Curr Opin Cardiol. 1995; 10:16-20. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003; 42:1959-1963. [DOI] [PubMed] [Google Scholar]
- 4.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Drenckhahn J, Michely B, Sasse‐Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze‐Bahr E, Thierfelder L. Mutations in the desmosomal protein plakophilin‐2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004; 36:1162-1164. [DOI] [PubMed] [Google Scholar]
- 5.Awad MM, Dalal D, Cho E, Amat‐Alarcon N, James C, Tichnell C, Tucker A, Russell SD, Bluemke DA, Dietz HC, Calkins H, Judge DP. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Hum Genet. 2006; 79:136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rampazzo A, Nava A, Malacrida S, Beffagna G, Bauce B, Rossi V, Zimbello R, Simionati B, Basso C, Thiene G, Towbin JA, Danieli GA. Mutation in human desmoplakin domain binding to plakoglobin causes a dominant form of arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2002; 71:1200-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet. 2000; 355:2119-2124. [DOI] [PubMed] [Google Scholar]
- 8.Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen‐Chowdhry S, McKenna WJ. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin‐2. Am J Hum Genet. 2006; 79:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Haan AD, Tan BY, Zikusoka MN, Lladó LI, Jain R, Daly A, Tichnell C, James C, Amat‐Alarcon N, Abraham T, Russell SD, Bluemke DA, Calkins H, Dalal D, Judge DP. Comprehensive desmosome mutation analysis in North Americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet. 2009; 2:428-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox MG, van der Zwaag PA, van der Werf C, van der Smagt JJ, Noorman M, Bhuiyan ZA, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, van den Wijngaard A, Houweling AC, Jongbloed JD, Jordaens L, Cramer MJ, Doevendans PA, de Bakker JM, Wilde AA, van Tintelen JP, Hauer RN. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index‐patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype‐phenotype follow‐up study. Circulation. 2011; 123:2690-2700. [DOI] [PubMed] [Google Scholar]
- 11.Pilichou K, Nava A, Basso C, Beffagna G, Bauce B, Lorenzon A, Frigo G, Vettori A, Valente M, Towbin J, Thiene G, Danieli GA, Rampazzo A. Mutations in desmoglein‐2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006; 113:1171-1179. [DOI] [PubMed] [Google Scholar]
- 12.Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res. 2010; 107:700-714. [DOI] [PubMed] [Google Scholar]
- 13.Saffitz JE, Asimaki A, Huang H. Arrhythmogenic right ventricular cardiomyopathy: new insights into mechanisms of disease. Cardiovasc Pathol. 2010; 19:166-170. [DOI] [PubMed] [Google Scholar]
- 14.James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age‐related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy‐associated desmosomal mutation carriers. J Am Coll Cardiol. 2013; 62:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merner ND, Hodgkinson KA, Haywood AF, Connors S, French VM, Drenckhahn JD, Kupprion C, Ramadanova K, Thierfelder L, McKenna W, Gallagher B, Morris‐Larkin L, Bassett AS, Parfrey PS, Young TL. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet. 2008; 82:809-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, Cox MG, van Lochem LT, de Boer RA, Hofstra RM, Christiaans I, van Spaendonck‐Zwarts KY, Lekanne dit Deprez RH, Judge DP, Calkins H, Suurmeijer AJ, Hauer RN, Saffitz JE, Wilde AA, van den Berg MP, van Tintelen JP. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012; 14:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quarta G, Syrris P, Ashworth M, Jenkins S, Zuborne Alapi K, Morgan J, Muir A, Pantazis A, McKenna WJ, Elliott PM. Mutations in the Lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2012; 33:1128-1136. [DOI] [PubMed] [Google Scholar]
- 18.Heidbüchel H, Prior DL, La Gerche A. Ventricular arrhythmias associated with long‐term endurance sports: what is the evidence? Br J Sports Med. 2012; 46suppl 1:i44-i50. [DOI] [PubMed] [Google Scholar]
- 19.La Gerche A, Robberecht C, Kuiperi C, Nuyens D, Willems R, de Ravel T, Matthijs G, Heidbüchel H. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart. 2010; 96:1268-1274. [DOI] [PubMed] [Google Scholar]
- 20.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010; 121:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan BY, Jain R, den Haan AD, Chen Y, Dalal D, Tandri H, Amat‐Alarcon N, Daly A, Tichnell C, James C, Calkins H, Judge DP. Shared desmosome gene findings in early and late onset arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Transl Res. 2010; 3:663-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turkbey EB, Jorgensen NW, Johnson WC, Bertoni AG, Polak JF, Diez Roux AV, Tracy RP, Lima JA, Bluemke DA. Physical activity and physiological cardiac remodelling in a community setting: the Multi‐Ethnic Study of Atherosclerosis (MESA). Heart. 2010; 96:42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell JH, Haskell W, Snell P, Van Camp SP. Task Force 8: classification of sports. J Am Coll Cardiol. 2005; 45:1364-1367. [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Willett W, Colditz G. Walking and leisure‐time activity and risk of hip fracture in postmenopausal women. JAMA. 2002; 288:2300-2306. [DOI] [PubMed] [Google Scholar]
- 25.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005; 293:2479-2486. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor‐Locke C, Greer JL, Vezina J, Whitt‐Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011; 43:1575-1581. [DOI] [PubMed] [Google Scholar]
- 27.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988; 318:129-133. [DOI] [PubMed] [Google Scholar]
- 28.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006; 296:1593-1601. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhof P, Fabritz L, Zwiener M, Witt H, Schäfers M, Zellerhoff S, Paul M, Athai T, Hiller KH, Baba HA, Breithardt G, Ruiz P, Wichter T, Levkau B. Age‐ and training‐dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin‐deficient mice. Circulation. 2006; 114:1799-1806. [DOI] [PubMed] [Google Scholar]
- 30.Douglas PS, O'Toole ML, Hiller WD, Reichek N. Different effects of prolonged exercise on the right and left ventricles. J Am Coll Cardiol. 1990; 15:64-69. [DOI] [PubMed] [Google Scholar]
- 31.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H, Prior DL. Exercise‐induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012; 33:998-1006. [DOI] [PubMed] [Google Scholar]
- 32.Benito B, Gay‐Jordi G, Serrano‐Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L. Cardiac arrhythmogenic remodeling in a rat model of long‐term intensive exercise training. Circulation. 2011; 123:13-22. [DOI] [PubMed] [Google Scholar]
- 33.Breuckmann F, Möhlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, Sievers B, Schlosser T, Jöckel KH, Heusch G, Erbel R, Barkhausen J. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009; 251:50-57. [DOI] [PubMed] [Google Scholar]
- 34.Wilson M, O'Hanlon R, Prasad S, Deighan A, Macmillan P, Oxborough D, Godfrey R, Smith G, Maceira A, Sharma S, George K, Whyte G. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol. 2011; 110:1622-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vite A, Radice GL. N‐cadherin/catenin complex as a master regulator of intercalated disc function. Cell Commun Adhes. 2014; 21:169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Hengel J, Calore M, Bauce B, Dazzo E, Mazzotti E, De Bortoli M, Lorenzon A, Li Mura IE, Beffagna G, Rigato I, Vleeschouwers M, Tyberghein K, Hulpiau P, van Hamme E, Zaglia T, Corrado D, Basso C, Thiene G, Daliento L, Nava A, van Roy F, Rampazzo A. Mutations in the area composita protein αT‐catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2013; 34:201-210. [DOI] [PubMed] [Google Scholar]
- 37.Sen‐Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007; 115:1710-1720. [DOI] [PubMed] [Google Scholar]
- 38.Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005; 112:3823-3832. [DOI] [PubMed] [Google Scholar]


