Abstract
Background
Questions remain concerning to what extent age and sex may modify the suggested association between psoriasis and the metabolic syndrome in the general population.
Objectives
To investigate the association between psoriasis and the metabolic syndrome within a large population-based cohort by age and sex.
Methods
A cross-sectional study including 10 521 participants aged 30–79 years from the Tromsø Study cohort was performed; 1137 participants reported lifetime psoriasis of a mainly mild character. The new harmonized definition of metabolic syndrome was used in the multivariable logistic regression analysis.
Results
There was a uniformly higher prevalence of metabolic syndrome in men and women with psoriasis compared with those without across all age groups. In women, psoriasis was associated with a 3·8-times higher odds of metabolic syndrome at age 30 years (95% confidence interval 1·5–9·7), with a decreasing odds ratio with increasing age. In men, psoriasis was associated with a stable 1·35-times higher odds of metabolic syndrome (95% confidence interval 1·1–1·6) at all ages. Abdominal obesity was the most frequent metabolic syndrome component in women in this study, and there was indication of a dose–response relationship between psoriasis severity, indicated through treatment, and having a high waistline in women.
Conclusions
This study suggests age and sex variations in the risk of metabolic syndrome among individuals with psoriasis. Given the high prevalence of psoriasis and the significantly elevated burden of metabolic syndrome in this patient group, there may be a benefit from targeted screening of metabolic syndrome among individuals with psoriasis regardless of age and disease severity.
What's already known about this topic?
Evidence suggests an association between psoriasis and the metabolic syndrome.
Population-based studies including mild-to-severe cases, age and sex variation, and information on confounding factors are scarce.
What does this study add?
In this population-based study individuals reporting psoriasis displayed elevated prevalence of metabolic syndrome.
The strongest association was present in young women, where those with psoriasis had up to fourfold increased odds of metabolic syndrome, independent of lifestyle risk factors.
The results support a possible benefit from targeted screening for metabolic risk factors in all patients with psoriasis, irrespective of age and disease severity.
There is growing evidence of an association between the chronic relapsing inflammatory skin disease psoriasis, and the obesity-related systemic inflammatory and prothrombotic condition metabolic syndrome (MetS).1–4 MetS is a cluster of risk factors associated with a doubling of cardiovascular disease risk and a five times greater risk for developing type 2 diabetes.5 Obesity and MetS have reached pandemic proportions during the last few decades.6,7 Meanwhile, studies suggest a parallel increase in the prevalence and incidence of psoriasis in some populations,8–11 with the highest prevalence being reported among adults in Scandinavia.9,12,13 However, important questions remain concerning the link between psoriasis and MetS in the general unselected population.1,14,15
Two recent meta-analyses including heterogeneous observational studies reported a pooled odds ratio (OR) for MetS of 1·8–2·3 among subjects with psoriasis compared with their reference groups.3,16 A limitation of these analyses was the scarcity of studies with data on the natural spectrum of psoriasis among adults outside of clinical settings or patient databases, including uniform screening procedures for psoriasis and MetS, and adequate adjustment for confounding lifestyle factors.16–18 There was also indication of publication bias.3,16 To our knowledge, only two population-based health surveys investigating this association have been performed. A U.S. survey reported a twofold increase in the odds of MetS among persons with psoriasis, and suggested that the association may be restricted to women,1 while a study from Denmark reported no association.13 Interestingly, age and sex modification of the association between psoriasis and the odds of MetS was observed in a U.K. general practice database study, with the highest effect estimates in middle-aged subjects and women.2
Expert groups have long recommended screening for cardiovascular risk factors in patients with psoriasis.19,20 However, studies from the clinic and insurance databases vs. general population cohorts investigating cardiovascular risk factors and end points have shown partly conflicting results, and it is not clear whether the suggested increased cardiovascular risk also applies to persons with mild or inactive psoriasis.16,21–23 Thus, the recent U.S. public health agenda for psoriasis points to the need for more population-based cohort studies analysing the age and sex disparities of relationships with cardiovascular risk factors and disease,15 and underlines that there is not a sufficient evidence base to recommend targeted preventive measures for patients with psoriasis.
The purpose of this study was to describe the association between psoriasis and MetS according to age and sex in a general adult population including a large age range, uniform screening procedures and clinical measurements.
Patients and methods
Study population and design
The Tromsø Study is a single-centre multipurpose population-based study with repeated high-quality health surveys of inhabitants in the subarctic municipality of Tromsø, Norway, population approximately 65 000 (2007).24 The design and cohort profile have been described in detail.25,26 In total 9625 men and 10 137 women aged 30–87 years were invited to the sixth Tromsø Study in 2007–8, and 12 984 participants of mainly white descent attended (66%).26 Two participants have since withdrawn their consent.
Trained health professionals at the screening centre conducted clinical examinations (height, weight, hip and waist circumference, blood pressure) and collected blood samples according to standardized procedures. Details concerning the measurements, biological specimens and analytical methods have been published elsewhere.26 Two questionnaires were used to collect data on general health, education, disease, medication use and lifestyle (Data S1; see Supporting Information).24,26
All subjects over the age of 79 years (n = 531), pregnant women (n = 28) and participants with missing data on self-reported psoriasis (n = 1176) or measured MetS components (n = 726) were excluded, giving a total of 10 521 individuals (5499 women and 5022 men) for the present cross-sectional analysis.
Ethics
Each participant gave written informed consent prior to the examinations. The study was approved by the Norwegian Data Protection Authority and the Regional Committee for Medical and Health Research Ethics, North Norway.
Psoriasis diagnosis and severity
Psoriasis was assessed by self-report of lifetime psoriasis using the following questions: ‘Do you have or have you ever had psoriasis?’ and/or ‘Have you ever been diagnosed with psoriasis by a physician?’ Answers to both questions were coded as yes or no, and those answering yes to one or both questions were regarded as having psoriasis. Self-reported present severity of psoriasis was assessed through rating current symptoms using a scale from 0–10 (Data S1; see Supporting Information).
Information on medication from the Norwegian Prescription Database was used as a proxy for disease severity (Data S1).
Metabolic syndrome
MetS was assessed using a slightly adapted version of the unified definition of the International Diabetes Federation and the National Heart, Lung and Blood Institute, and others.5 In order to be defined as having MetS, participants were required to have at least three of the following five criteria related to MetS: (i) central obesity defined by large waist circumference (WC), (ii) raised triglycerides, (iii) reduced high-density lipoprotein cholesterol, (iv) raised blood pressure and (v) raised fasting glucose. Due to the use of nonfasting blood samples, serum glycated haemoglobin was used instead of glucose (Data S1; see Supporting Information).
Central obesity was defined using the traditional cut-off values WC ≥ 102 cm in men and ≥ 88 cm in women of white descent (WChigher), as well as the newly presented lower cut-off values WC ≥ 94 cm in men and ≥ 80 cm in women (WClower).27 For most analyses, data were presented using both waistline cut-offs in order to compare the data with prior studies, as well as to see how the effect estimates depend on the definition used. For a subset of analyses, the WC variable most strongly associated with psoriasis was presented for simplification.
Statistical analysis
Due to indicated sex differences in the data, most analyses were stratified by sex. Descriptive characteristics are reported as means ± SDs for continuous variables and proportions for binary variables. Age-adjusted differences in means or proportions between individuals with and without psoriasis were assessed using linear or logistic regression models. Comparisons of the prevalence of MetS and its components by psoriasis status were done in age-stratified analysis (30–44, 45–59 and 60–79 years), according to an approximate cut-off for premenopause in women (< 45 years) and premature cardiovascular disease (< 60 years). The OR for MetS according to the presence of psoriasis was assessed in an age-adjusted logistic regression model (n = 10 521/n = 9 662), and in a multivariable model adjusted for age, sex, smoking, educational level and recreational physical activity (n = 9662, observations with missing covariates excluded) (Data S1; see Supporting Information). Alcohol and statin use did not affect the analysis and were not included in the final model. In order to account for a possible nonlinear effect of age, and for age being an effect modifier, age was also modelled using second-degree fractional polynomial terms. The best-fitting fractional polynomials were of degree 2 with powers equal to 1 and 3 for women and degree 1 with power equal to 1 for men. The main effect of age terms and two-way interactions with psoriasis were included in the models.
For the investigation of a possible dose–response relationship between psoriasis and MetS, medication was used as a surrogate marker of psoriasis severity (continuous variable of disease severity: 1, no prescription; 2, prescription without systemic medication; 3, prescription with systemic medication), and WChigher as a surrogate end point of MetS in the age-adjusted logistic regression model. Due to a restricted set of variables when linking our data with the Norwegian Prescription Database, full relationship between psoriasis severity and MetS could not be evaluated (Data S1).
All P-values were two sided using a 5% significance level. The analyses were performed with SAS 9·2 (SAS Institute Inc., Cary, NC, U.S.A.) and SPSS 20 (IBM, Armonk, NY, U.S.A.). Data were reported according to the STROBE criteria.
Results
Among the 5499 women, with a mean age 55·9 years, the mean body mass index (BMI) was 26·5 kg m−2, and the mean WC was 90·6 cm. The 5022 men had a mean age of 56·5 years, a mean BMI of 27·3 kg m−2 and a mean WC of 99·3 cm. In total 1137 participants reported lifetime psoriasis, giving an overall prevalence of 10·8%, 10·1% in women and 11·6% in men (P = 0·019). Among 550 women and 569 men with psoriasis also answering the question on doctor's diagnosis, 91% of women and 84% of men confirmed a doctor's diagnosis (P ≤ 0·001). Overall 828 participants with psoriasis (73%) provided data on self-reported present psoriasis severity; 16% stated no symptoms, 56% mild, 23% moderate and 5% severe symptoms. Furthermore, 584 individuals (49% of those with appropriate data) received a registered prescription indicative of psoriasis during the time period 2006–9; systemic drugs were prescribed to 66 patients (6%) in total (Data S1; see Supporting Information). Psoriasis was associated with older age, lower educational level and current smoking (Table 1). Among women, psoriasis was also associated with low leisure-time physical activity. Psoriasis was positively associated with markers of adiposity and high-sensitivity C-reactive protein (hs-CRP) (Table 2). In women, psoriasis was also associated with more unfavourable serum lipid and glucose profiles, as well as higher diastolic blood pressure.
Table 1.
Characteristics of the study population: women and men with and without psoriasis (n = 10 521)
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Psoriasis | No psoriasis | P-valuea | Psoriasis | No psoriasis | P-valuea | |
| Numberb | 557 | 4942 | 580 | 4442 | ||
| Age (years) | 57·3 ± 11·0 | 55·7 ± 11·9 | 0·003 | 57·6 ± 11·5 | 56·3 ± 11·2 | 0·01 |
| Living with spouse | 70·5 | 72·0 | 0·45 | 84·1 | 82·8 | 0·44 |
| College/university education | 31·9 | 39·0 | 0·002 | 37·1 | 42·1 | 0·03 |
| Low income | 23·4 | 21·1 | 0·22 | 13·8 | 12·9 | 0·49 |
| Present use of statins | 11·2 | 11·2 | 0·99 | 18·1 | 15·6 | 0·10 |
| Present smoker | 31·0 | 20·1 | < 0·001 | 22·8 | 18·6 | 0·02 |
| Alcohol higher | 19·9 | 21·2 | 0·48 | 23·7 | 25·5 | 0·33 |
| Low leisure activity | 22·0 | 18·0 | 0·03 | 21·8 | 20·4 | 0·44 |
Data from the sixth Tromsø Study. Values are age-adjusted percentages or means ± SD.
Means were assessed by the generalized linear model, binary logistics for dichotomous variables or scale response for linear variables, adjusted for age.
Totals may vary due to missing information.
P-values were generated through Wald statistics. Low income, proportion with approximately lowest income quartile; low leisure activity, proportion reporting mostly sedentary leisure-time activity; alcohol higher, proportion with alcohol intake at least 2–3 times a week.
Table 2.
Mean ± SD data of metabolic risk factors in women and men with and without psoriasis (n = 10 521)
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Psoriasis | No psoriasis | P-valuea | Psoriasis | No psoriasis | P-valuea | |
| Weight (kg) | 72·4 ± 13·1 | 70·9 ± 13·1 | 0·01 | 86·5 ± 13·8 | 85·6 ± 13·0 | 0·11 |
| Body mass index (kg m−2) | 27·1 ± 4·6 | 26·4 ± 4·6 | 0·002 | 27·6 ± 3·9 | 27·2 ± 3·7 | 0·03 |
| Waist circumference (cm) | 92·3 ± 12·2 | 90·4 ± 12·1 | 0·001 | 100·3 ± 11·0 | 99·2 ± 10·4 | 0·01 |
| Waist-to-hip ratio | 0·88 ± 0·07 | 0·87 ± 0·07 | < 0·001 | 0·96 ± 0·06 | 0·95 ± 0·07 | 0·003 |
| Systolic blood pressure (mmHg) | 133·1 ± 24·7 | 132·0 ± 23·8 | 0·22 | 137·5 ± 19·6 | 137·4 ± 20·2 | 0·95 |
| Diastolic blood pressure (mmHg) | 75·8 ± 10·3 | 74·7 ± 10·1 | 0·01 | 80·8 ± 10·4 | 81·3 ± 10·2 | 0·24 |
| Triglycerides (mmol L−1) | 1·54 ± 0·9 | 1·35 ± 0·7 | < 0·001 | 1·75 ± 1·0 | 1·67 ± 1·0 | 0·06 |
| Total cholesterol (mmol L−1) | 5·79 ± 1·1 | 5·66 ± 1·1 | 0·006 | 5·55 ± 1·1 | 5·52 ± 1·1 | 0·53 |
| LDL-cholesterol (mmol L−1) | 3·64 ± 1·0 | 3·52 ± 1·0 | 0·003 | 3·59 ± 0·9 | 3·57 ± 0·9 | 0·50 |
| HDL-cholesterol (mmol L−1) | 1·59 ± 0·4 | 1·66 ± 0·4 | 0·001 | 1·34 ± 0·4 | 1·36 ± 0·4 | 0·15 |
| Nonfasting glucose (mmol L−1) | 5·16 ± 1·2 | 5·08 ± 1·0 | 0·06 | 5·40 ± 1·3 | 5·38 ± 1·4 | 0·73 |
| HbA1c (%) | 5·62 ± 0·6 | 5·56 ± 0·6 | 0·006 | 5·70 ± 0·7 | 5·68 ± 0·7 | 0·56 |
| hs-CRP (mg L−1) | 3·07 ± 6·2 | 2·32 ± 4·3 | < 0·001 | 2·99 ± 4·9 | 2·37 ± 4·5 | 0·002 |
Data from the sixth Tromsø Study. Data may be from varying totals due to missing information.
Means were assessed by the generalized linear model, scale response for continuous variables, adjusted for age.
P-values were generated through Wald statistics. LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; HbA1c, glycated haemoglobin; hs-CRP, serum high-sensitivity C-reactive protein.
MetS, explored using the two established WC criteria (WChigher and WClower), was more frequent in men, 28% and 37%, than in women, 23% and 27%, respectively; the P-value for the difference between sexes was < 0·001 using both WC cut-offs. Psoriasis was associated with a higher prevalence of MetS in women of all age groups; however, statistical significance was not reached in middle-aged women using WChigher (Fig. 1). The difference in prevalence was largest in women aged 30–44 years and thereafter slightly decreased and remained stable. In men, the difference in prevalence of MetS was almost uniform across all age groups and reached statistical significance from middle age, depending on the waist criteria.
Fig 1.
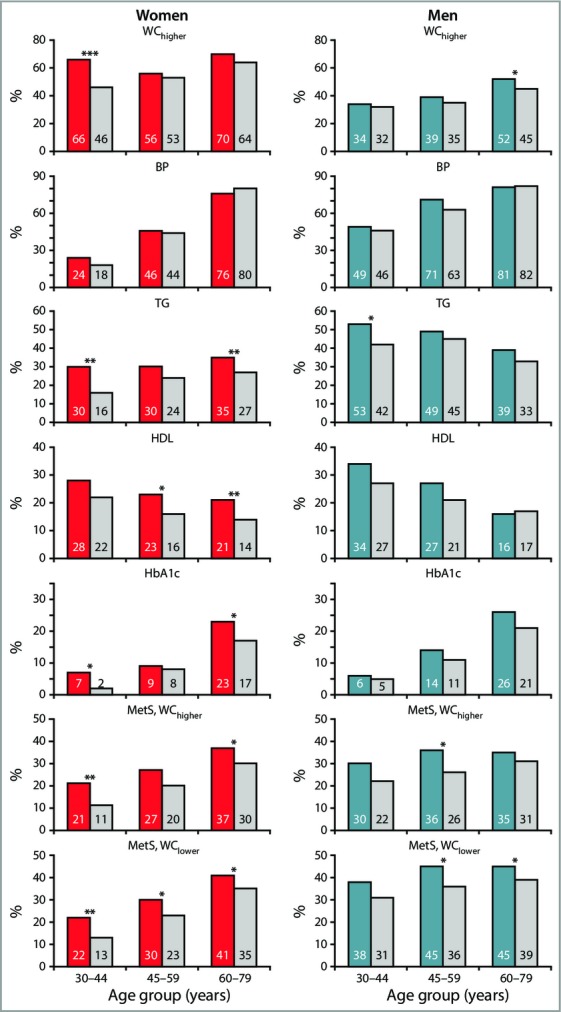
Prevalence of metabolic syndrome (MetS) components and the full syndrome by sex and age, the sixth Tromsø Study. Women, n = 5499: red bars, psoriasis; grey bars, no psoriasis. Men, n = 5022: blue bars, psoriasis; grey bars, no psoriasis. In the study sample (n = 10 521), the number of individuals with/without psoriasis within strata of women was: 30–44 years, 99/1249; 45–59 years, 176/1461; 60–79 years, 282/2232; and men: 30–44 years, 105/996; 45–59 years, 173/1342; 60–79 years, 302/2104. WChigher, waist circumference women ≥ 88 cm and men ≥ 102 cm. WClower, waist circumference women ≥ 80 cm and men ≥ 94 cm. BP, systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg, or treatment for hypertension; TG, triglyceride level ≥ 1·7 mmol L−1; HDL, high-density lipoprotein cholesterol < 1·03 mmol L−1 (male) and < 1·29 mmol L−1 (female); HbA1c, glycated haemoglobin ≥ 6·1% or treatment for diabetes. MetS, WChigher, MetS using WChigher. MetS, WClower, MetS using WClower. *P < 0·05; **P < 0·01; ***P < 0·001.
In the total population, 33% of individuals with psoriasis, vs. 25% of those without, had MetS defined using WChigher. In the corresponding age-adjusted logistic regression analysis, the odds of MetS were 43% higher among individuals with psoriasis [n = 10 521, OR 1·43, 95% confidence interval (CI) 1·25–1·63; for 9662 individuals with complete data on covariates, OR 1·41, 95% CI 1·22–1·62]. This association persisted when the model was further adjusted for sex, smoking, physical activity and educational level (n = 9662, OR 1·35, 95% CI 1·17–1·56).
Due to the indicated interaction between age and sex we used fractional polynomial models to explore further the association between psoriasis and MetS using WChigher (Fig. 2; test for interaction between sex and psoriasis: overall P = 0·06; age < 40 years P = 0·056; age ≥ 40 years P = 0·61). We observed a U-shaped pattern among women, with significantly increased odds in younger women and nonsignificantly increased odds in older women (multivariable model, test for psoriasis–age interaction in women, P = 0·10). In women, the strongest association was observed at age 30 years, where psoriasis was associated with a 3·8-fold increased odds of MetS defined by WChigher (Fig. 1 and Table 3). In men, the multivariable adjusted OR for MetS in men with vs. without psoriasis did not vary by age (test for psoriasis–age interaction, P = 0·67). Thus, men with psoriasis had an approximately 35% increased risk of MetS (OR 1·35, 95% CI 1·11–1·64). Overall, the same patterns were found using both waist criteria; lower ORs were evident in women using WClower, while estimates in men were almost unchanged (Table 3).
Fig 2.
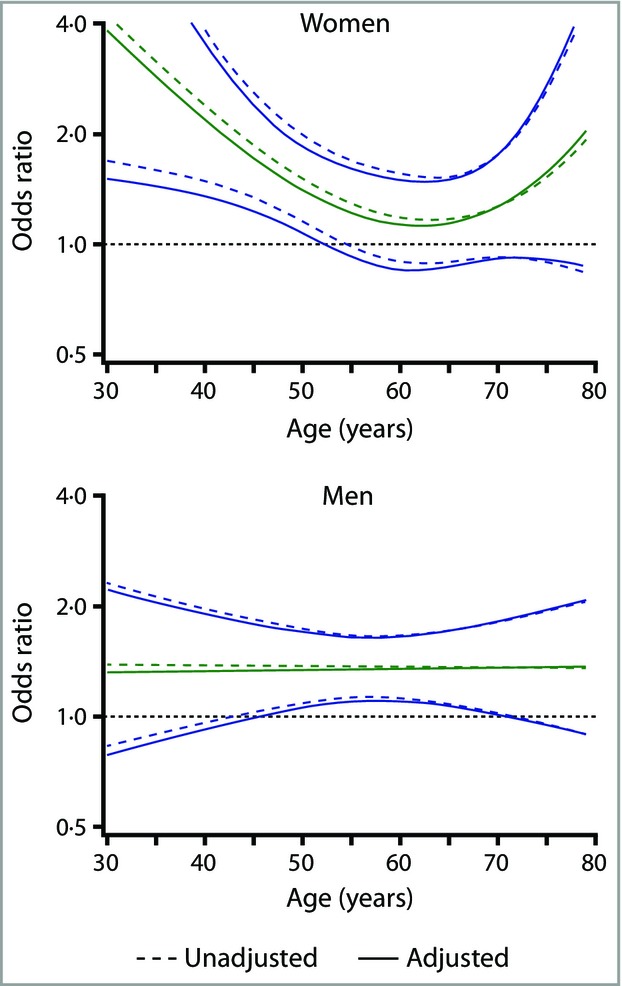
Odds ratios for metabolic syndrome (green line) with 95% confidence intervals (blue lines) among women and men with psoriasis compared with the reference population; data from the sixth Tromsø Study. Metabolic syndrome was defined using the higher waist circumference criteria (women ≥ 88 cm; men ≥ 102 cm). The fractional polynomial model was unadjusted (dotted lines; women, n = 5499; men, n = 5022) and adjusted for smoking, educational level and physical activity in leisure time (solid lines; women, n = 4971; men, n = 4691).
Table 3.
Odds ratios and 95% confidence intervals for metabolic syndrome (MetS) in persons with psoriasis compared with persons without psoriasis, estimated from logistic regression analysis with age modelled using second-degree fractional polynomial terms
| Total n | 30 years | 40 years | 50 years | 60 years | 70 years | 79 years | |
|---|---|---|---|---|---|---|---|
| Women | |||||||
| MetS, WChigher | |||||||
| Unadjusted | 5499 | 3·86 (1·59–9·38) | 2·27 (1·43–3·61) | 1·50 (1·16–1·95) | 1·23 (0·95–1·60) | 1·39 (1·04–1·86) | 2·23 (1·03–4·81) |
| Unadjusteda | 4971 | 4·21 (1·68–10·54) | 2·38 (1·48–3·83) | 1·51 (1·15–1·97) | 1·18 (0·89–1·56) | 1·27 (0·92–1·74) | 1·92 (0·83–4·42) |
| Adjustedb | 4971 | 3·82 (1·51–9·66) | 2·18 (1·35–3·53) | 1·40 (1·07–1·84) | 1·13 (0·85–1·50) | 1·27 (0·92–1·75) | 2·03 (0·87–4·75) |
| MetS, WClower | |||||||
| Unadjusted | 5499 | 2·98 (1·25–7·10) | 1·99 (1·27–3·12) | 1·45 (1·12–1·86) | 1·24 (0·96–1·60) | 1·35 (1·02–1·80) | 1·92 (0·90–4·10) |
| Unadjusteda | 4971 | 3·21 (1·31–7·88) | 2·07 (1·30–3·29) | 1·45 (1·12–1·89) | 1·21 (0·92–1·58) | 1·28 (0·93–1·74) | 1·76 (0·78–4·01) |
| Adjustedb | 4971 | 2·89 (1·16–7·16) | 1·89 (1·18–3·02) | 1·35 (1·04–1·77) | 1·15 (0·88–1·52) | 1·27 (0·92–1·74) | 1·84 (0·80–4·22) |
| Men | |||||||
| MetS, WChigher | |||||||
| Unadjusted | 5022 | 1·50 (0·92–2·47) | 1·45 (1·03–2·05) | 1·40 (1·12–1·75) | 1·35 (1·12–1·63) | 1·30 (0·99–1·71) | 1·26 (0·85–1·87) |
| Unadjusteda | 4691 | 1·38 (0·83–2·31) | 1·38 (0·96–1·97) | 1·37 (1·09–1·73) | 1·37 (1·12–1·66) | 1·36 (1·02–1·81) | 1·36 (0·89–2·05) |
| Adjustedb | 4691 | 1·32 (0·78–2·21) | 1·33 (0·92–1·91) | 1·34 (1·06–1·69) | 1·35 (1·10–1·65) | 1·36 (1·01–1·82) | 1·37 (0·90–2·08) |
| MetS, WClower | |||||||
| Unadjusted | 5022 | 1·36 (0·85–2·17) | 1·36 (0·98–1·89) | 1·37 (1·10–1·69) | 1·37 (1·14–1·64) | 1·37 (1·04–1·78) | 1·38 (0·94–2·01) |
| Unadjusteda | 4691 | 1·35 (0·84–2·19) | 1·35 (0·96–1·89) | 1·34 (1·08–1·67) | 1·34 (1·11–1·61) | 1·33 (1·01–1·76) | 1·33 (0·89–1·98) |
| Adjustedb | 4691 | 1·30 (0·80–2·12) | 1·31 (0·93–1·84) | 1·31 (1·05–1·64) | 1·32 (1·09–1·59) | 1·32 (1·00–1·75) | 1·33 (0·89–1·99) |
Data from the sixth Tromsø Study.
Unadjusted analysis including individuals with complete information on covariates used in the adjusted analysis.
Adjusted model includes smoking, physical activity in leisure time and educational level.
MetS, WChigher, harmonized definition of MetS using the higher waist circumference criteria (women ≥ 88 cm; men ≥ 102 cm). MetS, WClower, harmonized definition of MetS using the lower waist circumference criteria (women ≥ 80 cm; men ≥ 94 cm).
A sensitivity analysis with a redefined definition of the exposure variable was made; only participants reporting a doctor's diagnosis were considered to have psoriasis, and participants with only self-report of diagnosis were excluded. The psoriasis−MetS association remained almost unchanged in multivariable analyses (WChigher; total n = 9566; cases n = 886; OR 1·33, 95% CI 1·14–1·55). The same patterns were observed in both sexes (data not shown).
To assess present medication use as a proxy for severity of psoriasis, we used data from the Norwegian Prescription Database; we observed a dose–response relationship between psoriasis treatment in women with psoriasis (n = 587), and odds of high WC in age-adjusted analysis (WChigher; OR 1·33, 95% CI 1·00–1·77). There was no such association in men with psoriasis (n = 605).
Discussion
In this population-based study, including cases of mainly mild psoriasis, there was indication that the association between psoriasis and risk of MetS was modified by age and sex. There was a uniformly higher prevalence of MetS in men and women with psoriasis than in those without across all age groups. However, the strongest association between psoriasis and MetS was found in young women, with almost fourfold increased odds. While the odds decreased with age in women, men with psoriasis had a 35% increase in the odds of MetS compared with men without psoriasis across all ages. These associations were independent of potentially confounding lifestyle factors. Our data suggest a potential biological interaction between sex and psoriasis. However, the broad CIs, particularly in the youngest women, demand caution in the interpretation of the age and sex patterns.
This study included the natural spectrum of psoriasis in adults with both active and inactive disease. Further strengths include to our knowledge the largest sample from a general population survey to date, with a wide age range, high attendance, comprehensive assessment of lifestyle factors, and clinical examinations using standardized and validated methods by trained health professionals, performed within a short time frame.26 Also, MetS was assessed using the new harmonized criteria including two cut-off values for measured WC.
As the study is based on a general health survey with no publicity to recruit individuals with psoriasis, we may assume that selection bias in terms of psoriasis diagnosis was minimal.28 However, there were relatively few persons invited and even fewer attending aged < 45 years, with attendance of 44% in men and 58% in women. This gives greater uncertainty to the validity of the estimates from the youngest age groups, mainly in men. Longitudinal studies have demonstrated a tendency to recruit healthier individuals.25,26,29 Thus, overweight participants may be under-represented in health surveys,29 which may lead to attenuation of the effect estimates. When excluding individuals with incomplete information on covariates, there were no evident changes in the age-adjusted ORs (Table 3), indicating limited bias due to missing data.
Psoriasis has a major impact on a person's quality of life.30,31 This may lead to unhealthy lifestyle choices, which in turn increase the risk of several diseases including MetS. This study evolves the present knowledge of the psoriasis−MetS relationship, by its thorough adjustment of important lifestyle confounders, something that has been missing in most studies investigating this association.15,17 However, factors that have not been evaluated in this study, including genetic predisposition, mental health and diet, may lead to residual confounding of the association. Also, a potential added effect on MetS from concomitant psoriasis arthritis could not be investigated. All blood samples were taken nonfasting, which could lead to nondifferential misclassification of serum lipid levels, and bias the psoriasis−MetS association towards the null value. Several drugs given for psoriasis are known to induce weight gain or affect the blood lipid profile. Due to the low number of persons on systemic drugs in this study this was not investigated further.
Self-report of data is a widely used method in epidemiological studies of skin disease;1,13,32,33 however, there are concerns about the accuracy of psoriasis diagnosis with this approach (Data S1; see Supporting Information).9 Approximately 90% of cases have classical plaque phenotypes,34 which are adequately diagnosed by trained general practitioners,21,35,36 who care for the majority of patients with psoriasis in Norway. A recent population-based Norwegian study similar to the Tromsø Study found acceptable overlap between self-reported psoriasis diagnosis through a questionnaire and general practitioner medical records (Data S1).29 Also, several studies have pointed out that more than half of cases of mild psoriasis may go undiagnosed by a doctor,37–39 something that could potentially attenuate the effect estimates. It is unlikely that a misclassification of the diagnosis explains the higher odds of MetS among persons with psoriasis in this cohort. One might expect an even stronger association given a more specific definition of psoriasis.1,40 However, limiting the analysis to include only participants reporting a physician's diagnosis led to a slight reduction in the effect estimates. This may reflect a detection bias in physician's diagnosis, where persons with a less favourable metabolic profile consult their physician less frequently.
The results of this study confirm findings from the smaller North American National Health and Nutrition Examination Survey (NHANES),1 as well as the large-scale U.K. general practice database;2 these studies also suggested that the association between psoriasis and MetS may be stronger among women. Despite having a database design, a limited age range, different assessment criteria for MetS and incomplete information on potential confounders, the U.K. study reported an adjusted OR of 1·41, which is very similar to our finding, and MetS was found in 34% of individuals with psoriasis vs. 26% of controls.2 Comparably, in our population, 33–39% of participants with psoriasis vs. 25–31% of controls were affected, depending on the used WC criteria, supporting the external validity of the findings. In the NHANES study, including younger subjects, 40% of patients with psoriasis vs. 23% of controls fulfilled the definition of MetS. However, the U.S. study population had an increased WC compared with the Tromsø population,1 while a Danish study, which found no difference in the distribution of MetS in persons with and without psoriasis, had a lower mean WC.13
In our data the absolute difference in the prevalence of MetS between individuals with and without psoriasis remains fairly stable into old age in both sexes. The mechanisms contributing to the U-shaped pattern by age in the psoriasis-associated odds of MetS in women are not clearly understood (Fig. 2). Abdominal obesity, which was the most frequent MetS component in women in this study, is also a key initiator of insulin resistance,41 and for a given level of WC women exhibit more adverse metabolic disturbances than men.42 In women aged 30–44 years, approximately 75% of those with psoriasis and 45% of those without psoriasis had a WC ≥ 88 cm. There have been large secular changes in lifestyle in the Tromsø Study population, and younger female birth cohorts have increasingly higher BMI and WC compared with prior generations.9,24,43 Among the elderly the prevalence of MetS, and hypertension in particular, is high; about 80% of this age group have hypertension, independent of their psoriasis status. This age-related increase in metabolic components is driven primarily by factors other than psoriasis, possibly attenuating the OR estimates. Furthermore, the suggested higher cardiovascular mortality among individuals with psoriasis44,45 could produce lower effect estimates in the older age groups, due to a healthy survivor bias.
Interestingly, there was indication of a dose–response relationship between psoriasis severity, indicated through treatment, and an increased waistline in women. This is in line with findings of a dose-dependent increase in the odds of MetS and its components by disease severity from the U.K. general practice database.2 Due to our restricted dataset, the full association between MetS and psoriasis could unfortunately not be established. However, abdominal overweight is known to be the key component of MetS.41,42 The high discrepancy in waistline between individuals with and without psoriasis in young and middle-aged women compared with men may explain why this association could be established only in women.
Longitudinal studies suggested that obesity-related chronic inflammation may increase the risk of developing psoriasis.46,47 Evidence also indicates that weight loss and reduction in WC may improve psoriasis disease severity.48,49 Insulin resistance has been seen to block keratinocyte differentiation, and may contribute to the pathogenesis of both psoriasis and metabolic and cardiovascular disease.50–52 The cross-sectional design of this study limits the possibility of drawing conclusions in terms of causality. However, it seems unlikely that the substantially elevated prevalence of MetS among patients with psoriasis in this cohort is due to the inflammatory load from the psoriasis plaques alone.53 Although women with psoriasis displayed a particularly unfavourable metabolic profile, both sexes had substantially elevated hs-CRP, indicating low-grade systemic inflammation. It is plausible that psoriasis serves as a marker of a common genetic susceptibility, bridging the association between psoriasis and systemic manifestations such as MetS and cardiovascular disease, given the right environmental conditions.54–57
The increased burden of MetS among individuals with psoriasis in this population-based study is grounds for concern from a public health perspective. While the total prevalence of psoriasis is highest in Scandinavia,9,12,13 doubled figures are also reported in the U.S.A. and Asia, suggesting a global trend.8,10 In this cohort with mainly mild psoriasis, the prevalence of MetS is substantially increased in patients with psoriasis, with high odds especially among young women, also after adjusting for relevant confounding factors. These findings support the possibility of a benefit from regular screening for MetS and its components among all adults with psoriasis when visiting their general practitioner or dermatologist, regardless of age and disease severity, in order to reduce their risk of secondary diabetes and cardiovascular disease.
Acknowledgments
We wish to thank the participants and personnel of the sixth Tromsø Study. We are grateful to Rod Wolstenholme, UiT the Arctic University of Norway, for technical assistance in preparing the figures.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Data S1. Supplementary methods, results and discussion.
References
- 1.Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003–2006. Arch Dermatol. 2011;147:419–24. doi: 10.1001/archdermatol.2010.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556–62. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68:654–62. doi: 10.1016/j.jaad.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Goldminz AM, Buzney CD, Kim N, et al. Prevalence of the metabolic syndrome in children with psoriatic disease. Pediatr Dermatol. 2013;30:700–5. doi: 10.1111/pde.12218. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, D'Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 7.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Icen M, Crowson CS, McEvoy MT, et al. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol. 2009;60:394–401. doi: 10.1016/j.jaad.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danielsen K, Olsen AO, Wilsgaard T, Furberg AS. Is the prevalence of psoriasis increasing? A 30-year follow-up of a population-based cohort. Br J Dermatol. 2013;168:1303–10. doi: 10.1111/bjd.12230. [DOI] [PubMed] [Google Scholar]
- 10.Ding X, Wang T, Shen Y, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. 2012;22:663–7. doi: 10.1684/ejd.2012.1802. [DOI] [PubMed] [Google Scholar]
- 11.Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62:979–87. doi: 10.1016/j.jaad.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bo K, Thoresen M, Dalgard F. Smokers report more psoriasis, but not atopic dermatitis or hand eczema: results from a Norwegian population survey among adults. Dermatology. 2008;216:40–5. doi: 10.1159/000109357. [DOI] [PubMed] [Google Scholar]
- 13.Jensen P, Thyssen JP, Zachariae C, et al. Cardiovascular risk factors in subjects with psoriasis: a cross-sectional general population study. Int J Dermatol. 2013;52:681–3. doi: 10.1111/j.1365-4632.2011.05408.x. [DOI] [PubMed] [Google Scholar]
- 14.Ryan C, Korman NJ, Gelfand JM, et al. Research gaps in psoriasis: opportunities for future studies. J Am Acad Dermatol. 2013;70:146–67. doi: 10.1016/j.jaad.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Helmick CG, Sacks JJ, Gelfand JM, et al. Psoriasis and psoriatic arthritis: a public health agenda. Am J Prev Med. 2013;44:424–6. doi: 10.1016/j.amepre.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69:1014–24. doi: 10.1016/j.jaad.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 17.Nijsten T, Wakkee M. Complexity of the association between psoriasis and comorbidities. J Invest Dermatol. 2009;129:1601–3. doi: 10.1038/jid.2009.55. [DOI] [PubMed] [Google Scholar]
- 18.Stern RS, Nijsten T. Going beyond associative studies of psoriasis and cardiovascular disease. J Invest Dermatol. 2012;132:499–501. doi: 10.1038/jid.2011.452. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald VE, Cather JC, Gelfand JM, et al. AJC editor's consensus: psoriasis and coronary artery disease. Am J Cardiol. 2008;102:1631–43. doi: 10.1016/j.amjcard.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Kimball AB, Gladman D, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Am Acad Dermatol. 2008;58:1031–42. doi: 10.1016/j.jaad.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowlatshahi EA, Kavousi M, Nijsten T, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: the Rotterdam Study. J Invest Dermatol. 2013;133:2347–54. doi: 10.1038/jid.2013.131. [DOI] [PubMed] [Google Scholar]
- 22.Li WQ, Han JL, Manson JE, et al. Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. 2012;166:811–18. doi: 10.1111/j.1365-2133.2011.10774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 24.UiT The Arctic University of Norway. The Tromsø Study. Available at: http://www.tromsostudy.com (last accessed 13 December 2014)
- 25.Jacobsen BK, Eggen AE, Mathiesen EB, et al. Cohort profile: the Tromsø Study. Int J Epidemiol. 2012;41:961–7. doi: 10.1093/ije/dyr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggen AE, Mathiesen EB, Wilsgaard T, et al. The sixth survey of the Tromsø Study (Tromsø 6) in 2007–08: collaborative research in the interface between clinical medicine and epidemiology: study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand J Public Health. 2013;41:65–80. doi: 10.1177/1403494812469851. [DOI] [PubMed] [Google Scholar]
- 27.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson TP, Wislar JS. Response rates and nonresponse errors in surveys. JAMA. 2012;307:1805–6. doi: 10.1001/jama.2012.3532. [DOI] [PubMed] [Google Scholar]
- 29.Langhammer A, Krokstad S, Romundstad P, et al. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol. 2012;12:143. doi: 10.1186/1471-2288-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136–9. doi: 10.1046/j.1087-0024.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong AW, Schupp C, Wu J, Bebo B. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLoS ONE. 2012;7:e52935. doi: 10.1371/journal.pone.0052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsen AO, Grjibovski A, Magnus P, et al. Psoriasis in Norway as observed in a population-based Norwegian twin panel. Br J Dermatol. 2005;153:346–51. doi: 10.1111/j.1365-2133.2005.06613.x. [DOI] [PubMed] [Google Scholar]
- 33.Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23–6. doi: 10.1016/j.jaad.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 34.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 35.Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537–41. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 36.Basarab T, Munn SE, Jones RR. Diagnostic accuracy and appropriateness of general practitioner referrals to a dermatology out-patient clinic. Br J Dermatol. 1996;135:70–3. [PubMed] [Google Scholar]
- 37.Jagou M, Bastuji-Garin S, Bourdon-Lanoy E, et al. Poor agreement between self-reported and dermatologists’ diagnoses for five common dermatoses. Br J Dermatol. 2006;155:1006–12. doi: 10.1111/j.1365-2133.2006.07402.x. [DOI] [PubMed] [Google Scholar]
- 38.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009;60:218–24. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima XT, Minnillo R, Spencer JM, Kimball AB. Psoriasis prevalence among the 2009 AAD National Melanoma/Skin Cancer Screening Program participants. J Eur Acad Dermatol Venereol. 2013;27:680–5. doi: 10.1111/j.1468-3083.2012.04531.x. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd edn. Philadelphia: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 41.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–94. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 42.Vega GL, Adams-Huet B, Peshock R, et al. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–66. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 43.Wilsgaard T, Arnesen E. Change in serum lipids and body mass index by age, sex, and smoking status: the Tromsø study 1986–1995. Ann Epidemiol. 2004;14:265–73. doi: 10.1016/j.annepidem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:493–9. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 45.Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167:1345–50. doi: 10.1111/bjd.12002. [DOI] [PubMed] [Google Scholar]
- 46.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med. 2007;167:1670–5. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 47.Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Arch Dermatol. 2007;143:1559–65. doi: 10.1001/archderm.143.12.1559. [DOI] [PubMed] [Google Scholar]
- 48.Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795–801. doi: 10.1001/jamadermatol.2013.722. [DOI] [PubMed] [Google Scholar]
- 49.Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634–42. doi: 10.1111/bjd.12735. [DOI] [PubMed] [Google Scholar]
- 50.Buerger C, Richter B, Woth K, et al. Interleukin-1β interferes with epidermal homeostasis through induction of insulin resistance: implications for psoriasis pathogenesis. J Invest Dermatol. 2012;132:2206–14. doi: 10.1038/jid.2012.123. [DOI] [PubMed] [Google Scholar]
- 51.Capon F, Burden AD, Trembath RC, Barker JN. Psoriasis and other complex trait dermatoses: from loci to functional pathways. J Invest Dermatol. 2012;132:915–22. doi: 10.1038/jid.2011.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20:303–7. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 53.Kimball AB. Psoriasis and cardiovascular disease: another contribution in the hierarchy of evidence. Br J Dermatol. 2012;167:1198–9. doi: 10.1111/bjd.12089. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Begovich AB. Unraveling the genetics of complex diseases: susceptibility genes for rheumatoid arthritis and psoriasis. Semin Immunol. 2009;21:318–27. doi: 10.1016/j.smim.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Tian S, Krueger JG, Li K, et al. Meta-analysis derived (MAD) transcriptome of psoriasis defines the ‘core’ pathogenesis of disease. PLoS ONE. 2012;7:e44274. doi: 10.1371/journal.pone.0044274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Y, Chen H, Nikamo P, et al. Association of cardiovascular and metabolic disease genes with psoriasis. J Invest Dermatol. 2013;133:836–9. doi: 10.1038/jid.2012.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enamandram M, Kimball AB. Psoriasis epidemiology: the interplay of genes and the environment. J Invest Dermatol. 2013;133:287–9. doi: 10.1038/jid.2012.434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary methods, results and discussion.


