Abstract
Implantable ventricular assist devices (VADs) have proven efficient in advanced heart failure patients as a bridge-to-transplant or destination therapy. However, VAD usage often leads to infection, bleeding, and thrombosis, side effects attributable to the damage to blood cells and plasma proteins. Measuring hemolysis alone does not provide sufficient information to understand total blood damage, and research exploring the impact of currently available pumps on a wider range of blood cell types and plasma proteins such as von Willebrand factor (vWF) is required to further our understanding of safer pump design. The extracorporeal CentriMag (Thoratec Corporation, Pleasanton, CA, USA) has a hemolysis profile within published standards of normalized index of hemolysis levels of less than 0.01 g/100 L at 100 mm Hg but the effect on leukocytes, vWF multimers, and platelets is unknown. Here, the CentriMag was tested using bovine blood (n = 15) under constant hemodynamic conditions in comparison with a static control for total blood cell counts, hemolysis, leukocyte death, vWF multimers, microparticles, platelet activation, and apoptosis. The CentriMag decreased the levels of healthy leukocytes (P < 0.006), induced leukocyte microparticles (P < 10−5), and the level of high molecular weight of vWF multimers was significantly reduced in the CentriMag (P < 10−5) all compared with the static treatment after 6 h in vitro testing. Despite the leukocyte damage, microparticle formation, and cleavage of vWF multimers, these results show that the CentriMag is a hemocompatible pump which could be used as a standard in blood damage assays to inform the design of new implantable blood pumps.
Keywords: CentriMag centrifugal pump, Platelet activation, Cell-derived microparticles, Hemolysis, von Willebrand factor, Leukocyte damage, Apoptosis
Implantable rotary left ventricular assist devices (LVADs) have emerged as mainstream treatment for severely symptomatic heart failure in selected nontransplant eligible patients (1,2). Survival data from INTERMACS suggest that LVAD outcomes now rival transplantation particularly in patients with ischemic cardiomyopathy (3). This has stimulated interest in even smaller devices aimed at improving quality of life and minimizing potentially life-threatening complications. Pump thrombosis and systemic thromboembolism persist as the major limitation of this technology and has proven difficult to predict from in vivo testing in sheep or calves (4). Nonetheless, extensive preclinical testing is mandatory prior to clinical application of any new LVAD (5). Hemocompatibility testing plays a major role in this and as much as possible should be learnt from in vitro work before animal implants. Shear stress encountered by blood in transit through a rotary pump can affect both cells and large proteins in the plasma. Thus, blood testing requires more than evaluation of hemolysis as platelets, leukocytes, and plasma proteins all contribute to the propensity for infection, gastrointestinal (GI) bleeding, and thrombus formation (6–12). It is important to assess overall damage by a new device against a baseline as early as possible in the development process so that design iterations can be made if required.
First-generation pulsatile pumps caused damage to CD4+ lymphocytes, reducing both functionality and cell numbers, resulting in an immunological phenotype resembling that of patients suffering from HIV infection (8). This decline in the CD4+/CD8+ cell ratio occurred as early as 1 month after implantation with the HeartMate VAD (7). A study on patients implanted with either HeartMate II, HeartWare, or PVAD (Thoratec Corporation, Pleasanton, CA, USA) demonstrated that the HeartMate II pump activated granulocytes. This was measured as CD11b (Mac1)-positive CD15-expressing cells by flow cytometry by postoperative day (POD) 14 compared with the other pumps, and this increase remained until POD 120 (11). Activated leukocytes have also been demonstrated to form circulating microparticles, measured using flow cytometry as CD11b+ or CD62L+ 1.5 μM small events in a group of patients implanted with various assisting devices including HeartMate II, Thoratec VAD, Ventrassist, Circulite, and one patient on extracorporeal membrane oxygenation (ECMO) (10). The same study also identified an increase in CD31+/CD61+ platelet microparticles as a sign of platelet activation.
However, current temporary extracorporeal blood pumps, such as the widely used CentriMag centrifugal pump (CP) (Thoratec Corporation), often manifest a lesser blood damage profile than implanted LVADs because of lack of size constraints. This allows for larger slower moving rotors which provide lower shear stress. Combined with relatively low-cost replaceable pump heads and convenient flow connectors, this makes extracorporeal blood pumps a good choice as a baseline control for incorporation into a disposable test loop for in vitro hemocompatibility testing. The CentriMag manifests very low hemolysis levels (NIH of 0.0029 in vitro [13],[14]), so we sought to characterize the overall hemocompatibility profile of this device in vitro with the intention of using this pump as a benchmark control for development of a new long-term rotary LVAD.
Materials and Methods
Preparation of test blood
Blood was collected and prepared as described previously (9). In brief, whole bovine blood was collected into 14% citrate phosphate dextrose adenine (CPDA-1) anticoagulant solution and antibiotics (50 mg/L gentamycin and 10 mL/L antimycotic solution [Sigma Aldrich, Poole, UK]) (15,16). Hematocrit levels were adjusted to 30 ± 2% by dilution with phosphate buffered saline (PBS) (Life Technologies Ltd., Paisley, UK) and transferred into the test circuit within 4 h after sample collection (15).
Device operation and specifications
CentriMag CP was tested using an in vitro testing circuit under constant hemodynamic conditions in accordance with ASTM Standards. The test parameters were controlled such that: the flow rate was 5 ± 0.25 L/min, measured using an ultrasonic flow sensor (ME8-PXL, Transonic Systems Inc., Ithaca, NY, USA); the differential pressure across the pump was adjusted using a custom-made clamp to 100 ± 3 mm Hg, measured using pressure sensors (PRESS-S-000, PendoTECH, Princeton, NJ, USA); the volume of blood in the loop was 450 ± 45 mL; the temperature of the loop was maintained at 37 ± 1°C by the immersion of the blood reservoir and connecting tubing in a temperature controlled water bath, with any exposed tubing lagged to minimize temperature gradients. The test circuit was initially filled with PBS which was circulated by the action of the pump for 20 min to ensure all surfaces were wetted; PBS was drained prior to blood transfusion. Care was taken during warming (37 ± 1°C) of the blood to eliminate micro air bubbles. Prior to the test being started, the loops were purged of any air bubbles such that no air-blood interface existed. Blood samples were taken at hourly intervals via a sampling port in the blood reservoir. The first milliliter was discarded followed by a second draw of 5 mL which was used for the assays described below. A static control sample as recommended by ASTM Standards (15,16) was maintained at 37°C in a water bath throughout the whole test and sampled in the same way at the same time points.
Cell counts
The total numbers of leukocytes, platelets, and erythrocytes were measured using the clinical automatic hematology analyzer CELL-DYN Ruby (Abbott Diagnostics, Berkshire, UK). The accuracy of this for bovine blood was confirmed by analyzing a subset of samples on an analyzer optimized for veterinary purposes to confirm comparable results were obtained (Langford Veterinary Services, University of Bristol, UK).
Hemolysis assay
Three 1 mL aliquots of the hourly blood samples were centrifuged at 4200 × g for 7 min. Plasma supernatant (100 μL) was transferred from each aliquot into three cuvettes (Thermo Scientific, Loughborough, UK) and diluted with 1 mL 0.1% Na2CO3 solution. The absorbance was measured using a UV/visible spectrophotometer (6700 Series, Jenway, Essex, UK) at three wavelengths: 380, 415, and 450 nm; the plasma-free hemoglobin (pfHb) and normalized index of hemolysis (NIH) were calculated as described by Eqs. 1 and 2, respectively (17).
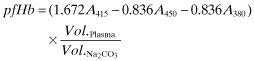 |
(1) |
| (2) |
where:
Leukocyte death assay
Hourly blood samples (20 μL) were stained with 1 μL CD45-PE, clone 1.11.32, mouse IgG1 (Thermo Scientific) for 30 min on ice in the dark (n = 7). The red blood cells were lysed by adding 1 mL ammonium chloride (Stem Cell Technologies, Grenoble, France), followed by vortexing and 10 min incubation on ice in the dark. The cells were pelleted at 515 × g, 7 min, 5°C, and washed once with 2 mL FACS buffer (PBS, Life Technologies Ltd.; 0.2% bovine serum albumin, Sigma Aldrich; and 0.05% sodium azide). Cells were resuspended in 100 μL FACS buffer and stained with 2 μL 7AAD solution (eBioscience, Hatfield, UK) at room temperature in the dark for at least 15 min before acquisition. Antibody capture beads (Life Technologies Ltd.) were used as single-stained compensation controls for CD45-PE. As a positive control for 7AAD staining of dead cells, 1 mL peripheral blood was treated with 3 μM Staurosporin solution (Sigma Aldrich) at room temperature for at least 4 h prior to staining with CD45-PE and 7AAD. Untreated blood single-stained with CD45-PE was used as a negative control in the 7AAD gate (see Figure 3). Samples were acquired and analyzed using BD FACSAria I with FACSDiva 6.1.3 software (BD Bioscience, Oxford, UK) and 10 000 leukocytes or beads were recorded uncompensated using the following filter configuration: AnnexinV-eFluor450 = 405 nm laser and 450/40 filter, 7AAD = 488 nm laser and 695/40 filter, and all PE-conjugated antibodies = 488 nm laser, 585/42 filter. FCS files were exported and analyzed in Kaluza 1.2 (Beckman Coulter, London, UK) using automatic compensation. All events were displayed on a density dot plot with forward scatter (FSC) against side scatter (SSC), both on logarithmic axes. The leukocytes were gated and displayed on a contour density dot plot with 7AAD against CD45-PE, both on logicle axes. The healthy cell gate was set using the Staurosporin-treated control to just outside the main contour of the healthy 7AAD-negative population (Supporting Information Fig. S1). Any events to the right of this border were considered 7AAD-positive necrotic leukocytes. Fragmented white blood cells (leukocyte microparticles; MPs) still display CD45 but no longer contain DNA and because of their reduced size and complexity, and therefore lower SSC, end up below the healthy leukocytes on an SSC axis in the gate designated MP.
FIG 3.
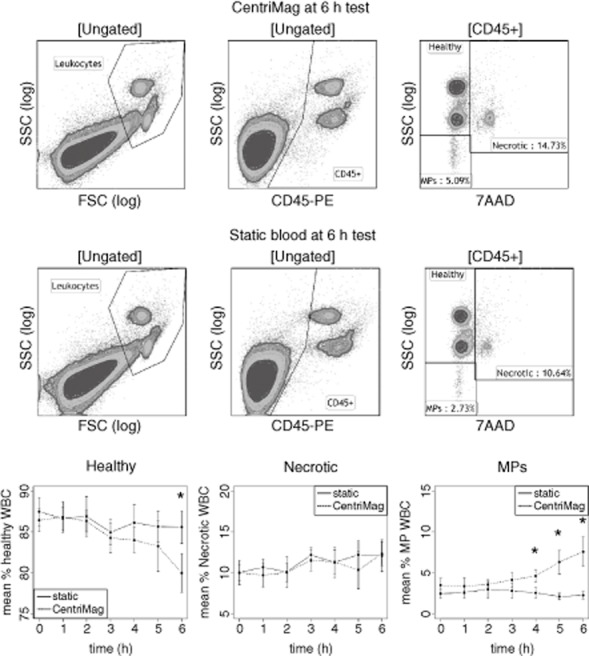
Leukocyte damage measured by flow cytometry. Whole bovine blood tested in the CentriMag (top row) versus static control (middle row) at 6 h. Leukocytes gate used to record 10 000 events. Ungated events displayed on CD45-PE versus SSC density plot and CD45+ events gated. CD45+ events displayed on 7AAD versus SSC density plot and healthy (7AAD-high SSC), necrotic (7AAD+), and microparticles (MPs; 7AAD- low SSC) events recorded for each sample. CentriMag caused a significant decrease in relative healthy events (bottom left) (P < 0.006) and significant increase in relative MP events after 3 h (P < 10−5), indicating that leukocytes are fragmented during the pumping process. Significant difference between CentriMag and static control.
Platelet activation assay
A 20 μL aliquot of the hourly blood samples, along with a positive control of blood treated with 4 μM phorbol 12-myristate 13-acetate (PMA) for 60 min at room temperature with gentle agitation, were stained individually with 5 μL of BAQ125 (1.0 mg/mL, binds activated bovine platelets but antigen still unknown [18],[19]) or CAPP2a (1.0 mg/dL, an anti-ruminant CD41/61 antibody [20]) (both from Monoclonal Antibody Center, Washington State University), diluted 1:50 with FACS buffer, and were left to incubate on ice for 30 min. Cells were washed once with FACS buffer and then stained with 5 μL of 1.0 mg/dL R-phycoerythrin F(ab′)2 fragment of goat anti-mouse IgG (H + L) (Life Technologies Ltd.) diluted 1:20 with FACS buffer and incubated in the dark on ice for 20 min. The red blood cells were lysed with 2 mL BD FACS Lysing Solution (BD Bioscience) according to the manufacturer's instructions. Cells were washed once with FACS buffer and fixed with 200 μL BD Stabilizing Fixative (BD Bioscience) and stored at 4°C overnight prior to acquisition using the instrument set-up described above.
All events were displayed in the FSC versus SSC graph on logarithmic scale. The platelet population was gated to include both platelets in a resting and activated state using the unstimulated control and the PMA-stimulated sample as guidance (Supporting Information Fig. S2); 10 000 events within this gate were recorded. BAQ125 and CAPP2a are expressed on resting inactivated platelets and decreased with platelet activation. Markers were drawn on BAQ125 and CAPP2a histograms to measure the percentage of negative events, and therefore percentage of activated platelets (Supporting Information Fig. S2B and C).
Platelet apoptosis assay
A 20 μL aliquot of the hourly blood sample, as well as the PMA-treated positive control, were stained with 2 μL of Annexin V-eFluor450 (eBioscience) (n = 3), on ice in the dark for 15 min. Red blood cells were lysed with 2 mL BD FACS Lysing Solution (BD Bioscience). Cells were washed once with Annexin V binding buffer (eBioscience) and fixed with 200 μL BD Stabilizing Fixative (BD Bioscience) and stored at 4°C overnight prior to acquisition using the instrument set-up described above. Gating was carried out as above and the platelet activation was displayed on a separate Annexin V-histogram (Supporting Information Fig. S2D). Annexin V positive events were gated to measure the percentage of apoptotic platelets.
High molecular weight (HMW) von Willebrand factor (vWF) degradation
Platelet-rich plasma (PRP) was prepared by centrifuging hourly blood samples for 7 min at 4200 × g at room temperature. Platelet-poor plasma (PPP) was prepared by further centrifuging PRP for 5 min at 13 000 × g at room temperature. The PPP was extracted and analyzed using an automated hematology analyzer CELL-DYN Ruby (Abbott Diagnostics, Abbott Park, IL, USA) to ensure that the plasma was void of blood cells. PPP was used instead of whole blood to ensure that any potential effect of shear on vWF was not a result of cellular interactions. PPP was subjected to electrophoresis on high gelling temperature agarose (0.6% agarose, w/v, Sea Kem, FMC Bioproducts, Rockland, ME, USA) in a horizontal gel apparatus (81-2325, Galileo Bioscience, Cambridge, MA, USA) at 4°C. Electrophoresis was performed at 30 mA (after 30 min, the current was increased to 50 mA until the dye front had migrated 10–12 cm from the origin; total run time was roughly 6 h). vWF multimers separated on agarose gel were transferred to polyvinylidene difluoride 0.45 μM membrane (IPVH304F0, Immobilon-P, Millipore Corporation, Billerica, MA, USA) for 15–17 h at 70 mA in the electroblotting tank (91-2020-TB, Galileo Bioscience). Detection using anti-human vWF primary (ab6994, Abcam, Cambridge, UK) and horseradish peroxidise (HRP)-conjugated secondary (ab6721, Abcam) antibodies, and chemiluminescence substrate (170-5060, Bio-Rad, Hercules, CA, USA) was performed as described by Krizek et al. (38). Pump-induced changes in the size distribution of plasma vWF multimers were studied by densitometric scanning initiated from the origin (Quantity One software v4.6.8, BioRad, Hertfordshire, UK). The loss of HMW vWF multimers was quantified by the rate of change in the gradient of HMW vWF.
Statistical analysis
Averages and standard deviations were calculated for each assay. Due to multiple measurements taken from each subject (both at the same time point to compare CentriMag with static, and at the six different time points), a set of repeated measures analysis of variance (using the R statistical environment, v 3.0.2 [R Core Team, Vienna, Austria], and RStudio v 0.97.551 [RStudio, Boston, MA, USA]) were applied. One analysis of variance (ANOVA) was performed each for the healthy, necrotic, and leukocyte MP analysis in the blood samples. In each case the different subjects were treated as a random effect, and the time points and CentriMag/static as a fixed effect. While the three ANOVAs are not independent (as the sum of the events in the three flow cytometry gates will be 100%), analyzing all separately aids the interpretation.
Results
Cell counts
There was no change in the concentration of leukocytes, erythrocytes, or platelets throughout the 6 h testing period in the CentriMag or the static control (see Fig. 1).
FIG 1.
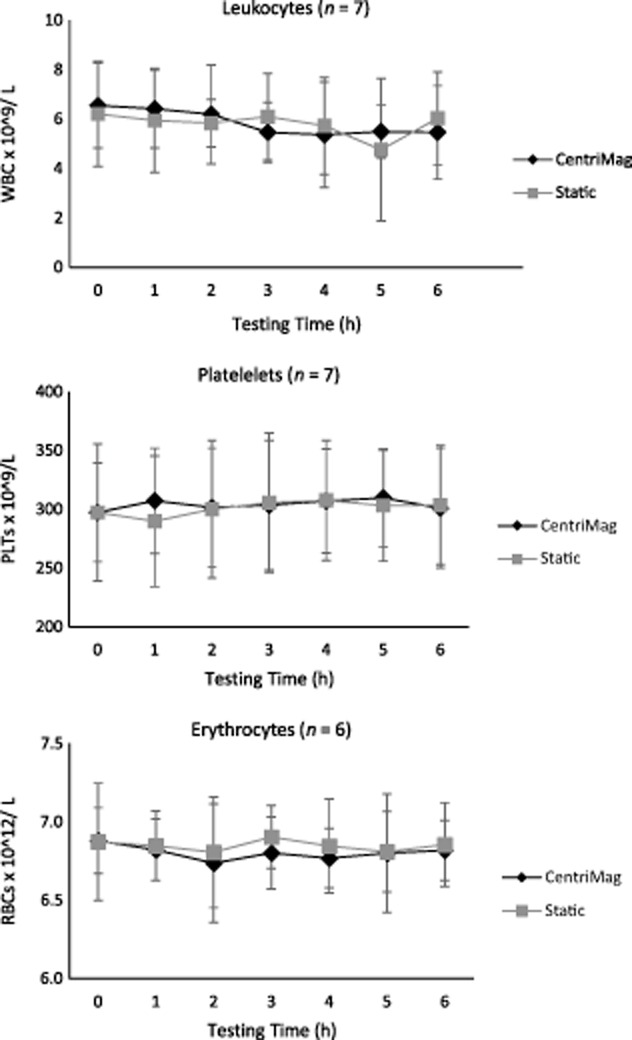
CentriMag's effect on blood cell levels. Whole bovine blood diluted to hematocrit of 30 ± 2% with PBS and pumped for 6 h through a circuit by the CentriMag and compared with a static control kept in a 37°C water bath. Total leukocytes, platelets, and erythrocytes blood counts obtained by the CELL-DYN Ruby automatic hematology analyzer plotted against time (n = 6–7, x ± SD). There is no difference between CentriMag and static blood in terms of blood cell levels.
Hemolysis
Hemolysis was evident in both the pumped sample and in the static control (n = 15). There was a steady increase in pfHb every hour with a statistically significant difference between hours 0 and 6 for both CentriMag (P = 0.016) and static control (P ≤ 0.001) (see Fig. 2). When comparing the 6 h samples, there was no significant difference between the CentriMag and the static control (P = 0.487), indicating that the CentriMag causes minimal hemolysis. NIH for the CentriMag was 0.0011 ± 0.0005 g/100 L (n = 15; x ± SD).
FIG 2.
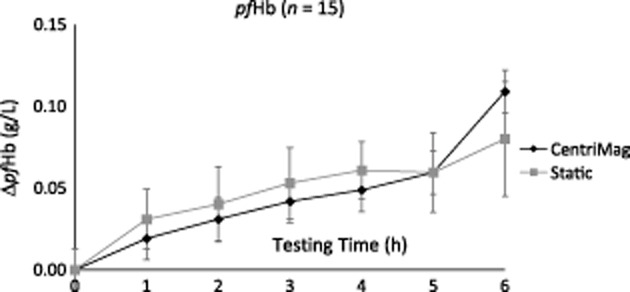
Hemolysis evaluation. Comparison of changes in plasma-free hemoglobin (ΔpfHb) between the CentriMag temporary mechanical circulatory support device and the static control (n = 15). There was no significant difference between CentriMag and static at hour 6 (P = 0.487), indicating that the CentriMag does not cause high-level hemolysis. NIH for the CentriMag was 0.0011 ± 0.0005 g/100 L (n = 15; x ± SD).
Leukocyte death
The percentage of healthy leukocytes (i.e., 7AAD negative and not microparticles) was significantly reduced in the CentriMag compared with the static control (P < 0.006). The effect of time was highly significant (P < 0.001), with the difference between the groups emerging at about 3 h and increasing throughout the test (Fig. 3). Leukocyte MPs also show a highly significant difference between the CentriMag and the static control (P < 10−5) but in the opposing direction; the CentriMag results in a significant increase, and the difference increases over time (P < 0.003, Fig. 3). There was no difference between CentriMag and static samples in terms of necrotic (7AAD-positive) leukocytes. However, there was a significant effect of time (P < 0.02, Fig. 3), with a general small increase in both the CentriMag and the static control.
Platelet activation and apoptosis
There was no significant difference between CentriMag and the static control for platelet activation or apoptosis (P ≥ 0.9) (n = 7; Fig. 4).
FIG 4.
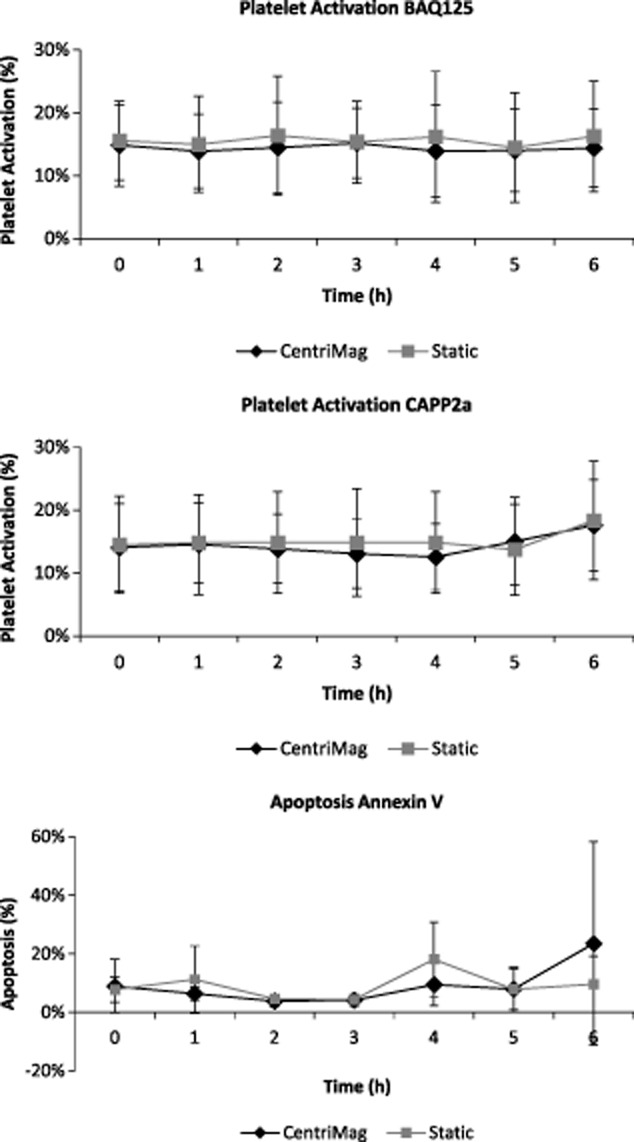
Platelet activation and apoptosis. Whole blood sampled hourly from the pump circuit (CentriMag) or the control (static) single-stained with BAQ125, CAPP2a, or Annexin V, and gated as described in Supporting Information Fig. S2. Top: percentage BAQ125- events; middle: percentage CAPP2a- events; and bottom: percentage Annexin V+ events (n = 7, x ± SD). No significant difference is observed between CentriMag and static control during the 6 h tests.
HMW vWF degradation
The level of HMW vWF multimers was reduced significantly in the CentriMag compared with the static treatment (P < 10−5). The time effect was also significant (P < 0.02). The difference between the groups appeared at about 2–3 h and continued to increase over the time period (Fig. 5).
FIG 5.
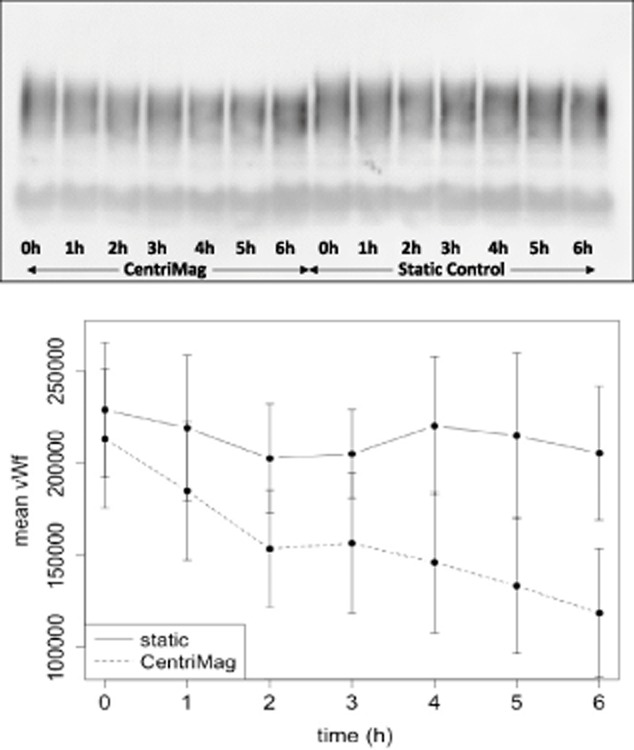
HMW vWF degradation. Platelet-poor plasma isolated from blood sampled hourly from the pump circuit (CentriMag) or the static control (n = 7) and analyzed by immunoblotting (top) and densitometry of the blot (bottom). Bottom graph: time versus rate of change in the gradient of HMW vWF. The level of the HMW vWF multimers is significantly lower in the CentriMag compared with the static control (P < 10−5) from around 3 h, and this increases over time.
Discussion
The CentriMag temporary centrifugal VAD has proven safety and clinical effectiveness in left and/or right ventricular and ECMO support for long-term use periods exceeding 300 days (22). Pump-related hemolysis and adverse event rates are consistently low, with outcomes depending predominantly upon patient status rather than VAD complications. As such, we chose to evaluate the CentriMag centrifugal pump as a control device against which new VADs in development can be tested. Our comprehensive in vitro baseline assessment using bovine-citrated blood confirms the low blood damage profile of CentriMag as experienced in clinical practice. However, there were significant differences in the total blood damage profile of circulating blood driven by CentriMag and static blood during the 6 h in vitro testing using a broader range of assays. This includes a decrease in healthy leukocytes, an increase in leukocyte fragmentation generating CD45+ microparticles, and a reduction in HMW vWF multimers. The effects became apparent around the 3 h mark and steadily increased over time.
Patients with VADs have shown increased platelet, leukocyte, and endothelial MPs compared with healthy controls. The MPs have been suggested to be involved in thrombus formation, through binding of platelets to activated endothelium and through activation of the intrinsic coagulation pathway via Factor XII exposure to negatively charged membrane particles (10,23,24). Shear stress has been suggested to mechanistically underpin MP release as demonstrated by increased platelet-derived MPs (PMPs) in patients implanted with prosthetic heart valves who typically experience high levels of shear, and whose elevated PMPs are the suspected cause of cerebrovascular events. Therefore, it is of importance to measure the MP-levels caused by VADs in vitro, to ensure new designs are made with minimal shear and are as safe as possible.
GI bleeding is one complication associated with the placement of a VAD, with rates as high as 65% within the first year after VAD placement (21,25). The mechanism underlying this problem in patients on long-term continuous flow mechanical support is not well understood (12,21,26–33). This cannot be explained by the anticoagulation regimen alone, but may be symptomatic of acquired von Willebrand syndrome. This disease can be explained partially by shear stress, as those suffering from aortic valve stenosis and those implanted with axial flow VADs both experience high shear and have reported bleeding and loss of HMW vWF multimers (34,35). Thus, comparing the degradation of vWF in VADs provides important feedback to device developers about shear stress levels.
Over a 6 h period in the recirculating loop, hemolysis and platelet damage remained low. Specifically, there was no difference between CentriMag and static samples in relation to necrotic 7AAD+ leukocytes, nor significant differences in platelet activation or apoptosis. Both antibody studies showed that background platelet activation occurs throughout the testing period, but this did not differ to the static control at 6 h. The Annexin V platelet apoptosis assay also indicates a low blood damage profile. We intend to confirm these findings using human blood as it is known that bovine platelets are less reactive than human platelets in response to shear stress (36,37). Our laboratory has previously carried out hemolysis and leukocyte profile assays using the temporary RotaFlow CP and the now obsolete VentrAssist implantable rotary blood pump. These studies showed that hemolysis and leukocyte damage do not necessarily correlate closely, and that a pump with low hemolysis can still have a significant damaging effect on leukocytes as shown for the VentrAssist (9). With similar results using our total blood damage profiling approach, both the CentriMag and RotaFlow CP appear to be good comparators when undertaking in vitro testing of new implantable LVADs, although RotaFlow CP's effect on platelet activation would need to be established first.
The field of in vitro blood damage profiling remains at an early stage of development with a need to address further aspects such as leukocyte activation, origin of the leukocyte microparticles, activation of the coagulation cascade, and the degradation of vWF multimers. Recent results show that increasing lactate dehydrogenase (LDH) levels can be monitored as a possible predictor of pump thrombosis in VAD patients (4). Developing a greater understanding of other hemocompatibility parameters such as changes in vWF, platelet, or leukocyte activation, may provide even more sensitive or early prediction of adverse reactions in clinical trials of blood pumps.
Conclusions
In summary, we have demonstrated that the CentriMag centrifugal blood pump has a low blood damage profile during in vitro testing of bovine blood. In our previous work, apart from the platelet activation and vWF degradation assay, the RotaFlow CP had similar low blood damage results compared to the CentriMag CP. We therefore conclude that these extracorporeal blood pumps, with their spacious design and low shear stress, have an excellent hemocompatibility profile and are good baseline controls for in vitro testing of new implantable VADs. Although bovine platelets were not activated in the CentriMag, new evidence has shown that platelet activation may be species-specific and device developers should validate results using several species and/or human blood where possible.
Acknowledgments
The authors would like to thank the Technology Strategy Board for the Biomedical Catalyst Award (reference: 101462) provided to Calon Cardio-Technology Ltd and Swansea University, and the ILS2020 project which have funded this research. The ILS2020 project is funded by the Academic Expertise for Business (A4B) fund financed by the Welsh Government and the European Union. The authors would also like to thank Lleucu Davies for blood sample collection.
Glossary
- NIH:
normalized index of hemolysis (g/100 L)
- ΔpfHb:
increase of plasma-free hemoglobin concentration (g/L) over the sampling time interval
- V:
circuit volume (L)
- Q:
flow rate (L/min)
- Ht:
hematocrit (%)
- T:
sampling time (min)
Conflict of Interest
Authors Alyssa Jones, Gemma Radley, and Graham Foster are employees of Calon Cardio-Technology Ltd (Calon Cardio). Stephen Westaby is one of the founders of Calon Cardio-Technology Ltd, and both Stephen Westaby and Graham Foster own stock in the company. Chris H.H. Chan and Ina Laura Pieper are employees of Swansea University but funded by Calon Cardio, fully and partially, respectively. Rebecca Hambly is a PhD student partially funded by Calon Cardio.
Authors' contributions
Chris H.H. Chan: concept/design, data collection, data analysis/interpretation, drafting article, critical revision of article, approval of article
Ina Laura Pieper: concept/design, data collection, data analysis/interpretation, drafting article, critical revision of article, approval of article
Rebecca Hambly: data collection, data analysis/interpretation, drafting article, critical revision of article, approval of article
Alyssa Jones: data collection, data analysis/interpretation, drafting article, critical revision of article, approval of article
Gemma Radley: data collection, data analysis/interpretation, drafting article, critical revision of article, approval of article
Yasmin Friedmann: data analysis/interpretation, drafting article, critical revision of article, approval of article
Karl M. Hawkins: critical revision of article, approval of article
Stephen Westaby: drafting article, critical revision of article, approval of article
Graham Foster: secured funding, drafting article, critical revision of article, approval of article
Catherine A. Thornton: concept/design, data analysis/interpretation, secured funding, critical revision of article, approval of article
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Leukocyte death gating strategy. Gating steps 1–3: whole blood treated with Staurosporin to induce leukocyte death was used to set up the gating strategy for CD45-PE and 7AAD. (1) A leukocyte gate was used to record 10 000 events. (2) Ungated events on a CD45-PE vs SSC (log) dot plot identifies CD45+ leukocytes. (3) CD45+ events on a 7AAD versus SSC (log) dot plot distinguishes necrotic 7AAD+ events from 7AAD- healthy and fragmented leukocytes (MPs). Negative control: whole untreated blood single-stained with CD45-PE.
Fig. S2. Platelet activation and apoptosis gating strategy. (A) Ungated events displayed on FSC versus SSC on logarithmic axes. Platelets were gated and 10 000 events recorded. (B and C) Ungated population displayed on a histogram of BAQ125-PE (B) or CAPP2a-PE (C) intensity and activated platelets (negative events) were gated. (D) Platelet population displayed on a histogram of Annexin V-eFluor450 intensity and Annexin V-positive (apoptotic) platelets were gated.
References
- 1.Westaby S, Deng M. Continuous flow blood pumps: the new gold standard for advanced heart failure? Eur J Cardiothorac Surg. 2013;44:4–8. doi: 10.1093/ejcts/ezt248. [DOI] [PubMed] [Google Scholar]
- 2.Westaby S. Cardiac transplant or rotary blood pump: contemporary evidence. J Thorac Cardiovasc Surg. 2013;145:24–31. doi: 10.1016/j.jtcvs.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Pagani FD, et al. Long-term mechanical circulatory support (destination therapy): on track to compete with heart transplantation? J Thorac Cardiovasc Surg. 2012;144:584–603. doi: 10.1016/j.jtcvs.2012.05.044. discussion 597–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:1466–1467. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 5.Acker MA, Pagani FD, Stough WG, et al. Statement regarding the pre and post market assessment of durable, implantable ventricular assist devices in the United States. Circulation: Heart Failure. 2013;6:e1–11. doi: 10.1161/HHF.0b013e318279f6b5. [DOI] [PubMed] [Google Scholar]
- 6.Garbade J, Bittner HB, Barten MJ, Mohr F-W. Current trends in implantable left ventricular assist devices. Cardiol Res Pract. 2011;2011:290516–290519. doi: 10.4061/2011/290561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ankersmit HJ, Edwards NM, Schuster M, et al. Quantitative changes in T-cell populations after left ventricular assist device implantation: relationship to T-cell apoptosis and soluble CD95. Circulation. 1999;100:II211–215. doi: 10.1161/01.cir.100.suppl_2.ii-211. [DOI] [PubMed] [Google Scholar]
- 8.Ankersmit HJ, Tugulea S, Spanier T, et al. Activation-induced T-cell death and immune dysfunction after implantation of left-ventricular assist device. Lancet. 1999;354:550–555. doi: 10.1016/s0140-6736(98)10359-8. [DOI] [PubMed] [Google Scholar]
- 9.Chan CH, Hilton A, Foster G, Hawkins KM, Badiei N, Thornton CA. The evaluation of leukocytes in response to the in vitro testing of ventricular assist devices. Artif Organs. 2013;37:793–801. doi: 10.1111/aor.12161. [DOI] [PubMed] [Google Scholar]
- 10.Diehl P, Aleker M, Helbing T, et al. Enhanced microparticles in ventricular assist device patients predict platelet, leukocyte and endothelial cell activation. Interact Cardiovasc Thorac Surg. 2010;11:133–137. doi: 10.1510/icvts.2010.232603. [DOI] [PubMed] [Google Scholar]
- 11.Woolley JR, Teuteberg JJ, Bermudez CA, et al. Temporal leukocyte numbers and granulocyte activation in pulsatile and rotary ventricular assist device patients. Artif Organs. 2013;38:447–455. doi: 10.1111/aor.12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielsen LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II) J Am Coll Cardiol. 2009;53:2162–2167. doi: 10.1016/j.jacc.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 13.Zhang JT, Gellman B, Koert A, et al. Computational and experimental evaluation of the fluid dynamics and hemocompatibility of the CentriMag blood pump. Artif Organs. 2006;30:168–177. doi: 10.1111/j.1525-1594.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 14.Sobieski MA, Giridharan GA, Ising M, Koenig SC, Slaughter MS. Blood trauma testing of CentriMag and RotaFlow centrifugal flow devices: a pilot study. Artif Organs. 2012;36:677–682. doi: 10.1111/j.1525-1594.2012.01514.x. [DOI] [PubMed] [Google Scholar]
- 15.ASTM. 2005. F1841-97. Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps.
- 16.ASTM. 2005. F1830-97. Standard Practice for Selection of Blood for In Vitro Evaluation of Blood Pumps.
- 17.Chan CH, Hilton A, Foster G, Hawkins K. Reevaluation of the Harboe assay as a standardized method of assessment for the hemolytic performance of ventricular assist devices. Artif Organs. 2012;36:724–730. doi: 10.1111/j.1525-1594.2012.01515.x. [DOI] [PubMed] [Google Scholar]
- 18.Snyder TA, Tsukui H, Kihara S, et al. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: coating comparison and platelet activation. J Biomed Mater Res A. 2007;81A:85–92. doi: 10.1002/jbm.a.31006. [DOI] [PubMed] [Google Scholar]
- 19.Snyder TA, Watach MJ, Litwak KN, Wagner WR. Platelet activation, aggregation, and life span in calves implanted with axial flow ventricular assist devices. Ann Thorac Surg. 2002;73:1933–1938. doi: 10.1016/s0003-4975(02)03549-x. [DOI] [PubMed] [Google Scholar]
- 20.Snyder TA, Litwak KN, Tsukui H, et al. Leukocyte-platelet aggregates and monocyte tissue factor expression in bovines implanted with ventricular assist devices. Artif Organs. 2007;31:126–131. doi: 10.1111/j.1525-1594.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 21.Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137:208–215. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Griffith K, Jenkins E, Stulak J, Paugh T, Pagani F. Long-term use of the CentriMag ventricular assist system as a right ventricular assist device: a case report. Perfusion. 2012;27:65–70. doi: 10.1177/0267659111424634. [DOI] [PubMed] [Google Scholar]
- 23.Vogler EA, Siedlecki CA. Contact activation of blood-plasma coagulation. Biomaterials. 2009;30:1857–1869. doi: 10.1016/j.biomaterials.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiser T, Sturzenegger M, Genewein U, Haeberli A, Beer JH. Mechanisms of cerebrovascular events as assessed by procoagulant activity, cerebral microemboli, and platelet microparticles in patients with prosthetic heart valves. Stroke. 1998;29:1770–1777. doi: 10.1161/01.str.29.9.1770. [DOI] [PubMed] [Google Scholar]
- 25.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. NEJM. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 26.Malehsa D, Meyer AL, Bara C, Struber M. Acquired von Willebrand syndrome after exchange of the HeartMate XVE to the HeartMate II ventricular assist device. Eur J Cardiothorac Surg. 2009;35:1091–1093. doi: 10.1016/j.ejcts.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 27.Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circulation: Heart Failure. 2010;3:675–681. doi: 10.1161/CIRCHEARTFAILURE.109.877597. [DOI] [PubMed] [Google Scholar]
- 28.Eckman PM. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–3047. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 29.Letsou GV, Shah N, Gregoric ID, Myers TJ, Delgado R, Frazier OH, et al. Gastrointestinal bleeding from arteriovenous malformations in patients supported by the Jarvik 2000 axial-flow left ventricular assist device. J Heart Lung Transplant. 2005;24:105–109. doi: 10.1016/j.healun.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Uriel N, Pak S-W, Jorde UP. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–1269. doi: 10.1016/j.athoracsur.2010.04.099. discussion 1269. [DOI] [PubMed] [Google Scholar]
- 32.Heilmann C, Geisen U, Beyersdorf F, et al. Acquired von Willebrand syndrome in patients with ventricular assist device or total artificial heart. Thromb Haemost. 2010;103:962–967. doi: 10.1160/TH09-07-0497. [DOI] [PubMed] [Google Scholar]
- 33.Geisen U, Heilmann C, Beyersdorf F, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 34.Pareti FI, Lattuada AL, Bressi C, et al. Proteolysis of von Willebrand factor and shear stress-induced platelet aggregation in patients with aortic valve stenosis. Circulation. 2000;102:1290–1295. doi: 10.1161/01.cir.102.11.1290. [DOI] [PubMed] [Google Scholar]
- 35.Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 36.Lu Q, Hofferbert BV, Koo G, Malinauskas RA. In vitro shear stress-induced platelet activation: sensitivity of human and bovine blood. Artif Organs. 2013;37:894–903. doi: 10.1111/aor.12099. [DOI] [PubMed] [Google Scholar]
- 37.Saeed D, Fukamachi K. In vivo preclinical anticoagulation regimens after implantation of ventricular assist devices. Artif Organs. 2009;33:491–503. doi: 10.1111/j.1525-1594.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 38.Krizek DR, Rick ME. A rapid method to visualize von Willebrand factor multimers by using agarose gel electrophoresis, immunolocalization and luminographic detection. Thromb Res. 2000;97:457–462. doi: 10.1016/s0049-3848(99)00196-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Leukocyte death gating strategy. Gating steps 1–3: whole blood treated with Staurosporin to induce leukocyte death was used to set up the gating strategy for CD45-PE and 7AAD. (1) A leukocyte gate was used to record 10 000 events. (2) Ungated events on a CD45-PE vs SSC (log) dot plot identifies CD45+ leukocytes. (3) CD45+ events on a 7AAD versus SSC (log) dot plot distinguishes necrotic 7AAD+ events from 7AAD- healthy and fragmented leukocytes (MPs). Negative control: whole untreated blood single-stained with CD45-PE.
Fig. S2. Platelet activation and apoptosis gating strategy. (A) Ungated events displayed on FSC versus SSC on logarithmic axes. Platelets were gated and 10 000 events recorded. (B and C) Ungated population displayed on a histogram of BAQ125-PE (B) or CAPP2a-PE (C) intensity and activated platelets (negative events) were gated. (D) Platelet population displayed on a histogram of Annexin V-eFluor450 intensity and Annexin V-positive (apoptotic) platelets were gated.


