Abstract
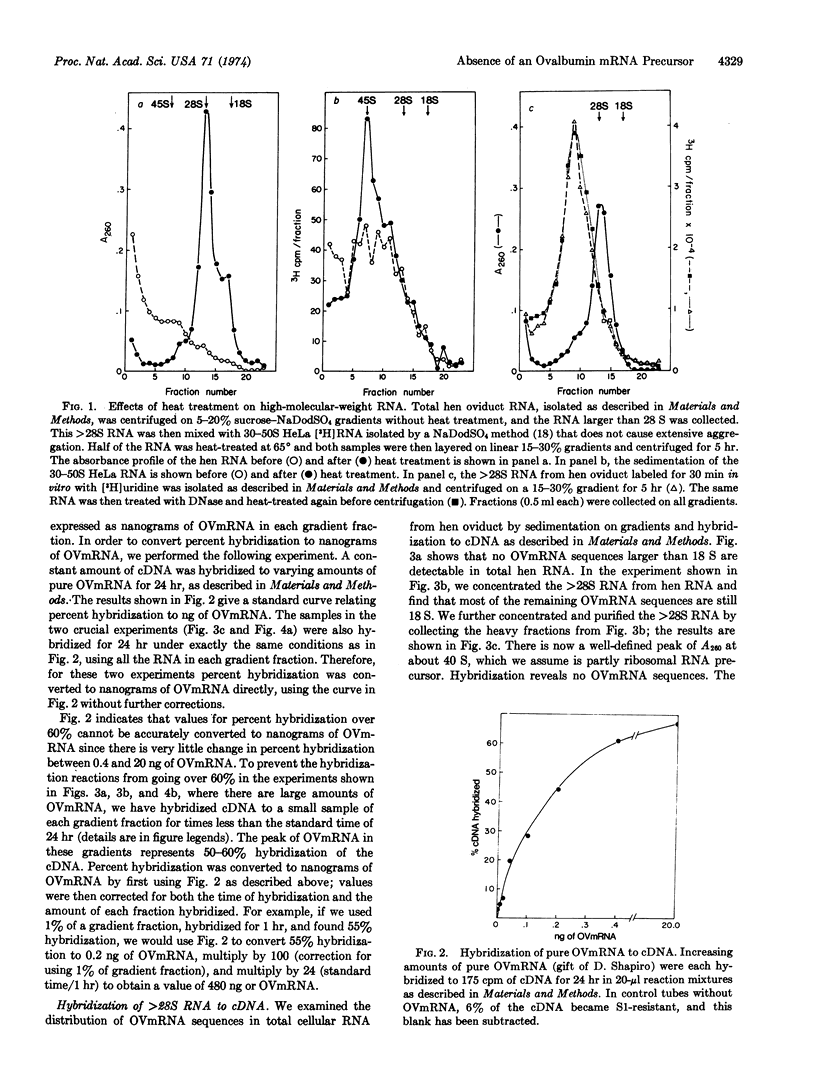
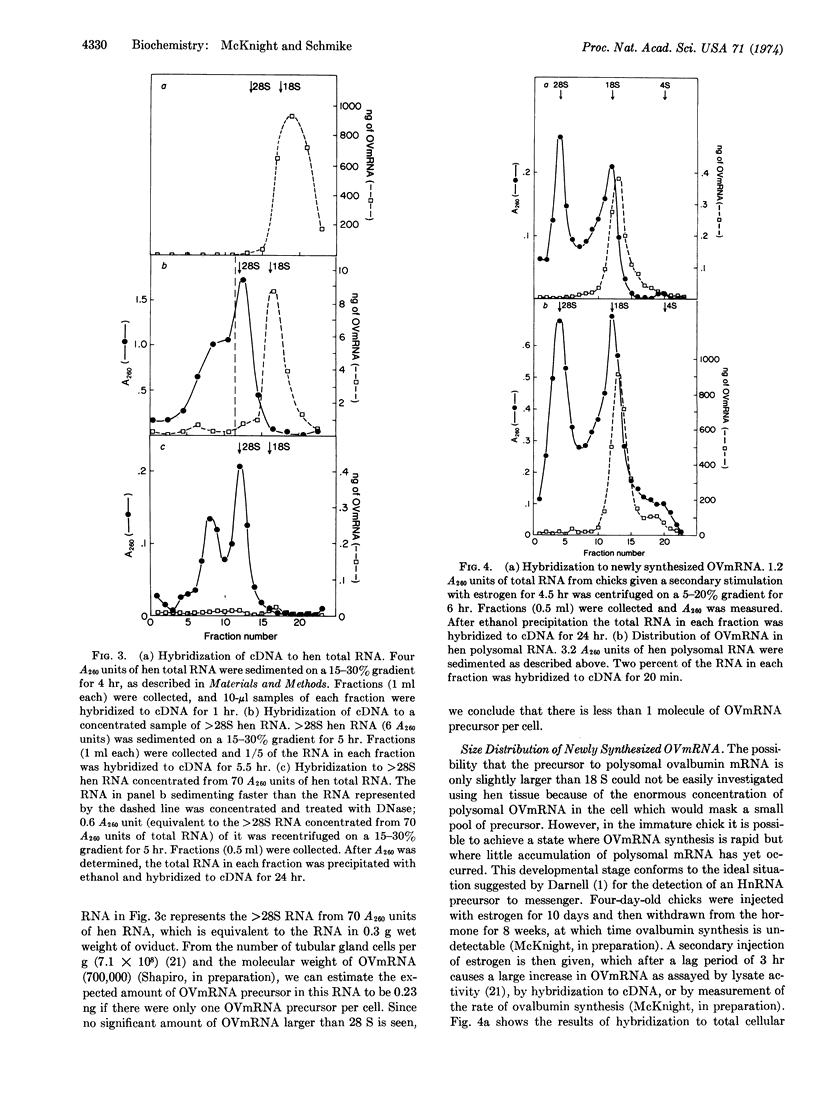
The messenger RNA for ovalbumin, the major secretory protein of the chick oviduct, appears not to be made as a high-molecular-weight precursor when artifacts due to aggregation are eliminated. No ovalbumin messenger RNA sequences that will hybridize to complementary DNA made against ovalbumin mRNA are found in concentrated samples of hen oviduct RNA larger than 28 S. The sensitivity of the hybridization assay is sufficient to detect less than one molecule of ovalbumin mRNA precursor per tubular gland cell. Newly synthesized ovalbumin messenger RNA isolated from immature chicks stimulated briefly by estrogen is the same size as that found in hen polyribosomes. We conclude that ovalbumin messenger RNA does not undergo any significant change in molecular weight from its initial transcription to its incorporation into polyribosomes.
Keywords: complementary DNA, hybridization, RNA processing, oviduct
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Lodish H. F. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):295–314. doi: 10.1016/0022-2836(73)90007-7. [DOI] [PubMed] [Google Scholar]
- Haines M. E., Carey N. H., Palmiter R. S. Purification and properties of ovalbumin messenger RNA. Eur J Biochem. 1974 Apr 16;43(3):549–560. doi: 10.1111/j.1432-1033.1974.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Identification of discrete polyadenylate-containing RNA components transcribed from HeLa cell mitochondrial DNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):563–567. doi: 10.1073/pnas.71.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Palacios R., Sullivan D., Summers N. M., Kiely M. L., Schimke R. T. Purification of ovalbumin messenger ribonucleic acid by specific immunoadsorption of ovalbumin-synthesizing polysomes and millipore partition of ribonucleic acid. J Biol Chem. 1973 Jan 25;248(2):540–548. [PubMed] [Google Scholar]
- Palmiter R. D., Christensen A. K., Schimke R. T. Organization of polysomes from pre-existing ribosomes in chick oviduct by a secondary administration of either estradiol or progesterone. J Biol Chem. 1970 Feb 25;245(4):833–845. [PubMed] [Google Scholar]
- Palmiter R. D. Ovalbumin messenger ribonucleic acid translation. Comparable rates of polypeptide initiation and elongation on ovalbumin and globin messenger ribonucleic acid in a rabbit reticulocyte lysate. J Biol Chem. 1973 Mar 25;248(6):2095–2106. [PubMed] [Google Scholar]
- Palmiter R. D. Rate of ovalbumin messenger ribonucleic acid synthesis in the oviduct of estrogen-primed chicks. J Biol Chem. 1973 Dec 10;248(23):8260–8270. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Ruiz-Carrilo A., Beato M., Schutz G., Feigelson P., Allfrey V. G. Cell-free translation of the globin message within polydisperse high-molecular-weight ribonucleic acid of avian erythrocytes. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3641–3645. doi: 10.1073/pnas.70.12.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D., Palacios R., Stavnezer J., Taylor J. M., Faras A. J., Kiely M. L., Summers N. M., Bishop J. M., Schimke R. T. Synthesis of a deoxyribonucleic acid sequence complementary to ovalbumin messenger ribonucleic acid and quantification of ovalbumin genes. J Biol Chem. 1973 Nov 10;248(21):7530–7539. [PubMed] [Google Scholar]
- Williamson R., Drewienkiewicz C. E., Paul J. Globin messenger sequences in high molecular weight RNA from embryonic mouse liver. Nat New Biol. 1973 Jan 17;241(107):66–68. doi: 10.1038/newbio241066a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]



